An Evaluation of Serum 25-Hydroxy Vitamin D Levels in Patients with COVID-19 in New York City
et al., Journal of the American College of Nutrition, doi:10.1080/07315724.2020.1869626, Feb 2021
Vitamin D for COVID-19
8th treatment shown to reduce risk in
October 2020, now with p < 0.00000000001 from 136 studies, recognized in 18 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 437 mostly serious condition (85% hospitalized) patients in New York, showing vitamin D deficiency associated with increased likelihood of oxygen support, but no association with mortality and hospitalization. Multivariate analysis excluded variables with p > 0.2 in univariate analysis. Adjustment for factors correlated with vitamin D may obscure the effect of vitamin D levels.
This is the 49th of 228 COVID-19 sufficiency studies for vitamin D, which collectively show higher levels reduce risk with p<0.0000000001.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
This may explain in part the very high mortality seen in this study.
Results may differ in countries with improved SOC.
|
risk of death, 4.7% higher, RR 1.05, p = 0.83, high D levels 80 of 260 (30.8%), low D levels 52 of 177 (29.4%), >20ng/ml.
|
|
risk of death, 44.8% lower, RR 0.55, p < 0.001, high D levels 102 of 376 (27.1%), low D levels 30 of 61 (49.2%), NNT 4.5, >10ng/ml.
|
|
risk of oxygen therapy, 55.2% lower, RR 0.45, p < 0.001, high D levels 127 of 260 (48.8%), low D levels 116 of 177 (65.5%), NNT 6.0, adjusted per study, inverted to make RR<1 favor high D levels, >20ng/ml, multivariate.
|
|
risk of hospitalization, 3.6% lower, RR 0.96, p = 0.41, high D levels 218 of 260 (83.8%), low D levels 154 of 177 (87.0%), NNT 32, >20ng/ml.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Gavioli et al., 19 Feb 2021, retrospective, USA, peer-reviewed, 4 authors.
An Evaluation of Serum 25-Hydroxy Vitamin D Levels in Patients with COVID-19 in New York City
Journal of the American Nutrition Association, doi:10.1080/07315724.2020.1869626
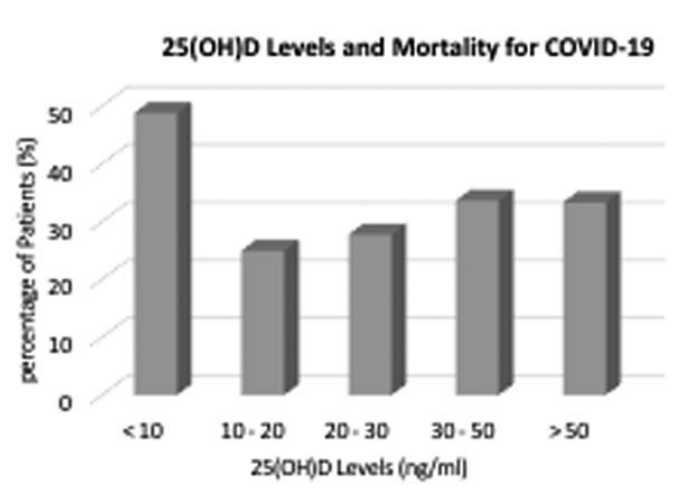
Aim: Deterioration of patients from COVID-19 is associated with cytokine release syndrome attributed to an elevation in pro-inflammatory cytokines. Vitamin D reduces proinflammatory cytokines, and has the possibility of reducing complications from respiratory tract illnesses. Method: This was a retrospective, observational, cohort study of patients with COVID-19 disease within a New York City Health System. Adult patients were included if they tested positive for SARS-CoV-2, and had a serum 25-hydroxy vitamin D level (25(OH)D) within the three previous months prior to their detected SARS-CoV-2 test. Patients were compared and evaluated based upon their 25(OH)D levels. The primary endpoints were hospitalization, need for oxygen support, and 90-day mortality. Results: 437 COVID-19 patients were included [67 (IQR: 56-79) years] within this cohort. Deficient plasma 25(OH)D levels (<20 ng/ml) were associated with an increased likelihood of oxygen support [OR:2.23 (95% CI: 1.46-3.44, p ¼ 0.0002)] from COVID-19. Deficient plasma 25(OH)D levels were not independently associated with 90-day mortality or risk of hospitalization. Hospitalization rates (98%), oxygen support (93%), and mortality rates (49%) were highest in patients who had 25(OH)D levels less than 10 ng/ml when compared to other 25(OH)D levels. Conclusion: Serum 25-hydroxy vitamin D levels may affect the need for oxygen support therapy in patients with COVID-19.
Disclosure statement The authors declare that they do not have a conflict of interest. Appendix A. Multivariate analysis between 25(OH)D levels and outcomes from COVID-19 infection
References
Abrishami, Dalili, Torbati, Asgari, Arab-Ahmadi et al., Possible association of vitamin D status with lung involvement and outcome in patients with COVID-19: a retrospective study, Eur J Nutr, doi:10.1007/s00394-020-02411-0
Aihara, Azuma, Akaike, Ikeda, Yamashita et al., Disruption of nuclear vitamin D receptor gene causes enhanced thrombogenicity in mice, J Biol Chem, doi:10.1074/jbc.M404865200
Alquaiz, Kazi, Fouda, Alyousefi, Age and gender differences in the prevalence and correlates of vitamin D deficiency, Arch Osteoporos, doi:10.1007/s11657-018-0461-5
Annweiler, Corvaisier, Gautier, Dub Ee, Legrand et al., Vitamin D supplementation associated to better survival in hospitalized frail elderly COVID-19 patients: The GERIA-COVID quasi-experimental study, Nutrients, doi:10.3390/nu12113377
Arvinte, Singh, Marik, Serum levels of vitamin C and vitamin D in a cohort of critically ill COVID-19 patients of a North American Community Hospital Intensive Care Unit in May 2020: a pilot study, Med Drug Discov, doi:10.1016/j.medidd.2020.100064
Aygun, Vitamin D can prevent COVID-19 infection-induced multiple organ damage, Naunyn Schmiedebergs Arch Pharmacol, doi:10.1007/s00210-020-01911-4
D'avolio, Avataneo, Manca, Cusato, Nicol O et al., 25-Hydroxyvitamin D concentrations are lower in patients with positive PCR for SARS-CoV-2, Nutrients, doi:10.3390/nu12051359
De Lucena, Da, Santos, De Lima, De et al., Mechanism of inflammatory response in associated comorbidities in COVID-19, Diabetes Metab Syndr, doi:10.1016/j.dsx.2020.05.025
Grimes, Schulz, False alarms and pseudo-epidemics: the limitations of observational epidemiology, Obstet Gynecol, doi:10.1097/AOG.0b013e31826af61a
Guo, Cao, Hong, Tan, Chen et al., The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak -an update on the status, Mil Med Res, doi:10.1186/s40779-020-00240-0
Han, Jones, Tangpricha, Brown, Brown et al., High dose vitamin D administration in ventilated intensive care unit patients: a pilot double blind randomized controlled trial, J Clin Transl Endocrinol, doi:10.1016/j.jcte.2016.04.004
Ilie, Stefanescu, Smith, The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality, Aging Clin Exp Res, doi:10.1007/s40520-020-01570-8
Ishimura, Nishizawa, Inaba, Matsumoto, Emoto et al., Serum levels of 1,25-dihydroxyvitamin D, 24,25-dihydroxyvitamin D, and 25-hydroxyvitamin D in nondialyzed patients with chronic renal failure, Kidney Int, doi:10.1046/j.1523-1755.1999.0550031019.x
Jain, Parsanathan, Can vitamin D and L-cysteine co-supplementation reduce 25(OH)-vitamin D deficiency and the mortality associated with COVID-19 in African Americans?, J Am Coll Nutr, doi:10.1080/07315724.2020.1789518
Khoo, Chai, Koenen, Joosten, Netea et al., Translating the role of vitamin D3 in infectious diseases, Crit Rev Microbiol, doi:10.3109/1040841X.2011.622716
Kumar, Gupta, Banerjee, Letter: does vitamin D have a potential role against COVID-19?, Aliment Pharmacol Ther, doi:10.1111/apt.1580
Laclair, Hellman, Karp, Kraus, Ofner et al., Prevalence of calcidiol deficiency in CKD: a cross-sectional study across latitudes in the United States, Am J Kidney Dis, doi:10.1053/j.ajkd.2005.02.029
Martineau, Jolliffe, Greenberg, Aloia, Bergman et al., Vitamin D supplementation to prevent acute respiratory infections: individual participant data meta-analysis, Health Technol Assess, doi:10.3310/hta23020
Merzon, Tworowski, Gorohovski, Vinker, Cohen et al., Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: an Israeli population-based study, FEBS J, doi:10.1111/febs.15495
Mikami, Miyashita, Yamada, Risk factors for mortality in patients with COVID-19 in New York City
Mohammad, Mishra, Ashraf, Emerging role of vitamin D and its associated molecules in pathways related to pathogenesis of thrombosis, Biomolecules, doi:10.3390/biom9110649
Moulas, Vaiou, Vitamin D fortification of foods and prospective health outcomes, J Biotechnol, doi:10.1016/j.jbiotec.2018.08.010
Ohaegbulam, Swalih, Patel, Smith, Perrin, Vitamin D supplementation in COVID-19 patients: a clinical case series, Am J Ther, doi:10.1097/MJT.0000000000001222
Panarese, Shahini, Letter: Covid-19, and vitamin D, Aliment Pharmacol Ther, doi:10.1111/apt.15752
Rajakumar, De Las Heras, Chen, Lee, Holick et al., Vitamin D status, adiposity, and lipids in black American and Caucasian children, J Clin Endocrinol Metab, doi:10.1210/jc.2010-2388
Rastogi, Bhansali, Khare, Suri, Yaddanapudi et al., Short term, high-dose vitamin D supplementation for COVID-19 disease: a randomised, placebo-controlled, study (SHADE study), Postgrad Med J, doi:10.1136/postgradmedj-2020-139065
Vyas, Kurian, Bagchi, Manu, Saravu et al., Vitamin D in prevention and treatment of COVID-19: current perspective and future prospects, J Am Coll Nutr, doi:10.1080/07315724.2020
Wang, Degruttola, Lei, Mayer, Redline et al., The vitamin D for COVID-19 (VIVID) trial: a pragmatic cluster-randomized design, Contemp Clin Trials, doi:10.1016/j.cct.2020.106176
Yang, Gu, Zhao, Wang, Cao et al., Angiotensin-converting enzyme 2 (ACE2) mediates influenza H7N9 virus-induced acute lung injury, Sci Rep, doi:10.1038/srep07027
Zhou, Yu, Du, Fan, Liu et al., Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, Lancet, doi:10.1016/S0140-6736(20)30566-3
DOI record:
{
"DOI": "10.1080/07315724.2020.1869626",
"ISSN": [
"2769-7061",
"2769-707X"
],
"URL": "http://dx.doi.org/10.1080/07315724.2020.1869626",
"alternative-id": [
"10.1080/07315724.2020.1869626"
],
"assertion": [
{
"label": "Peer Review Statement",
"name": "peerreview_statement",
"order": 1,
"value": "The publishing and review policy for this title is described in its Aims & Scope."
},
{
"URL": "http://www.tandfonline.com/action/journalInformation?show=aimsScope&journalCode=uacn21",
"label": "Aim & Scope",
"name": "aims_and_scope_url",
"order": 2,
"value": "http://www.tandfonline.com/action/journalInformation?show=aimsScope&journalCode=uacn21"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2020-11-25"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Revised",
"name": "revised",
"order": 1,
"value": "2020-12-22"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "2020-12-23"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 3,
"value": "2021-02-19"
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-6436-0748",
"affiliation": [
{
"name": "Arnold & Marie Schwartz College of Pharmacy and Health Sciences, Brooklyn, New York, USA"
},
{
"name": "Mount Sinai Beth Israel, New York, New York, USA"
}
],
"authenticated-orcid": false,
"family": "Gavioli",
"given": "Elizabeth Marie",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Mount Sinai Beth Israel, New York, New York, USA"
}
],
"family": "Miyashita",
"given": "Hirotaka",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Arnold & Marie Schwartz College of Pharmacy and Health Sciences, Brooklyn, New York, USA"
}
],
"family": "Hassaneen",
"given": "Omar",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Mount Sinai Beth Israel, New York, New York, USA"
},
{
"name": "Icahn School of Medicine at Mount Sinai, New York, New York, USA"
}
],
"family": "Siau",
"given": "Evan",
"sequence": "additional"
}
],
"container-title": "Journal of the American Nutrition Association",
"container-title-short": "Journal of the American Nutrition Association",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"www.tandfonline.com"
]
},
"created": {
"date-parts": [
[
2021,
2,
19
]
],
"date-time": "2021-02-19T16:04:04Z",
"timestamp": 1613750644000
},
"deposited": {
"date-parts": [
[
2022,
2,
28
]
],
"date-time": "2022-02-28T13:29:48Z",
"timestamp": 1646054988000
},
"indexed": {
"date-parts": [
[
2023,
8,
22
]
],
"date-time": "2023-08-22T16:25:01Z",
"timestamp": 1692721501122
},
"is-referenced-by-count": 12,
"issue": "2",
"issued": {
"date-parts": [
[
2021,
2,
19
]
]
},
"journal-issue": {
"issue": "2",
"published-print": {
"date-parts": [
[
2022,
2,
17
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://www.tandfonline.com/doi/pdf/10.1080/07315724.2020.1869626",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "301",
"original-title": [],
"page": "201-206",
"prefix": "10.1080",
"published": {
"date-parts": [
[
2021,
2,
19
]
]
},
"published-online": {
"date-parts": [
[
2021,
2,
19
]
]
},
"published-print": {
"date-parts": [
[
2022,
2,
17
]
]
},
"publisher": "Informa UK Limited",
"reference": [
{
"DOI": "10.1186/s40779-020-00240-0",
"doi-asserted-by": "publisher",
"key": "CIT0001"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"doi-asserted-by": "publisher",
"key": "CIT0002"
},
{
"DOI": "10.1016/j.dsx.2020.05.025",
"doi-asserted-by": "publisher",
"key": "CIT0003"
},
{
"DOI": "10.3109/1040841X.2011.622716",
"doi-asserted-by": "publisher",
"key": "CIT0004"
},
{
"DOI": "10.1210/jc.2010-2388",
"doi-asserted-by": "publisher",
"key": "CIT0005"
},
{
"author": "Vyas N",
"first-page": "1",
"journal-title": "J Am Coll Nutr",
"key": "CIT0006",
"year": "2020"
},
{
"DOI": "10.1016/j.jbiotec.2018.08.010",
"doi-asserted-by": "publisher",
"key": "CIT0007"
},
{
"DOI": "10.1007/s00210-020-01911-4",
"doi-asserted-by": "publisher",
"key": "CIT0008"
},
{
"DOI": "10.1080/07315724.2020.1789518",
"doi-asserted-by": "publisher",
"key": "CIT0009"
},
{
"DOI": "10.1111/apt.15801",
"doi-asserted-by": "publisher",
"key": "CIT0010"
},
{
"DOI": "10.1038/srep07027",
"doi-asserted-by": "publisher",
"key": "CIT0011"
},
{
"DOI": "10.3390/biom9110649",
"doi-asserted-by": "publisher",
"key": "CIT0012"
},
{
"DOI": "10.1074/jbc.M404865200",
"doi-asserted-by": "publisher",
"key": "CIT0013"
},
{
"DOI": "10.3310/hta23020",
"doi-asserted-by": "publisher",
"key": "CIT0014"
},
{
"DOI": "10.3390/nu12051359",
"doi-asserted-by": "publisher",
"key": "CIT0015"
},
{
"DOI": "10.1111/febs.15495",
"doi-asserted-by": "publisher",
"key": "CIT0016"
},
{
"DOI": "10.1007/s40520-020-01570-8",
"doi-asserted-by": "publisher",
"key": "CIT0017"
},
{
"DOI": "10.1111/apt.15752",
"doi-asserted-by": "publisher",
"key": "CIT0018"
},
{
"author": "Mikami T",
"first-page": "1",
"journal-title": "J Gen Intern Med",
"key": "CIT0019",
"year": "2020"
},
{
"DOI": "10.1007/s11657-018-0461-5",
"doi-asserted-by": "publisher",
"key": "CIT0020"
},
{
"DOI": "10.1053/j.ajkd.2005.02.029",
"doi-asserted-by": "publisher",
"key": "CIT0021"
},
{
"DOI": "10.1046/j.1523-1755.1999.0550031019.x",
"doi-asserted-by": "publisher",
"key": "CIT0022"
},
{
"author": "Abrishami A",
"first-page": "1",
"journal-title": "Eur J Nutr",
"key": "CIT0023",
"year": "2020"
},
{
"DOI": "10.1097/MJT.0000000000001222",
"doi-asserted-by": "publisher",
"key": "CIT0024"
},
{
"DOI": "10.1136/postgradmedj-2020-139065",
"doi-asserted-by": "publisher",
"key": "CIT0025"
},
{
"DOI": "10.3390/nu12113377",
"doi-asserted-by": "publisher",
"key": "CIT0026"
},
{
"DOI": "10.1016/j.jcte.2016.04.004",
"doi-asserted-by": "publisher",
"key": "CIT0027"
},
{
"DOI": "10.1016/j.medidd.2020.100064",
"doi-asserted-by": "publisher",
"key": "CIT0028"
},
{
"DOI": "10.1097/AOG.0b013e31826af61a",
"doi-asserted-by": "publisher",
"key": "CIT0029"
},
{
"author": "Institute of Medicine",
"key": "CIT0030",
"volume-title": "Dietary reference intakes for calcium and vitamin D",
"year": "2010"
},
{
"DOI": "10.1016/j.cct.2020.106176",
"doi-asserted-by": "publisher",
"key": "CIT0031"
}
],
"reference-count": 31,
"references-count": 31,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.tandfonline.com/doi/full/10.1080/07315724.2020.1869626"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"title": "An Evaluation of Serum 25-Hydroxy Vitamin D Levels in Patients with COVID-19 in New York City",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1080/tandf_crossmark_01",
"volume": "41"
}
