Efficacy of a Multistrain Synbiotic Treatment in Acute and Post-Acute COVID-19 Patients: A Double-Blind, Placebo-Controlled Randomized Trial
et al., Microorganisms, doi:10.3390/microorganisms12071443, Jul 2024
Probiotics for COVID-19
20th treatment shown to reduce risk in
March 2021, now with p = 0.00000044 from 29 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
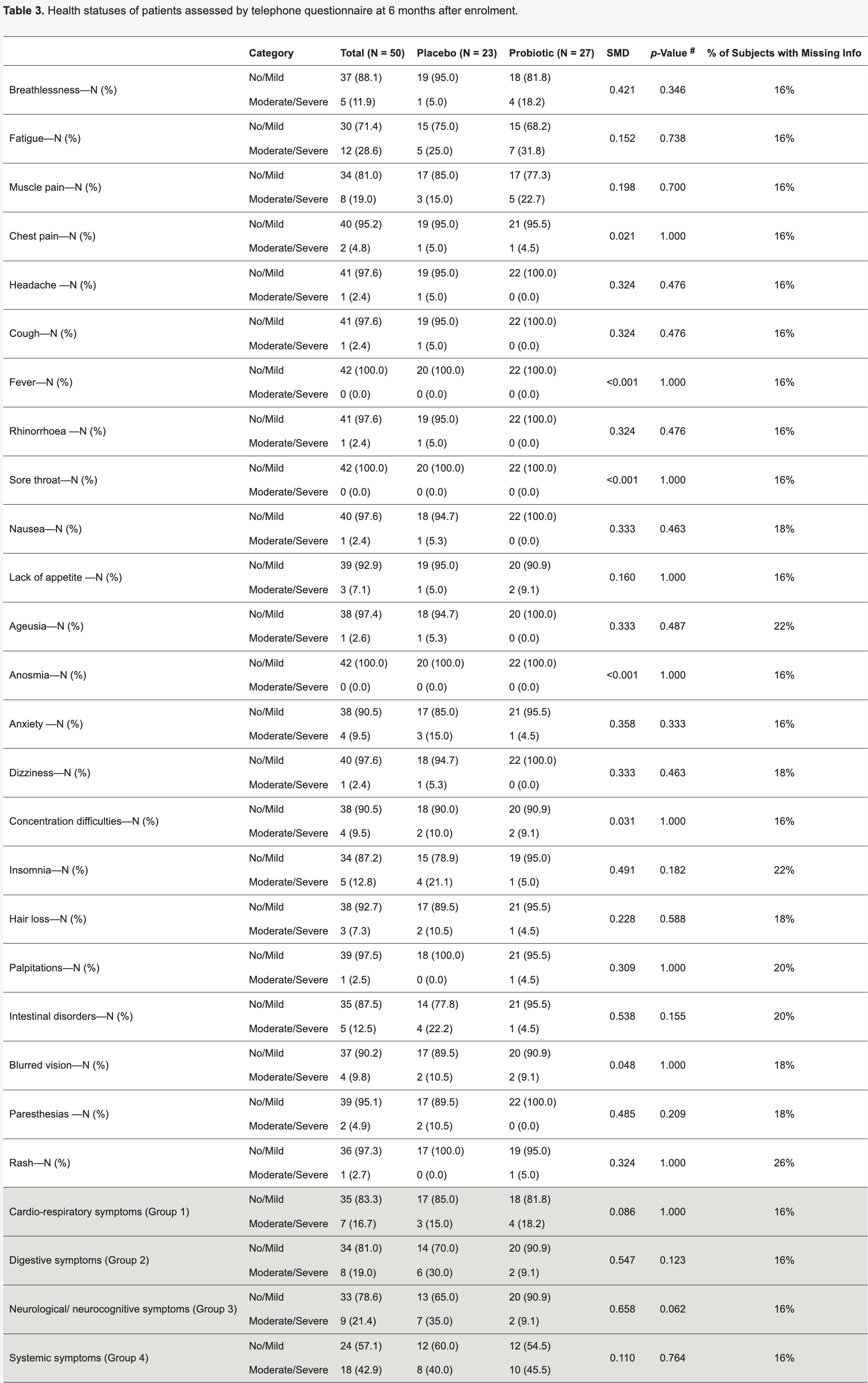
RCT 52 acute COVID-19 inpatients in Italy showing a multistrain synbiotic formula prevented a decrease in gut microbiota diversity and prevented decreases in lymphocyte count and hemoglobin levels compared to placebo. The probiotic group also had enrichment of beneficial bacteria and fewer neurological/neurocognitive symptoms at 6 months, although not statistically significant. Authors suggest modulating gut microbiota in acute COVID-19 through probiotics could be a useful supportive strategy.
Probiotic efficacy depends on the specific strains used. Specific microbes may decrease or increase COVID-19 risk1.
|
risk of death, 14.8% lower, RR 0.85, p = 1.00, treatment 1 of 27 (3.7%), control 1 of 23 (4.3%), NNT 155.
|
|
risk of moderate/severe symptoms, 33.2% lower, RR 0.67, p = 0.32, treatment 22, control 20, combined.
|
|
risk of moderate/severe symptoms, 21.2% higher, RR 1.21, p = 1.00, treatment 4 of 22 (18.2%), control 3 of 20 (15.0%), moderate/severe symptoms, day 180, cardio-respiratory symptoms.
|
|
risk of moderate/severe symptoms, 69.7% lower, RR 0.30, p = 0.12, treatment 2 of 22 (9.1%), control 6 of 20 (30.0%), NNT 4.8, moderate/severe symptoms, day 180, digestive symptoms.
|
|
risk of moderate/severe symptoms, 74.0% lower, RR 0.26, p = 0.06, treatment 2 of 22 (9.1%), control 7 of 20 (35.0%), NNT 3.9, moderate/severe symptoms, day 180, neurological/neurocognitive symptoms.
|
|
risk of moderate/severe symptoms, 13.6% higher, RR 1.14, p = 0.76, treatment 10 of 22 (45.5%), control 8 of 20 (40.0%), moderate/severe symptoms, day 180, systemic symptoms.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Giancola et al., 16 Jul 2024, Double Blind Randomized Controlled Trial, placebo-controlled, Italy, peer-reviewed, 16 authors, study period 18 January, 2022 - 21 March, 2023.
Contact: emanuele.nicastri@inmi.it (corresponding author), mletizia.giancola@inmi.it, antonio.mazzarelli@inmi.it, beccacece.alessia@gmail.com, patrizia.demarco@inmi.it, chiara.degiuli@inmi.it, germana.grassi@inmi.it, carla.fontana@inmi.it, a.fontana@operapadrepio.it, m.copetti@operapadrepio.it, panebianco.c@gmail.com, g.cocomazzi@operapadrepio.it, gbaldini97@gmail.com, viviana.contu@gradenigo.it, v.pazienza@operapadrepio.it.
Efficacy of a Multistrain Synbiotic Treatment in Acute and Post-Acute COVID-19 Patients: A Double-Blind, Placebo-Controlled Randomized Trial
Microorganisms, doi:10.3390/microorganisms12071443
Background and Aims: Several studies reported the effect of COVID-19 on inducing gut dysbiosis, which is also correlated with disease severity. This study aims to investigate the effect of a nutraceutical formula on the shift of microbiota profiles and, secondly, on the clinical-pathological parameters of acute and post-acute COVID-19 patients. Methods: In this randomised, doubleblind, placebo-controlled trial conducted at National Institute for Infectious diseases (INMI) Lazzaro Spallanzani (Italy), 52 patients were randomly assigned (1:1) to receive a multistrain synbiotic formula (Kebirah ® ) or placebo orally for 35 days at COVID-19 diagnosis. Health professionals, investigators, and patients were masked to group assignments. The V3-V4 hypervariable region of 16S rRNA gene sequencing was employed to study the gut microbiota composition in the two groups. Results: Supplementation with Kebirah ® prevented the decrease in the Shannon diversity index of gut microbiota, which was instead observed in patients receiving the placebo. In addition, decreases in lymphocyte count and haemoglobin levels were observed only in the placebo group and not in the treated group, which was also characterised by an amelioration of the gut microbial profile, with an enrichment in beneficial bacteria and a preservation of species diversity. Conclusions: Our data suggest that modulating the gut microbiota in acute disease through administration of a specific symbiotic formula could be a useful strategy in the frame of SARS-CoV-2 infections.
Conflicts of Interest: P.V. is the founder of PharmaBiotiX, with no financial or commercial interest related to the development of the products or the outcome of the work with this product. All other authors declare no conflicts of interests related to this manuscript.
References
Ahmed, Jafri, Hoodbhoy, Siddiqui, Prognostic Value of Serum Procalcitonin in COVID-19 Patients: A Systematic Review, Indian J. Crit. Care Med, doi:10.5005/jp-journals-10071-23706
Amrhein, Greenland, Mcshane, Scientists rise up against statistical significance, Nature, doi:10.1038/d41586-019-00857-9
Bellmann-Weiler, Lanser, Barket, Rangger, Schapfl et al., Prevalence and Predictive Value of Anemia and Dysregulated Iron Homeostasis in Patients with COVID-19 Infection, J. Clin. Med, doi:10.3390/jcm9082429
Catinean, Sida, Silvestru, Balan, Ongoing Treatment with a Spore-Based Probiotic Containing Five Strains of Bacillus Improves Outcomes of Mild COVID-19, Nutrients, doi:10.3390/nu15030488
Chen, Zhan, Huang, Wang, Coinfection and superinfection in ICU critically ill patients with severe COVID-19 pneumonia and influenza pneumonia: Are the pictures different? Front, Public Health, doi:10.3389/fpubh.2023.1195048
Corrêa, Castro, Moser, Ferreira, Quesniaux et al., Connecting the gut-lung axis to the management of pulmonary disorders, Front. Nutr, doi:10.3389/fnut.2022.1011732
Cowman, Rossi, Gendlina, Guo, Liu et al., Elucidating the role of procalcitonin as a biomarker in hospitalized COVID-19 patients, Diagn. Microbiol. Infect. Dis, doi:10.1016/j.diagmicrobio.2022.115721
Dang, Marsland, Microbes, metabolites, and the gut-lung axis, Mucosal Immunol, doi:10.1038/s41385-019-0160-6
Fan, Liu, Sun, Yang, Deng et al., Gut microbiota composition is associated with disease severity and host immune responses in COVID-19, Front. Cell Infect. Microbiol, doi:10.3389/fcimb.2023.1274690
Farsi, Tahvildari, Arbabi, Vazife, Sechi et al., Prognostic, and Therapeutic Roles of Gut Microbiota in COVID-19: A Comprehensive Systematic Review, Front Cell Infect Microbiol, doi:10.3389/fcimb.2022.804644
Gaibani, D'amico, Bartoletti, Lombardo, Rampelli et al., The Gut Microbiota of Critically Ill Patients With COVID-19, Front. Cell Infect. Microbiol, doi:10.3389/fcimb.2021.670424
Group, Clinical characteristics with inflammation profiling of long COVID and association with 1-year recovery following hospitalisation in the UK: A prospective observational study, Lancet Respir. Med, doi:10.1016/S2213-2600(22)00127-8
Guo, Zhang, Prajapati, Li, Lymphopenia Caused by Virus Infections and the Mechanisms Beyond, Viruses, doi:10.3390/v13091876
Gutiérrez-Castrellón, Gandara-Martí, Abreu, Abreu, Nieto-Rufino et al., Probiotic improves symptomatic and viral clearance in Covid19 outpatients: A randomized, quadruple-blinded, placebo-controlled trial, Gut Microbes, doi:10.1080/19490976.2021.2018899
Hirayama, Nishiwaki, Hamaguchi, Ito, Ueyama et al., Intestinal Collinsella may mitigate infection and exacerbation of COVID-19 by producing ursodeoxycholate, PLoS ONE, doi:10.1371/journal.pone.0260451
Ivashkin, Fomin, Moiseev, Brovko, Maslennikov et al., Efficacy of a Probiotic Consisting of Lacticaseibacillus rhamnosus PDV 1705, Bifidobacterium bifidum PDV 0903, Bifidobacterium longum subsp. infantis PDV 1911, and Bifidobacterium longum subsp. longum PDV 2301 in the Treatment of Hospitalized Patients with COVID-19: A Randomized Controlled Trial, Probiotics Antimicrob. Proteins, doi:10.1007/s12602-021-09858-5
Klindworth, Pruesse, Schweer, Peplies, Quast et al., Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies, Nucleic Acids Res, doi:10.1093/nar/gks808
Kumar, Karn, Trivedi, Kumar, Chauhan et al., Procalcitonin as a predictive marker in COVID-19: A systematic review and meta-analysis, PLoS ONE, doi:10.1371/journal.pone.0272840
Martín Giménez, Modrego, Gómez-Garre, Manucha, De Las Heras, Gut Microbiota Dysbiosis in COVID-19: Modulation and Approaches for Prevention and Therapy, Int. J. Mol. Sci, doi:10.3390/ijms241512249
Mazzarelli, Giancola, Farina, Marchioni, Rueca et al., 16S rRNA gene sequencing of rectal swab in patients affected by COVID-19, PLoS ONE, doi:10.1371/journal.pone.0247041
Mazzarelli, Giancola, Fontana, Piselli, Binda et al., Gut microbiota composition in COVID-19 hospitalized patients with mild or severe symptoms, Front Microbiol, doi:10.3389/fmicb.2022.1049215
Moreira-Rosário, Marques, Pinheiro, Araújo, Ribeiro et al., Gut Microbiota Diversity and C-Reactive Protein Are Predictors of Disease Severity in COVID-19 Patients, Front Microbiol, doi:10.3389/fmicb.2021.705020
Phetsouphanh, Darley, Wilson, Howe, Munier et al., Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection, Nat. Immunol, doi:10.1038/s41590-021-01113-x
Ren, Wang, Cui, Lu, Wang et al., Alterations in the human oral and gut microbiomes and lipidomics in COVID-19, Gut, doi:10.1136/gutjnl-2020-323826
Sawilowsky, New effect size rules of thumb, J. Mod. Appl. Stat. Method, doi:10.22237/jmasm/1257035100
Schwab, Janiaud, Dayan, Amrhein, Panczak et al., Ten simple rules for good research practice, PLoS Comput. Biol, doi:10.1371/journal.pcbi.1010139
Sheldon, What Does it all Mean?, Significance, doi:10.1111/j.1740-9713.2019.01296.x
Siddiqui, Cresci, The Immunomodulatory Functions of Butyrate, J. Inflamm. Res, doi:10.2147/JIR.S300989
Sohail, Cheema, Mithani, Shahid, Nawaz et al., Probiotics for the prevention and treatment of COVID-19: A rapid systematic review and meta-analysis, Front. Nutr, doi:10.3389/fnut.2023.1274122
Sui, Noubouossie, Gandotra, Cao, Elevated Plasma Fibrinogen Is Associated with Excessive Inflammation and Disease Severity in COVID-19 Patients, Front. Cell Infect. Microbiol, doi:10.3389/fcimb.2021.734005
Thachil, The protective rather than prothrombotic fibrinogen in COVID-19 and other inflammatory states, J. Thromb. Haemost, doi:10.1111/jth.14942
Thaweethai, Jolley, Karlson, Levitan, Levy et al., Development of a Definition of Postacute Sequelae of SARS-CoV-2 Infection, JAMA, doi:10.1001/jama.2023.8823
Yeoh, Zuo, Lui, Zhang, Liu et al., Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19, Gut, doi:10.1136/gutjnl-2020-323020
Yousef, Rob, Varghese, Rao, Zamir et al., The effect of microbiome therapy on COVID-19-induced gut dysbiosis: A narrative and systematic review, Life Sci, doi:10.1016/j.lfs.2024.122535
Zeng, Tang, Gut microbiota: A potential player in psychiatric symptoms during COVID-19, World J. Biol. Psychiatry, doi:10.1080/15622975.2024.2342846
Zhang, Deng, Li, Su, Hu et al., Changes of gut microbiota under different nutritional methods in elderly patients with severe COVID-19 and their relationship with prognosis, Front Immunol, doi:10.3389/fimmu.2023.1260112
Zhang, Han, Li, Chen, Xie et al., Probiotics use is associated with improved clinical outcomes among hospitalized patients with COVID-19, Ther. Adv. Gastroenterol, doi:10.1177/17562848211035670
Zhang, Lau, Liu, Su, Chan et al., Gut microbiota in COVID-19: Key microbial changes, potential mechanisms and clinical applications, Nat. Rev. Gastroenterol. Hepatol, doi:10.1038/s41575-022-00698-4
Zhao, Meng, Kumar, Wu, Huang et al., Lymphopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A systemic review and meta-analysis, Int. J. Infect Dis, doi:10.1016/j.ijid.2020.04.086
Zuo, Zhang, Lui, Yeoh, Li et al., Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization, Gastroenterology, doi:10.1053/j.gastro.2020.05.048
DOI record:
{
"DOI": "10.3390/microorganisms12071443",
"ISSN": [
"2076-2607"
],
"URL": "http://dx.doi.org/10.3390/microorganisms12071443",
"abstract": "<jats:p>Background and Aims: Several studies reported the effect of COVID-19 on inducing gut dysbiosis, which is also correlated with disease severity. This study aims to investigate the effect of a nutraceutical formula on the shift of microbiota profiles and, secondly, on the clinical–pathological parameters of acute and post-acute COVID-19 patients. Methods: In this randomised, double-blind, placebo-controlled trial conducted at National Institute for Infectious diseases (INMI) Lazzaro Spallanzani (Italy), 52 patients were randomly assigned (1:1) to receive a multistrain synbiotic formula (Kebirah®) or placebo orally for 35 days at COVID-19 diagnosis. Health professionals, investigators, and patients were masked to group assignments. The V3–V4 hypervariable region of 16S rRNA gene sequencing was employed to study the gut microbiota composition in the two groups. Results: Supplementation with Kebirah® prevented the decrease in the Shannon diversity index of gut microbiota, which was instead observed in patients receiving the placebo. In addition, decreases in lymphocyte count and haemoglobin levels were observed only in the placebo group and not in the treated group, which was also characterised by an amelioration of the gut microbial profile, with an enrichment in beneficial bacteria and a preservation of species diversity. Conclusions: Our data suggest that modulating the gut microbiota in acute disease through administration of a specific symbiotic formula could be a useful strategy in the frame of SARS-CoV-2 infections.</jats:p>",
"alternative-id": [
"microorganisms12071443"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-2481-6621",
"affiliation": [
{
"name": "National Institute for Infectious Diseases, INMI “Lazzaro Spallanzani”, IRCCS, 00149 Rome, Italy"
}
],
"authenticated-orcid": false,
"family": "Giancola",
"given": "Maria Letizia",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-6660-5315",
"affiliation": [
{
"name": "Biostatistic Unit, Fondazione-IRCCS “Casa Sollievo della Sofferenza” Hospital, 71013 San Giovanni Rotondo, FG, Italy"
}
],
"authenticated-orcid": false,
"family": "Fontana",
"given": "Andrea",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Gastroenterology Unit, Fondazione-IRCCS “Casa Sollievo della Sofferenza” Hospital, Opera di San Pio da Pietrelcina, 71013 San Giovanni Rotondo, FG, Italy"
}
],
"family": "Panebianco",
"given": "Concetta",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6285-4368",
"affiliation": [
{
"name": "National Institute for Infectious Diseases, INMI “Lazzaro Spallanzani”, IRCCS, 00149 Rome, Italy"
}
],
"authenticated-orcid": false,
"family": "Mazzarelli",
"given": "Antonio",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "National Institute for Infectious Diseases, INMI “Lazzaro Spallanzani”, IRCCS, 00149 Rome, Italy"
}
],
"family": "Beccacece",
"given": "Alessia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "National Institute for Infectious Diseases, INMI “Lazzaro Spallanzani”, IRCCS, 00149 Rome, Italy"
}
],
"family": "De Marco",
"given": "Patrizia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Gastroenterology Unit, Fondazione-IRCCS “Casa Sollievo della Sofferenza” Hospital, Opera di San Pio da Pietrelcina, 71013 San Giovanni Rotondo, FG, Italy"
}
],
"family": "Cocomazzi",
"given": "Giovanna",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "National Institute for Infectious Diseases, INMI “Lazzaro Spallanzani”, IRCCS, 00149 Rome, Italy"
}
],
"family": "De Giuli",
"given": "Chiara",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "National Institute for Infectious Diseases, INMI “Lazzaro Spallanzani”, IRCCS, 00149 Rome, Italy"
}
],
"family": "Grassi",
"given": "Germana",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2198-1947",
"affiliation": [
{
"name": "National Institute for Infectious Diseases, INMI “Lazzaro Spallanzani”, IRCCS, 00149 Rome, Italy"
}
],
"authenticated-orcid": false,
"family": "Fontana",
"given": "Carla",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5061-8007",
"affiliation": [
{
"name": "AO Consorziale Policlinico di Bari, Università Aldo Moro di Bari, 70121 Bari, BA, Italy"
}
],
"authenticated-orcid": false,
"family": "Baldini",
"given": "Giorgio Maria",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0009-0001-7774-365X",
"affiliation": [
{
"name": "Integrative Medicine Unit, Humanitas Gradenigo, Corso Regina Margherita 8/10, 10153 Torino, TO, Italy"
}
],
"authenticated-orcid": false,
"family": "Contu",
"given": "Viviana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Biostatistic Unit, Fondazione-IRCCS “Casa Sollievo della Sofferenza” Hospital, 71013 San Giovanni Rotondo, FG, Italy"
}
],
"family": "Copetti",
"given": "Massimiliano",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8314-8016",
"affiliation": [
{
"name": "Gastroenterology Unit, Fondazione-IRCCS “Casa Sollievo della Sofferenza” Hospital, Opera di San Pio da Pietrelcina, 71013 San Giovanni Rotondo, FG, Italy"
}
],
"authenticated-orcid": false,
"family": "Perri",
"given": "Francesco",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5606-8712",
"affiliation": [
{
"name": "National Institute for Infectious Diseases, INMI “Lazzaro Spallanzani”, IRCCS, 00149 Rome, Italy"
}
],
"authenticated-orcid": false,
"family": "Nicastri",
"given": "Emanuele",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3492-1153",
"affiliation": [
{
"name": "Gastroenterology Unit, Fondazione-IRCCS “Casa Sollievo della Sofferenza” Hospital, Opera di San Pio da Pietrelcina, 71013 San Giovanni Rotondo, FG, Italy"
}
],
"authenticated-orcid": false,
"family": "Pazienza",
"given": "Valerio",
"sequence": "additional"
}
],
"container-title": "Microorganisms",
"container-title-short": "Microorganisms",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
7,
17
]
],
"date-time": "2024-07-17T12:48:29Z",
"timestamp": 1721220509000
},
"deposited": {
"date-parts": [
[
2024,
7,
18
]
],
"date-time": "2024-07-18T15:58:10Z",
"timestamp": 1721318290000
},
"funder": [
{
"award": [
"line 1 on emerging and re-emerging infections."
],
"name": "Ricerca Corrente Program of the Italian Ministry of Health to the National Institute for Infectious Diseases Lazzaro Spallanzani IRCCS"
}
],
"indexed": {
"date-parts": [
[
2024,
7,
19
]
],
"date-time": "2024-07-19T00:22:53Z",
"timestamp": 1721348573092
},
"is-referenced-by-count": 0,
"issue": "7",
"issued": {
"date-parts": [
[
2024,
7,
16
]
]
},
"journal-issue": {
"issue": "7",
"published-online": {
"date-parts": [
[
2024,
7
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
7,
16
]
],
"date-time": "2024-07-16T00:00:00Z",
"timestamp": 1721088000000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2076-2607/12/7/1443/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "1443",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2024,
7,
16
]
]
},
"published-online": {
"date-parts": [
[
2024,
7,
16
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1001/jama.2023.8823",
"article-title": "Development of a Definition of Postacute Sequelae of SARS-CoV-2 Infection",
"author": "Thaweethai",
"doi-asserted-by": "crossref",
"first-page": "1934",
"journal-title": "JAMA",
"key": "ref_1",
"volume": "329",
"year": "2023"
},
{
"DOI": "10.3389/fmicb.2022.1049215",
"doi-asserted-by": "crossref",
"key": "ref_2",
"unstructured": "Mazzarelli, A., Giancola, M.L., Fontana, A., Piselli, P., Binda, E., Trivieri, N., Mencarelli, G., Marchioni, L., Vulcano, A., and De Giuli, C. (2022). Gut microbiota composition in COVID-19 hospitalized patients with mild or severe symptoms. Front Microbiol., 13."
},
{
"DOI": "10.1038/s41385-019-0160-6",
"article-title": "Microbes, metabolites, and the gut-lung axis",
"author": "Dang",
"doi-asserted-by": "crossref",
"first-page": "843",
"journal-title": "Mucosal Immunol.",
"key": "ref_3",
"volume": "12",
"year": "2019"
},
{
"DOI": "10.1371/journal.pone.0247041",
"doi-asserted-by": "crossref",
"key": "ref_4",
"unstructured": "Mazzarelli, A., Giancola, M.L., Farina, A., Marchioni, L., Rueca, M., Gruber, C.E.M., Bartolini, B., Ascoli Bartoli, T., Maffongelli, G., and Capobianchi, M.R. (2021). 16S rRNA gene sequencing of rectal swab in patients affected by COVID-19. PLoS ONE, 16."
},
{
"DOI": "10.1053/j.gastro.2020.05.048",
"article-title": "Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization",
"author": "Zuo",
"doi-asserted-by": "crossref",
"first-page": "944",
"journal-title": "Gastroenterology",
"key": "ref_5",
"volume": "159",
"year": "2020"
},
{
"DOI": "10.3389/fcimb.2022.804644",
"doi-asserted-by": "crossref",
"key": "ref_6",
"unstructured": "Farsi, Y., Tahvildari, A., Arbabi, M., Vazife, F., Sechi, L.A., Shahidi Bonjar, A.H., Jamshidi, P., Nasiri, M.J., and Mirsaeidi, M. (2022). Diagnostic, Prognostic, and Therapeutic Roles of Gut Microbiota in COVID-19: A Comprehensive Systematic Review. Front Cell Infect Microbiol., 12."
},
{
"DOI": "10.3389/fmicb.2021.705020",
"doi-asserted-by": "crossref",
"key": "ref_7",
"unstructured": "Moreira-Rosário, A., Marques, C., Pinheiro, H., Araújo, J.R., Ribeiro, P., Rocha, R., Mota, I., Pestana, D., Ribeiro, R., and Pereira, A. (2021). Gut Microbiota Diversity and C-Reactive Protein Are Predictors of Disease Severity in COVID-19 Patients. Front Microbiol., 12."
},
{
"DOI": "10.1136/gutjnl-2020-323020",
"article-title": "Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19",
"author": "Yeoh",
"doi-asserted-by": "crossref",
"first-page": "698",
"journal-title": "Gut",
"key": "ref_8",
"volume": "70",
"year": "2021"
},
{
"DOI": "10.1038/s41575-022-00698-4",
"article-title": "Gut microbiota in COVID-19: Key microbial changes, potential mechanisms and clinical applications",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "323",
"journal-title": "Nat. Rev. Gastroenterol. Hepatol.",
"key": "ref_9",
"volume": "20",
"year": "2023"
},
{
"DOI": "10.3390/ijms241512249",
"doi-asserted-by": "crossref",
"key": "ref_10",
"unstructured": "Martín Giménez, V.M., Modrego, J., Gómez-Garre, D., Manucha, W., and de Las Heras, N. (2023). Gut Microbiota Dysbiosis in COVID-19: Modulation and Approaches for Prevention and Therapy. Int. J. Mol. Sci., 24."
},
{
"DOI": "10.1177/17562848211035670",
"article-title": "Probiotics use is associated with improved clinical outcomes among hospitalized patients with COVID-19",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "17562848211035670",
"journal-title": "Ther. Adv. Gastroenterol.",
"key": "ref_11",
"volume": "14",
"year": "2021"
},
{
"DOI": "10.3390/nu15030488",
"doi-asserted-by": "crossref",
"key": "ref_12",
"unstructured": "Catinean, A., Sida, A., Silvestru, C., and Balan, G.G. (2023). Ongoing Treatment with a Spore-Based Probiotic Containing Five Strains of Bacillus Improves Outcomes of Mild COVID-19. Nutrients, 15."
},
{
"DOI": "10.1007/s12602-021-09858-5",
"article-title": "Efficacy of a Probiotic Consisting of Lacticaseibacillus rhamnosus PDV 1705, Bifidobacterium bifidum PDV 0903, Bifidobacterium longum subsp. infantis PDV 1911, and Bifidobacterium longum subsp. longum PDV 2301 in the Treatment of Hospitalized Patients with COVID-19: A Randomized Controlled Trial",
"author": "Ivashkin",
"doi-asserted-by": "crossref",
"first-page": "460",
"journal-title": "Probiotics Antimicrob. Proteins",
"key": "ref_13",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.1080/19490976.2021.2018899",
"article-title": "Probiotic improves symptomatic and viral clearance in Covid19 outpatients: A randomized, quadruple-blinded, placebo-controlled trial",
"author": "Abreu",
"doi-asserted-by": "crossref",
"first-page": "2018899",
"journal-title": "Gut Microbes",
"key": "ref_14",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1016/j.lfs.2024.122535",
"article-title": "The effect of microbiome therapy on COVID-19-induced gut dysbiosis: A narrative and systematic review",
"author": "Yousef",
"doi-asserted-by": "crossref",
"first-page": "122535",
"journal-title": "Life Sci.",
"key": "ref_15",
"volume": "342",
"year": "2024"
},
{
"DOI": "10.1001/jama.2012.5669",
"doi-asserted-by": "crossref",
"key": "ref_16",
"unstructured": "The ARDS Definition Task Force (2012). Acute Respiratory Distress Syndrome: The Berlin Definition. JAMA, 307, 2526–2533."
},
{
"DOI": "10.1093/nar/gks808",
"article-title": "Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies",
"author": "Klindworth",
"doi-asserted-by": "crossref",
"first-page": "e1",
"journal-title": "Nucleic Acids Res.",
"key": "ref_17",
"volume": "41",
"year": "2013"
},
{
"DOI": "10.22237/jmasm/1257035100",
"article-title": "New effect size rules of thumb",
"author": "Sawilowsky",
"doi-asserted-by": "crossref",
"first-page": "467",
"journal-title": "J. Mod. Appl. Stat. Method",
"key": "ref_18",
"volume": "8",
"year": "2009"
},
{
"DOI": "10.3389/fcimb.2021.734005",
"doi-asserted-by": "crossref",
"key": "ref_19",
"unstructured": "Sui, J., Noubouossie, D.F., Gandotra, S., and Cao, L. (2021). Elevated Plasma Fibrinogen Is Associated with Excessive Inflammation and Disease Severity in COVID-19 Patients. Front. Cell Infect. Microbiol., 11."
},
{
"DOI": "10.1111/jth.14942",
"article-title": "The protective rather than prothrombotic fibrinogen in COVID-19 and other inflammatory states",
"author": "Thachil",
"doi-asserted-by": "crossref",
"first-page": "1849",
"journal-title": "J. Thromb. Haemost.",
"key": "ref_20",
"volume": "18",
"year": "2020"
},
{
"DOI": "10.5005/jp-journals-10071-23706",
"article-title": "Prognostic Value of Serum Procalcitonin in COVID-19 Patients: A Systematic Review",
"author": "Ahmed",
"doi-asserted-by": "crossref",
"first-page": "77",
"journal-title": "Indian J. Crit. Care Med.",
"key": "ref_21",
"volume": "25",
"year": "2021"
},
{
"DOI": "10.1371/journal.pone.0272840",
"doi-asserted-by": "crossref",
"key": "ref_22",
"unstructured": "Kumar, A., Karn, E., Trivedi, K., Kumar, P., Chauhan, G., Kumari, A., Pant, P., Munisamy, M., Prakash, J., and Sarkar, P.G. (2022). Procalcitonin as a predictive marker in COVID-19: A systematic review and meta-analysis. PLoS ONE, 17."
},
{
"DOI": "10.1016/j.diagmicrobio.2022.115721",
"doi-asserted-by": "crossref",
"key": "ref_23",
"unstructured": "Cowman, K., Rossi, J., Gendlina, I., Guo, Y., Liu, S., Szymczak, W., Forest, S.K., Wolgast, L., Orner, E., and Bao, H. (2022). Elucidating the role of procalcitonin as a biomarker in hospitalized COVID-19 patients. Diagn. Microbiol. Infect. Dis., 103."
},
{
"DOI": "10.3390/v13091876",
"doi-asserted-by": "crossref",
"key": "ref_24",
"unstructured": "Guo, Z., Zhang, Z., Prajapati, M., and Li, Y. (2021). Lymphopenia Caused by Virus Infections and the Mechanisms Beyond. Viruses, 13."
},
{
"DOI": "10.1016/j.ijid.2020.04.086",
"article-title": "Lymphopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A systemic review and meta-analysis",
"author": "Zhao",
"doi-asserted-by": "crossref",
"first-page": "131",
"journal-title": "Int. J. Infect Dis.",
"key": "ref_25",
"volume": "96",
"year": "2020"
},
{
"DOI": "10.3390/jcm9082429",
"doi-asserted-by": "crossref",
"key": "ref_26",
"unstructured": "Bellmann-Weiler, R., Lanser, L., Barket, R., Rangger, L., Schapfl, A., Schaber, M., Fritsche, G., Wöll, E., and Weiss, G. (2020). Prevalence and Predictive Value of Anemia and Dysregulated Iron Homeostasis in Patients with COVID-19 Infection. J. Clin. Med., 9."
},
{
"DOI": "10.1038/d41586-019-00857-9",
"article-title": "Scientists rise up against statistical significance",
"author": "Amrhein",
"doi-asserted-by": "crossref",
"first-page": "305",
"journal-title": "Nature",
"key": "ref_27",
"volume": "567",
"year": "2019"
},
{
"DOI": "10.1111/j.1740-9713.2019.01296.x",
"article-title": "What Does it all Mean?",
"author": "Sheldon",
"doi-asserted-by": "crossref",
"first-page": "15",
"journal-title": "Significance",
"key": "ref_28",
"volume": "16",
"year": "2019"
},
{
"DOI": "10.1371/journal.pcbi.1010139",
"doi-asserted-by": "crossref",
"key": "ref_29",
"unstructured": "Schwab, S., Janiaud, P., Dayan, M., Amrhein, V., Panczak, R., Palagi, P.M., Hemkens, L.G., Ramon, M., Rothen, N., and Senn, S. (2022). Ten simple rules for good research practice. PLoS Comput. Biol., 18."
},
{
"DOI": "10.3389/fnut.2023.1274122",
"doi-asserted-by": "crossref",
"key": "ref_30",
"unstructured": "Sohail, A., Cheema, H.A., Mithani, M.S., Shahid, A., Nawaz, A., Hermis, A.H., Chinnam, S., Nashwan, A.J., Cherrez-Ojeda, I., and Awan, R.U. (2023). Probiotics for the prevention and treatment of COVID-19: A rapid systematic review and meta-analysis. Front. Nutr., 10."
},
{
"DOI": "10.1038/s41590-021-01113-x",
"article-title": "Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection",
"author": "Phetsouphanh",
"doi-asserted-by": "crossref",
"first-page": "210",
"journal-title": "Nat. Immunol.",
"key": "ref_31",
"volume": "23",
"year": "2022"
},
{
"key": "ref_32",
"unstructured": "PHOSP-COVID Collaborative Group (2022). Clinical characteristics with inflammation profiling of long COVID and association with 1-year recovery following hospitalisation in the UK: A prospective observational study. Lancet Respir. Med., 10, 761–775."
},
{
"DOI": "10.3389/fcimb.2021.670424",
"doi-asserted-by": "crossref",
"key": "ref_33",
"unstructured": "Gaibani, P., D’Amico, F., Bartoletti, M., Lombardo, D., Rampelli, S., Fornaro, G., Coladonato, S., Siniscalchi, A., Re, M.C., and Viale, P. (2021). The Gut Microbiota of Critically Ill Patients With COVID-19. Front. Cell Infect. Microbiol., 11."
},
{
"DOI": "10.1136/gutjnl-2020-323826",
"article-title": "Alterations in the human oral and gut microbiomes and lipidomics in COVID-19",
"author": "Ren",
"doi-asserted-by": "crossref",
"first-page": "1253",
"journal-title": "Gut",
"key": "ref_34",
"volume": "70",
"year": "2021"
},
{
"DOI": "10.1371/journal.pone.0260451",
"doi-asserted-by": "crossref",
"key": "ref_35",
"unstructured": "Hirayama, M., Nishiwaki, H., Hamaguchi, T., Ito, M., Ueyama, J., Maeda, T., Kashihara, K., Tsuboi, Y., and Ohno, K. (2021). Intestinal Collinsella may mitigate infection and exacerbation of COVID-19 by producing ursodeoxycholate. PLoS ONE, 16."
},
{
"DOI": "10.3389/fnut.2022.1011732",
"doi-asserted-by": "crossref",
"key": "ref_36",
"unstructured": "Corrêa, R.O., Castro, P.R., Moser, R., Ferreira, C.M., Quesniaux, V.F.J., Vinolo, M.A.R., and Ryffel, B. (2022). Butyrate: Connecting the gut-lung axis to the management of pulmonary disorders. Front. Nutr., 9."
},
{
"DOI": "10.2147/JIR.S300989",
"article-title": "The Immunomodulatory Functions of Butyrate",
"author": "Siddiqui",
"doi-asserted-by": "crossref",
"first-page": "6025",
"journal-title": "J. Inflamm. Res.",
"key": "ref_37",
"volume": "14",
"year": "2021"
},
{
"DOI": "10.1080/15622975.2024.2342846",
"article-title": "Gut microbiota: A potential player in psychiatric symptoms during COVID-19",
"author": "Zeng",
"doi-asserted-by": "crossref",
"first-page": "267",
"journal-title": "World J. Biol. Psychiatry",
"key": "ref_38",
"volume": "25",
"year": "2024"
},
{
"DOI": "10.3389/fpubh.2023.1195048",
"doi-asserted-by": "crossref",
"key": "ref_39",
"unstructured": "Chen, Z., Zhan, Q., Huang, L., and Wang, C. (2023). Coinfection and superinfection in ICU critically ill patients with severe COVID-19 pneumonia and influenza pneumonia: Are the pictures different?. Front. Public Health, 11."
},
{
"DOI": "10.3389/fcimb.2023.1274690",
"doi-asserted-by": "crossref",
"key": "ref_40",
"unstructured": "Fan, R., Liu, S., Sun, N., Yang, Y., Deng, X., Hu, B., Sun, C., Wen, C., Li, H., and Cheng, D. (2023). Gut microbiota composition is associated with disease severity and host immune responses in COVID-19. Front. Cell Infect. Microbiol., 13."
},
{
"DOI": "10.3389/fimmu.2023.1260112",
"article-title": "Changes of gut microbiota under different nutritional methods in elderly patients with severe COVID-19 and their relationship with prognosis",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "1260112",
"journal-title": "Front Immunol.",
"key": "ref_41",
"volume": "14",
"year": "2023"
}
],
"reference-count": 41,
"references-count": 41,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2076-2607/12/7/1443"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Efficacy of a Multistrain Synbiotic Treatment in Acute and Post-Acute COVID-19 Patients: A Double-Blind, Placebo-Controlled Randomized Trial",
"type": "journal-article",
"volume": "12"
}
