Eligibility and efficacy of a CPC‐ and CHX‐based antiviral mouthwash for the elimination of SARS‐CoV‐2 from the saliva: A randomized, double‐blind, controlled clinical trial
et al., Journal of Clinical Periodontology, doi:10.1111/jcpe.13905, Dec 2023
58th treatment shown to reduce risk in
September 2025, now with p = 0.0035 from 4 studies.
Lower risk for recovery and viral clearance.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 20 patients rinsing with CPC-CHX compared with 20 patients rinsing with water, showing no significant difference in short-term viral load via PCR and ELISA.
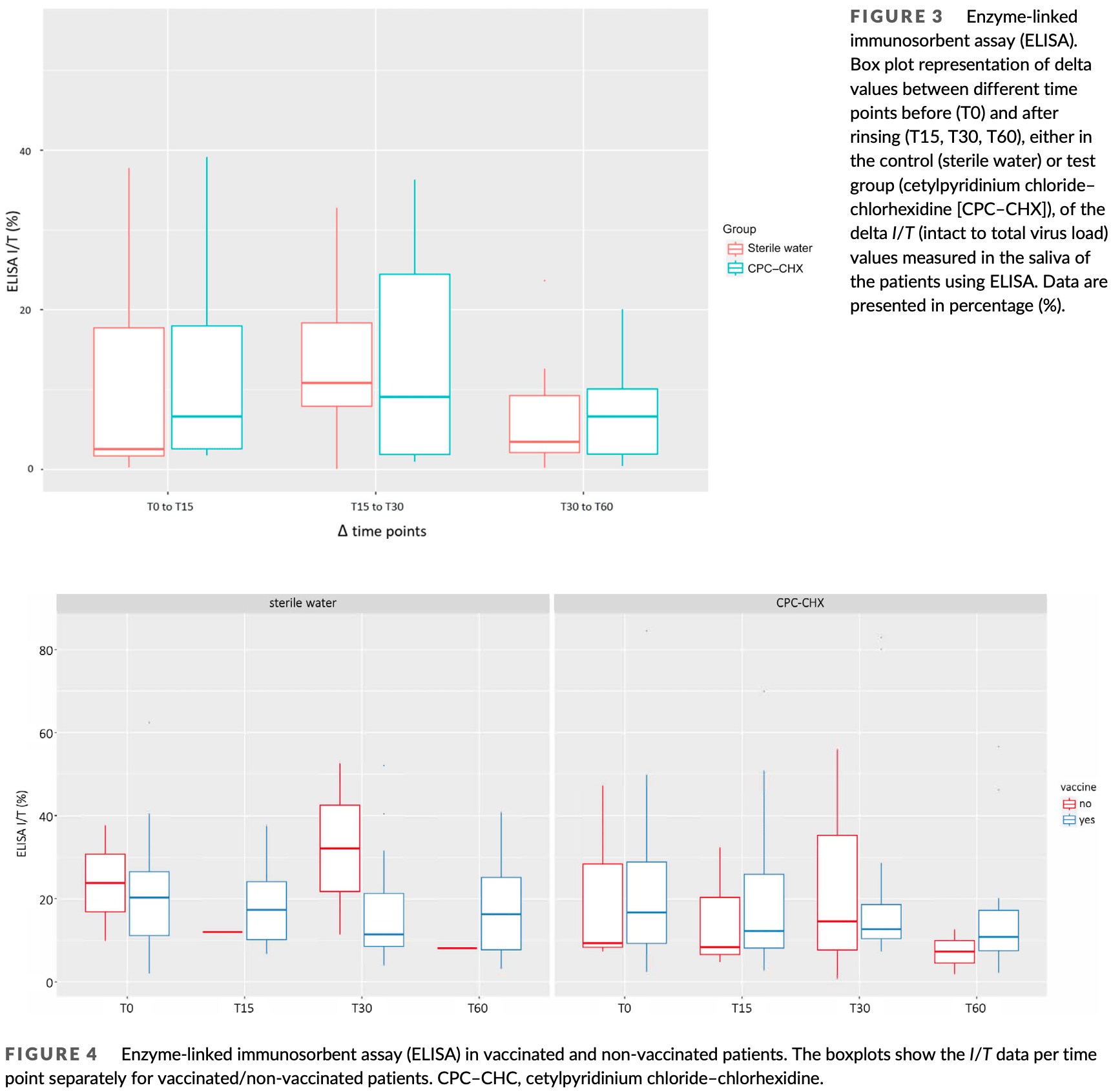
Results are not clear. Figure 3 shows an increase in ELISA I/T between all time points in both groups, which does not match Figure 4. For example, in Figure 4 I/T decreases for both water and CPC-CHX groups in both the vaccinated and unvaccinated subgroups.
Study covers cetylpyridinium chloride and chlorhexidine.
Giulia et al., 7 Dec 2023, Double Blind Randomized Controlled Trial, Germany, peer-reviewed, mean age 40.2, 9 authors, study period January 2022 - August 2022.
Contact: kathrin.becker@charite.de.
Eligibility and efficacy of a CPC‐ and CHX‐based antiviral mouthwash for the elimination of SARS‐CoV‐2 from the saliva: A randomized, double‐blind, controlled clinical trial
Journal of Clinical Periodontology, doi:10.1111/jcpe.13905
Aim: This study aimed at investigating the efficacy of a 0.05% cetylpyridinium chloride-0.05% chlorhexidine (CPC-CHX) mouthwash in reducing viral load in the saliva as compared with sterile water. Materials and Methods: Forty SARS-CoV-2 positive patients were asked to dispense 4 mL of saliva. Half the patients rinsed for 60 s with 15 mL CPC-CHX, and the remaining patients rinsed with sterile water (control). Four millilitres of saliva were collected after 15, 30 and 60 min after rinsing. Quantitative reverse transcriptase polymerase chain reaction (RT-qPCR) and enzyme-linked immunosorbent assay (ELISA) specific for SARS-CoV-2 nucleocapsid protein were performed. For ELISA, the intact (representing the active virus) to total virus load (I/T) was calculated. Results: SARS-CoV-2 copy numbers/mL from RT-qPCR tended to decrease in the control group, whereas in the CPC-CHX group, an increase was observed after T30. However, mixed linear model analysis revealed no statistical differences between groups (p = .124), time points (p = .616) and vaccinated or non-vaccinated patients (p = .953). Similarly, no impact of group (p = .880), time points (p = .306) and vaccination (p = .711) was observed for I/T ratio values. Conclusions: Within the limitation of this study, there was no evidence that the intervention reduced salivary SARS-CoV-2 viral load during the course of 60 min. Therefore, commonly used pre-procedural rinsing might not be clinically relevant.
AUTHOR CONTRIBUTIONS Conceptualisation: Giulia Brunello, Jürgen Becker and Kathrin Becker.
ACKNOWLEDGEMENT Open Access funding enabled and organized by Projekt DEAL.
FUNDING INFORMATION The study was self-funded by the Department of Oral Surgery, University Hospital Düsseldorf (Düsseldorf, Germany).
CONFLICT OF INTEREST STATEMENT The authors declare no conflicts of interest.
SUPPORTING INFORMATION Additional supporting information can be found online in the Supporting Information section at the end of this article.
References
Alemany, Perez-Zsolt, Raïch-Regué, Muñoz-Basagoiti, Ouchi et al., Cetylpyridinium chloride mouthwash to reduce shedding of infectious SARS-CoV-2: A doubleblind randomized clinical trial, Journal of Dental Research, doi:10.1177/00220345221102310
Becker, Gurzawska-Comis, Brunello, Klinge, Summary of European guidelines on infection control and prevention during COVID-19 pandemic, Clinical Oral Implants Research, doi:10.1111/clr.13784
Brunello, Gurzawska-Comis, Becker, Becker, Sivolella et al., Dental care during COVID-19 pandemic: Follow-up survey of experts' opinion, Clinical Oral Implants Research, doi:10.1111/clr.13783
Carrouel, Gadea, Esparcieux, Dimet, Langlois et al., Saliva quantification of SARS-CoV-2 in real-time PCR from asymptomatic or mild COVID-19 adults, Frontiers in Microbiology, doi:10.3389/fmicb.2021.786042
Core, R: A language and environment for statistical computing
Ferrer, Barrueco, Martinez-Beneyto, Mateos-Moreno, Ausina-Márquez et al., Clinical evaluation of antiseptic mouth rinses to reduce salivary load of SARS-CoV-2, Scientific Reports, doi:10.1038/s41598-021-03461-y
Huang, Pérez, Kato, Mikami, Okuda et al., SARS-CoV-2 infection of the oral cavity and saliva, Nature Medicine, doi:10.1038/s41591-021-01296-8
Jefferson, Spencer, Brassey, Heneghan, Viral cultures for coronavirus disease 2019 infectivity assessment: A systematic review, Clinical Infectious Diseases, doi:10.1093/cid/ciaa1764
Justo, Bueno, Barbosa, Perosa, Carvalho et al., Comparison of viral load between saliva and nasopharyngeal swabs for SARS-CoV2: The role of days of symptoms onset on diagnosis, Mem orias do Instituto Oswaldo Cruz, doi:10.1590/0074-02760210018
Koch-Heier, Hoffmann, Schindler, Lussi, Planz, Inactivation of SARS-CoV-2 through treatment with the mouth rinsing solutions ViruProX( ® ) and BacterX( ® ) pro, Microorganisms, doi:10.3390/microorganisms9030521
Komine, Yamaguchi, Okamoto, Yamamoto, Virucidal activity of oral care products against SARS-CoV-2 in vitro, Journal of Oral and Maxillofacial Pathology, doi:10.1016/j.ajoms.2021.02.002
Lai, German, Hong, Tai, Mcphaul et al., Comparison of saliva and midturbinate swabs for detection of SARS-CoV-2, Microbiol Spectrum, doi:10.1128/spectrum.00128-22
Meister, Brüggemann, Todt, Conzelmann, Müller et al., Virucidal efficacy of different Oral rinses against severe acute respiratory syndrome coronavirus 2, The Journal of Infectious Diseases, doi:10.1093/infdis/jiaa471
Meister, Gottsauner, Schmidt, Heinen, Todt et al., Mouthrinses against SARS-CoV-2-High antiviral effectivity by membrane disruption in vitro translates to mild effects in a randomized placebo-controlled clinical trial, Virus Research, doi:10.1016/j.virusres.2022.198791
Mostafa, Barhoum, Sehit, Gewaid, Mostafa et al., Current trends in COVID-19 diagnosis and its new variants in physiological fluids: Surface antigens, antibodies, nucleic acids, and RNA sequencing, Trends in Analytical Chemistry, doi:10.1016/j.trac.2022.116750
Muñoz-Basagoiti, Perez-Zsolt, Le On, Blanc, Raïch-Regué et al., Mouthwashes with CPC reduce the infectivity of SARS-CoV-2 variants in vitro, Journal of Dental Research, doi:10.1177/00220345211029269
Perussolo, Teh, Gkranias, Tiberi, Petrie et al., Efficacy of three antimicrobial mouthwashes in reducing SARS-CoV-2 viral load in the saliva of hospitalized patients: A randomized controlled pilot study, Scientific Reports, doi:10.1038/s41598-023-39308-x
Popkin, Zilka, Dimaano, Fujioka, Rackley et al., Cetylpyridinium chloride (CPC) exhibits potent, rapid activity against influenza viruses in vitro and in vivo, Pathogens & Immunity, doi:10.20411/pai.v2i2.200
Rathod, Poojari, Manna, Is lipid specificity key to the potential antiviral activity of mouthwash reagent chlorhexidine against SARS-CoV-2?, Membranes, doi:10.3390/membranes12060616
Rathore, Rathore, Singh, Kumar, Redefining aerosol in dentistry during COVID-19 pandemic, Dental Research Journal
Sbricoli, Schiavon, Brunello, Brun, Becker et al., Efficacy of different mouthwashes against COVID-19: A systematic review and network meta-analysis, Japanese Dental Science Review
Schulz, Altman, Moher, CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials, Trials, doi:10.1186/1745-6215-11-32
Sánchez Barrueco, Mateos-Moreno, Martínez-Beneyto, García-Vázquez, Campos González et al., Effect of oral antiseptics in reducing SARS-CoV-2 infectivity: Evidence from a randomized double-blind clinical trial, Emerging Microbes & Infections, doi:10.1080/22221751.2022.2098059
Tarrag O-Gil, Gil-Mosteo, Aza-Pascual-Salcedo, Alvarez, Ainaga et al., Randomized clinical trial to assess the impact of oral intervention with cetylpyridinium chloride to reduce salivary SARS-CoV-2 viral load, Journal of Clinical Periodontology, doi:10.1111/jcpe.13746
Villani, Aiuto, Paglia, Re, COVID-19 and dentistry: Prevention in dental practice, a literature review, International Journal of Environmental Research and Public Health, doi:10.3390/ijerph17124609
Walker, Houwaart, Finzer, Ehlkes, Tyshaieva et al., Characterization of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection clusters based on integrated genomic surveillance, outbreak analysis and contact tracing in an urban setting, Clinical Infectious Diseases, doi:10.1093/cid/ciab588
Wyllie, Fournier, Casanovas-Massana, Campbell, Tokuyama et al., Saliva or nasopharyngeal swab specimens for detection of SARS-CoV-2, New England Journal of Medicine
Xu, Li, Gan, Du, Yao, Salivary glands: Potential reservoirs for COVID-19 asymptomatic infection, Journal of Dental Research, doi:10.1177/0022034520918518
Xu, Li, Zhu, Liang, Fang et al., Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding, Nature Medicine, doi:10.1038/s41591-020-0817-4
Xu, Zhong, Deng, Peng, Dan et al., High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa, International Journal of Oral Science, doi:10.1038/s41368-020-0074-x
Zhang, Meng, Duo, Yang, Dong et al., Efficacy of mouthwash on reducing salivary SARS-CoV-2 viral load and clinical symptoms: A systematic review and meta-analysis, BMC Infectious Diseases, doi:10.1186/s12879-023-08669-z
DOI record:
{
"DOI": "10.1111/jcpe.13905",
"ISSN": [
"0303-6979",
"1600-051X"
],
"URL": "http://dx.doi.org/10.1111/jcpe.13905",
"abstract": "<jats:title>Abstract</jats:title><jats:sec><jats:title>Aim</jats:title><jats:p>This study aimed at investigating the efficacy of a 0.05% cetylpyridinium chloride–0.05% chlorhexidine (CPC–CHX) mouthwash in reducing viral load in the saliva as compared with sterile water.</jats:p></jats:sec><jats:sec><jats:title>Materials and Methods</jats:title><jats:p>Forty SARS‐CoV‐2 positive patients were asked to dispense 4 mL of saliva. Half the patients rinsed for 60 s with 15 mL CPC–CHX, and the remaining patients rinsed with sterile water (control). Four millilitres of saliva were collected after 15, 30 and 60 min after rinsing. Quantitative reverse transcriptase polymerase chain reaction (RT‐qPCR) and enzyme‐linked immunosorbent assay (ELISA) specific for SARS‐CoV‐2 nucleocapsid protein were performed. For ELISA, the intact (representing the active virus) to total virus load (<jats:italic>I</jats:italic>/<jats:italic>T</jats:italic>) was calculated.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>SARS‐CoV‐2 copy numbers/mL from RT‐qPCR tended to decrease in the control group, whereas in the CPC–CHX group, an increase was observed after T30. However, mixed linear model analysis revealed no statistical differences between groups (<jats:italic>p</jats:italic> = .124), time points (<jats:italic>p</jats:italic> = .616) and vaccinated or non‐vaccinated patients (<jats:italic>p</jats:italic> = .953). Similarly, no impact of group (<jats:italic>p</jats:italic> = .880), time points (<jats:italic>p</jats:italic> = .306) and vaccination (<jats:italic>p</jats:italic> = .711) was observed for <jats:italic>I</jats:italic>/<jats:italic>T</jats:italic> ratio values.</jats:p></jats:sec><jats:sec><jats:title>Conclusions</jats:title><jats:p>Within the limitation of this study, there was no evidence that the intervention reduced salivary SARS‐CoV‐2 viral load during the course of 60 min. Therefore, commonly used pre‐procedural rinsing might not be clinically relevant.</jats:p></jats:sec>",
"alternative-id": [
"10.1111/jcpe.13905"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2023-09-06"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2023-11-07"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2023-12-07"
}
],
"author": [
{
"affiliation": [
{
"name": "Department of Oral Surgery University Hospital Düsseldorf Düsseldorf Germany"
},
{
"name": "Department of Neurosciences, Dentistry Section University of Padua Padua Italy"
}
],
"family": "Giulia",
"given": "Brunello",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Oral Surgery University Hospital Düsseldorf Düsseldorf Germany"
}
],
"family": "Viktoria",
"given": "Wolf",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Orthodontics and Dentofacial Orthopaedics Charité–Universitätsmedizin Berlin Berlin Germany"
},
{
"name": "Department of Orthodontics University Hospital Düsseldorf Düsseldorf Germany"
}
],
"family": "Robert",
"given": "Kerberger",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Emergency Department University Hospital Düsseldorf Düsseldorf Germany"
}
],
"family": "Michael",
"given": "Bernhard",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Virology Heinrich‐Heine‐Universität Düsseldorf Düsseldorf Germany"
}
],
"family": "Nadine",
"given": "Lübke",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Oral Surgery University Hospital Düsseldorf Düsseldorf Germany"
}
],
"family": "Jürgen",
"given": "Becker",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute for Anatomy II Heinrich‐Heine‐Universität Düsseldorf Düsseldorf Germany"
}
],
"family": "Beryl",
"given": "Schwarz‐Herzke",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Institute of Virology Heinrich‐Heine‐Universität Düsseldorf Düsseldorf Germany"
}
],
"family": "Jörg",
"given": "Timm",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1936-4683",
"affiliation": [
{
"name": "Department of Orthodontics and Dentofacial Orthopaedics Charité–Universitätsmedizin Berlin Berlin Germany"
}
],
"authenticated-orcid": false,
"family": "Kathrin",
"given": "Becker",
"sequence": "additional"
}
],
"container-title": "Journal of Clinical Periodontology",
"container-title-short": "J Clinic Periodontology",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2023,
12,
8
]
],
"date-time": "2023-12-08T00:06:15Z",
"timestamp": 1701993975000
},
"deposited": {
"date-parts": [
[
2023,
12,
8
]
],
"date-time": "2023-12-08T00:06:19Z",
"timestamp": 1701993979000
},
"indexed": {
"date-parts": [
[
2023,
12,
8
]
],
"date-time": "2023-12-08T05:22:41Z",
"timestamp": 1702012961767
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
12,
7
]
]
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
12,
7
]
],
"date-time": "2023-12-07T00:00:00Z",
"timestamp": 1701907200000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1111/jcpe.13905",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"prefix": "10.1111",
"published": {
"date-parts": [
[
2023,
12,
7
]
]
},
"published-online": {
"date-parts": [
[
2023,
12,
7
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1177/00220345221102310",
"doi-asserted-by": "publisher",
"key": "e_1_2_14_2_1"
},
{
"DOI": "10.1111/clr.13784",
"doi-asserted-by": "publisher",
"key": "e_1_2_14_3_1"
},
{
"DOI": "10.1111/clr.13783",
"doi-asserted-by": "publisher",
"key": "e_1_2_14_4_1"
},
{
"DOI": "10.3389/fmicb.2021.786042",
"doi-asserted-by": "publisher",
"key": "e_1_2_14_5_1"
},
{
"DOI": "10.1038/s41598-021-03461-y",
"doi-asserted-by": "publisher",
"key": "e_1_2_14_6_1"
},
{
"DOI": "10.1038/s41591-021-01296-8",
"doi-asserted-by": "publisher",
"key": "e_1_2_14_7_1"
},
{
"DOI": "10.1093/cid/ciaa1764",
"doi-asserted-by": "publisher",
"key": "e_1_2_14_8_1"
},
{
"DOI": "10.1590/0074-02760210018",
"doi-asserted-by": "publisher",
"key": "e_1_2_14_9_1"
},
{
"DOI": "10.3390/microorganisms9030521",
"doi-asserted-by": "publisher",
"key": "e_1_2_14_10_1"
},
{
"DOI": "10.1016/j.ajoms.2021.02.002",
"doi-asserted-by": "publisher",
"key": "e_1_2_14_11_1"
},
{
"DOI": "10.1128/spectrum.00128-22",
"doi-asserted-by": "publisher",
"key": "e_1_2_14_12_1"
},
{
"DOI": "10.1093/infdis/jiaa471",
"doi-asserted-by": "publisher",
"key": "e_1_2_14_13_1"
},
{
"DOI": "10.1016/j.virusres.2022.198791",
"doi-asserted-by": "publisher",
"key": "e_1_2_14_14_1"
},
{
"DOI": "10.1016/j.trac.2022.116750",
"doi-asserted-by": "publisher",
"key": "e_1_2_14_15_1"
},
{
"DOI": "10.1177/00220345211029269",
"doi-asserted-by": "publisher",
"key": "e_1_2_14_16_1"
},
{
"DOI": "10.1038/s41598-023-39308-x",
"doi-asserted-by": "publisher",
"key": "e_1_2_14_17_1"
},
{
"DOI": "10.20411/pai.v2i2.200",
"doi-asserted-by": "publisher",
"key": "e_1_2_14_18_1"
},
{
"author": "R Core Team",
"key": "e_1_2_14_19_1",
"volume-title": "R: A language and environment for statistical computing",
"year": "2021"
},
{
"DOI": "10.3390/membranes12060616",
"doi-asserted-by": "publisher",
"key": "e_1_2_14_20_1"
},
{
"DOI": "10.4103/1735-3327.351342",
"article-title": "Redefining aerosol in dentistry during COVID‐19 pandemic",
"author": "Rathore K.",
"doi-asserted-by": "crossref",
"first-page": "53",
"journal-title": "Dental Research Journal",
"key": "e_1_2_14_21_1",
"volume": "19",
"year": "2022"
},
{
"DOI": "10.1080/22221751.2022.2098059",
"doi-asserted-by": "publisher",
"key": "e_1_2_14_22_1"
},
{
"DOI": "10.1016/j.jdsr.2023.09.003",
"article-title": "Efficacy of different mouthwashes against COVID‐19: A systematic review and network meta‐analysis",
"author": "Sbricoli L.",
"doi-asserted-by": "crossref",
"first-page": "334",
"journal-title": "Japanese Dental Science Review",
"key": "e_1_2_14_23_1",
"volume": "59",
"year": "2023"
},
{
"DOI": "10.1186/1745-6215-11-32",
"doi-asserted-by": "publisher",
"key": "e_1_2_14_24_1"
},
{
"DOI": "10.1111/jcpe.13746",
"doi-asserted-by": "publisher",
"key": "e_1_2_14_25_1"
},
{
"DOI": "10.3390/ijerph17124609",
"doi-asserted-by": "publisher",
"key": "e_1_2_14_26_1"
},
{
"DOI": "10.1093/cid/ciab588",
"doi-asserted-by": "publisher",
"key": "e_1_2_14_27_1"
},
{
"DOI": "10.1056/NEJMc2016359",
"doi-asserted-by": "publisher",
"key": "e_1_2_14_28_1"
},
{
"DOI": "10.1038/s41368-020-0074-x",
"doi-asserted-by": "publisher",
"key": "e_1_2_14_29_1"
},
{
"DOI": "10.1177/0022034520918518",
"doi-asserted-by": "publisher",
"key": "e_1_2_14_30_1"
},
{
"DOI": "10.1038/s41591-020-0817-4",
"doi-asserted-by": "publisher",
"key": "e_1_2_14_31_1"
},
{
"DOI": "10.1186/s12879-023-08669-z",
"doi-asserted-by": "publisher",
"key": "e_1_2_14_32_1"
}
],
"reference-count": 31,
"references-count": 31,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1111/jcpe.13905"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Periodontics"
],
"subtitle": [],
"title": "Eligibility and efficacy of a <scp>CPC‐</scp> and <scp>CHX</scp>‐based antiviral mouthwash for the elimination of <scp>SARS‐CoV</scp>‐2 from the saliva: A randomized, double‐blind, controlled clinical trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy"
}
