Leveraging SARS-CoV-2 Main Protease (Mpro) for COVID-19 Mitigation with Selenium-Based Inhibitors
et al., International Journal of Molecular Sciences, doi:10.3390/ijms25020971, Jan 2024
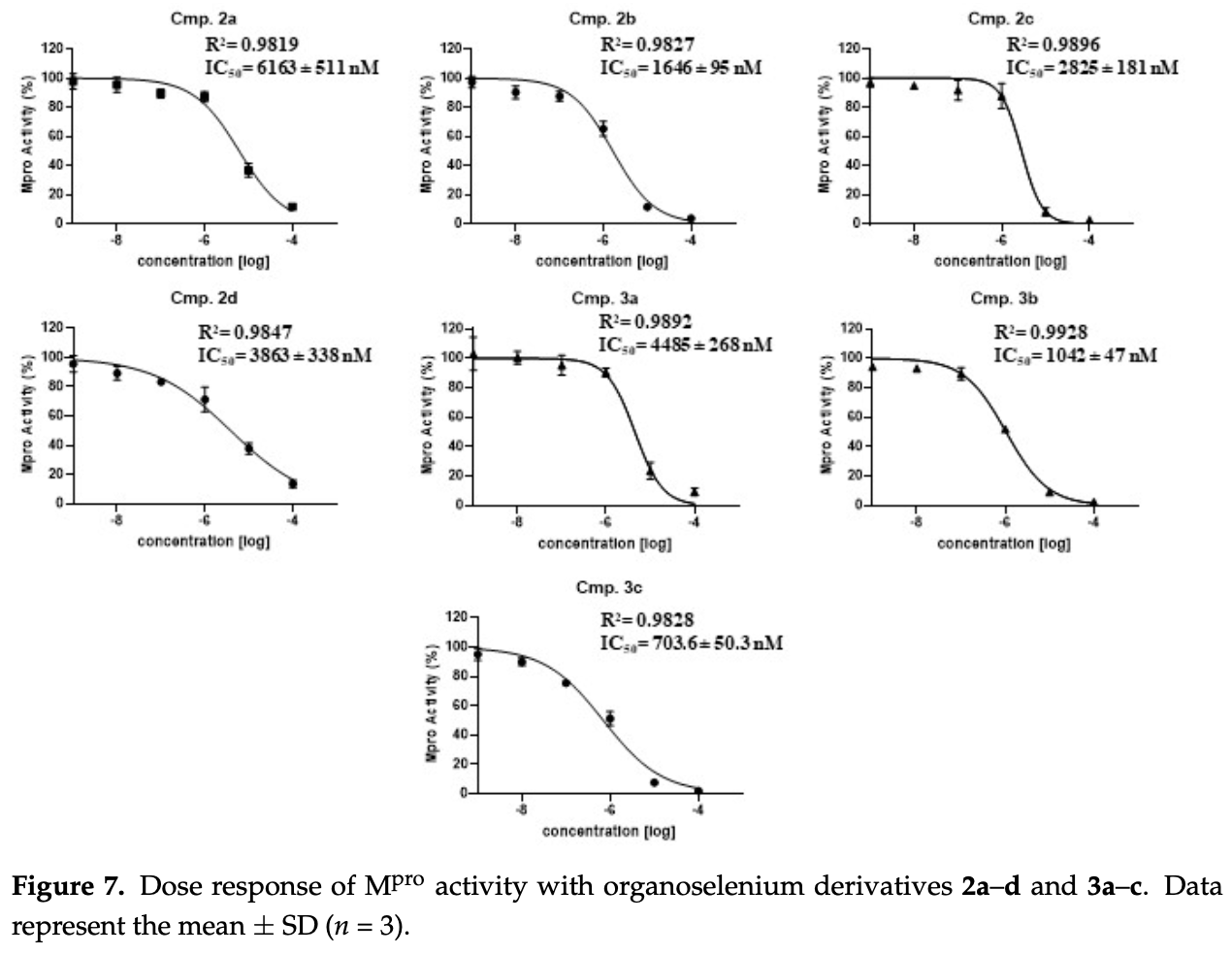
In vitro study showing that novel selenium-containing compounds including benzoselenoates and carbamoselenoates inhibit SARS-CoV-2 main protease (Mpro) activity. After successfully expressing and purifying recombinant Mpro, authors find selenium derivatives exhibit dose-dependent inhibition with IC50 values in the micromolar range. Notably, the selenocarbamate 3c demonstrates potent sub-micromolar inhibition at 703.6 nM. These results highlight the potential of organoselenium agents, through their interactions with the catalytic cysteine, as effective Mpro inhibitors and antiviral therapeutics against SARS-CoV-2.
3 preclinical studies support the efficacy of selenium for COVID-19:
Selenium has been identified by the European Food
Safety Authority (EFSA) as having sufficient evidence for a causal
relationship between intake and optimal immune system function4-6.
Selenium may be beneficial for COVID-19 by inhibiting
ferroptosis, an oxidative stress-induced cell death pathway implicated
in COVID-19 pathogenesis7.
Selenium enhances immune response, inhibits ROS
production, and protects against ferroptosis via GPX4 induction8.
1.
Sinha et al., Selective Impact of Selenium Compounds on Two Cytokine Storm Players, Preprints, doi:10.20944/preprints202308.1168.v1.
2.
Hajdrik et al., In Vitro Determination of Inhibitory Effects of Humic Substances Complexing Zn and Se on SARS-CoV-2 Virus Replication, Foods, doi:10.3390/foods11050694.
3.
Zhou et al., Metal-coding assisted serological multi-omics profiling deciphers the role of selenium in COVID-19 immunity, Chemical Science, doi:10.1039/d3sc03345g.
4.
Galmés et al., Suboptimal Consumption of Relevant Immune System Micronutrients Is Associated with a Worse Impact of COVID-19 in Spanish Populations, Nutrients, doi:10.3390/nu14112254.
5.
Galmés (B) et al., Current State of Evidence: Influence of Nutritional and Nutrigenetic Factors on Immunity in the COVID-19 Pandemic Framework, Nutrients, doi:10.3390/nu12092738.
6.
EFSA, Scientific Opinion on the substantiation of health claims related to selenium and protection of DNA, proteins and lipids from oxidative damage (ID 277, 283, 286, 1289, 1290, 1291, 1293, 1751), function of the immune system (ID 278), thyroid function (ID 279, 282, 286, 1289, 1290, 1291, 1293), function of the heart and blood vessels (ID 280), prostate function (ID 284), cognitive function (ID 285) and spermatogenesis (ID 396) pursuant to Article 13(1) of Regulation (EC) No 1924/2006, EFSA Journal, doi:10.2903/j.efsa.2009.1220.
De Luca et al., 12 Jan 2024, peer-reviewed, 10 authors.
Contact: claudiu.supuran@unifi.it (corresponding author), viviana.deluca@ibbr.cnr.it, vincenzo.carginale@cnr.it, andrea.angeli@unifi.it, alessio.nocentini@unifi.it, paola.gratteri@unifi.it, silvia.pratesi1@stud.unifi.it, damiano.tanini@unifi.it, antonella.capperucci@unifi.it, clemente.capasso@ibbr.cnr.it.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Leveraging SARS-CoV-2 Main Protease (Mpro) for COVID-19 Mitigation with Selenium-Based Inhibitors
International Journal of Molecular Sciences, doi:10.3390/ijms25020971
The implementation of innovative approaches is crucial in an ongoing endeavor to mitigate the impact of COVID-19 pandemic. The present study examines the strategic application of the SARS-CoV-2 Main Protease (M pro ) as a prospective instrument in the repertoire to combat the virus. The cloning, expression, and purification of M pro , which plays a critical role in the viral life cycle, through heterologous expression in Escherichia coli in a completely soluble form produced an active enzyme. The hydrolysis of a specific substrate peptide comprising a six-amino-acid sequence (TSAVLQ) linked to a p-nitroaniline (pNA) fragment together with the use of a fluorogenic substrate allowed us to determine effective inhibitors incorporating selenium moieties, such as benzoselenoates and carbamoselenoates. The new inhibitors revealed their potential to proficiently inhibit M pro with IC 50 -s in the low micromolar range. Our study contributes to the development of a new class of protease inhibitors targeting M pro , ultimately strengthening the antiviral arsenal against COVID-19 and possibly, related coronaviruses.
Supplementary Materials: The following supporting information can be downloaded at: https: //www.mdpi.com/article/10.3390/ijms25020971/s1.
Conflicts of Interest: The authors declare no conflicts of interest.
References
Ali, Benedetti, Handzlik, Zwergel, Battistelli, The innovative potential of seleniumcontaining agents for fighting cancer and viral infections, Drug Discov. Today, doi:10.1016/j.drudis.2020.10.014
Amporndanai, Meng, Shang, Jin, Rogers et al., Inhibition mechanism of SARS-CoV-2 main protease by ebselen and its derivatives, Nat. Commun, doi:10.1038/s41467-021-23313-7
Angeli, Carta, Donnini, Capperucci, Ferraroni et al., Selenolesterase enzyme activity of carbonic anhydrases, Chem. Commun, doi:10.1039/D0CC00995D
Angeli, Ferraroni, Capperucci, Tanini, Costantino et al., Selenocarbamates As a Prodrug-Based Approach to Carbonic Anhydrase Inhibition, ChemMedChem, doi:10.1002/cmdc.202200085
Angeli, Tanini, Nocentini, Capperucci, Ferraroni et al., A new class of carbonic anhydrase inhibitors, Chem. Commun, doi:10.1039/C8CC08562E
Badiani, Patel, Ziolkowski, Nielsen, Pfizer, The miracle vaccine for COVID-19?, Public Health Pract, doi:10.1016/j.puhip.2020.100061
Bhardwaj, Singh, Das, Purohit, Evaluation of acridinedione analogs as potential SARS-CoV-2 main protease inhibitors and their comparison with repurposed anti-viral drugs, Comput. Biol. Med, doi:10.1016/j.compbiomed.2020.104117
Buxeraud, Faure, Fougere, Nirmatrelvir/ritonavir (Paxlovid(R)), a treatment for COVID-19, Actual. Pharm
Cao, Goreshnik, Coventry, Case, Miller et al., De novo design of picomolar SARS-CoV-2 miniprotein inhibitors, Science, doi:10.1126/science.abd9909
Capasso, Nocentini, Supuran, Protease inhibitors targeting the main protease and papain-like protease of coronaviruses, Expert Opin. Ther. Pat, doi:10.1080/13543776.2021.1857726
Capperucci, Petrucci, Faggi, Tanini, Click Reaction of Selenols with Isocyanates: Rapid Access to Selenocarbamates as Peroxide-Switchable Reservoir of Thiol-peroxidase-Like Catalysts, Adv. Synth. Catal, doi:10.1002/adsc.202100611
Carabelli, Peacock, Thorne, Harvey, Hughes et al., SARS-CoV-2 variant biology: Immune escape, transmission and fitness, Nat. Rev. Microbiol, doi:10.1038/s41579-022-00841-7
Chavda, Vuppu, Mishra, Kamaraj, Patel et al., Recent review of COVID-19 management: Diagnosis, treatment and vaccination, Pharmacol. Rep, doi:10.1007/s43440-022-00425-5
Chen, Shao, Peng, Liang, Xu et al., Review of preclinical data of PF-07304814 and its active metabolite derivatives against SARS-CoV-2 infection, Front. Pharmacol, doi:10.3389/fphar.2022.1035969
Citarella, Scala, Piperno, Micale, SARS-CoV-2 M pro : A Potential Target for Peptidomimetics and Small-Molecule Inhibitors, Biomolecules, doi:10.3390/biom11040607
Da Silva, Do Nascimento, Mendes, Guarines, Da Silva et al., Two Years into the COVID-19 Pandemic: Lessons Learned, ACS Infect. Dis, doi:10.1021/acsinfecdis.2c00204
Debnath, Agarwal, Kumar, Bedi, Selenium-Based Drug Development for Antioxidant and Anticancer Activity, Future Pharmacol, doi:10.3390/futurepharmacol2040036
Deniz, Uysal, Capasso, Supuran, Ozensoy Guler, Is carbonic anhydrase inhibition useful as a complementary therapy of COVID-19 infection?, J. Enzym. Inhib. Med. Chem, doi:10.1080/14756366.2021.1924165
Elnaggar, Elgazar, Gamal, Hamed, Elsayed et al., Identification of sulphonamide-tethered N-((triazol-4-yl)methyl)isatin derivatives as inhibitors of SARS-CoV-2 main protease, J. Enzym. Inhib. Med. Chem, doi:10.1080/14756366.2023.2234665
Eltayb, Abdalla, Rabie, Novel Investigational Anti-SARS-CoV-2 Agent Ensitrelvir "S-217622": A Very Promising Potential Universal Broad-Spectrum Antiviral at the Therapeutic Frontline of Coronavirus Species, ACS Omega, doi:10.1021/acsomega.2c03881
Flynn, Samant, Schneider-Nachum, Barkan, Yilmaz et al., Comprehensive fitness landscape of SARS-CoV-2 M pro reveals insights into viral resistance mechanisms, eLife, doi:10.7554/eLife.77433
Ghosh, Mishevich, Mesecar, Mitsuya, Recent Drug Development and Medicinal Chemistry Approaches for the Treatment of SARS-CoV-2 Infection and COVID-19, ChemMedChem, doi:10.1002/cmdc.202200440
Harris, Nasally Administered Monoclonal Antibody for COVID-19, JAMA-J. Am. Med. Assoc, doi:10.1001/jama.2023.4000
Hashemian, Sheida, Taghizadieh, Memar, Hamblin et al., Nirmatrelvir/Ritonavir): A new approach to COVID-19 therapy?, Biomed. Pharmacother, doi:10.1016/j.biopha.2023.114367
Hoffman, Kania, Brothers, Davies, Ferre et al., Discovery of Ketone-Based Covalent Inhibitors of Coronavirus 3CL Proteases for the Potential Therapeutic Treatment of COVID-19, J. Med. Chem, doi:10.1021/acs.jmedchem.0c01063
Hou, Dong, Zhang, Wang, Su et al., Selenium as an emerging versatile player in heterocycles and natural products modification, Drug Discov. Today, doi:10.1016/j.drudis.2022.03.020
Hu, Xiong, Zhu, Zhang, Zhang et al., The SARS-CoV-2 main protease (M pro ): Structure, function, and emerging therapies for COVID-19, MedComm, doi:10.1002/mco2.151
Iacobucci, COVID-19: Evusheld protects the most vulnerable patients, analysis shows, BMJ-Br. Med. J, doi:10.1136/bmj.o2690
Ishihara, Koketsu, Fukuta, Nada, Reaction of lithium aluminum hydride with elemental selenium: Its application as a selenating reagent into organic molecules, J. Am. Chem. Soc, doi:10.1021/ja005800o
Jiang, Zou, Zeng, Zeng, Zhou et al., Crystal structures of main protease (M pro ) mutants of SARS-CoV-2 variants bound to PF-07304814, Mol. Biomed, doi:10.1186/s43556-023-00134-2
Jin, Du, Xu, Deng, Liu et al., Structure of M pro from SARS-CoV-2 and discovery of its inhibitors, Nature, doi:10.1038/s41586-020-2223-y
Kabinger, Stiller, Schmitzova, Dienemann, Kokic et al., Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis, Nat. Struct. Mol. Biol, doi:10.1038/s41594-021-00651-0
Kamal, Ramadan, Farraj, Bahig, Ezzat, The pill of recovery; Molnupiravir for treatment of COVID-19 patients; a systematic review, Saudi Pharm. J, doi:10.1016/j.jsps.2022.03.002
Karniadakis, Mazonakis, Tsioutis, Papadakis, Markaki et al., Oral Molnupiravir and Nirmatrelvir/Ritonavir for the Treatment of COVID-19: A Literature Review with a Focus on Real-World Evidence, Infect. Dis. Rep, doi:10.3390/idr15060061
Kelley, De Moor, Douglas, Renshaw, Traviglia, Monoclonal antibody therapies for COVID-19: Lessons learned and implications for the development of future products, Curr. Opin. Biotechnol, doi:10.1016/j.copbio.2022.102798
Kip, Mccreary, Collins, Minnier, Snyder et al., Evolving Real-World Effectiveness of Monoclonal Antibodies for Treatment of COVID-19 A Cohort Study, Ann. Intern. Med, doi:10.7326/M22-1286
Kovacs, Kurtan, Varga, Nagy, Panyi et al., remdesivir) formulations inhibit initial membranecoupled events of SARS-CoV-2 infection due to their sulfobutylether-beta-cyclodextrin content, Br. J. Pharmacol, doi:10.1111/bph.16063
Li, Lin, Zhou, Zhong, Zeng et al., Structural Basis of Main Proteases of Coronavirus Bound to Drug Candidate PF-07304814, J. Mol. Biol, doi:10.1016/j.jmb.2022.167706
Li, Niu, A commentary on "The socio-economic implications of the coronavirus pandemic (COVID-19): A review, Int. J. Surg, doi:10.1016/j.ijsu.2021.106048
Li, Wang, Tian, Pang, Yang et al., COVID-19 vaccine development: Milestones, lessons and prospects, Signal Transduct. Target. Ther, doi:10.1038/s41392-022-00996-y
Liu, Wu, Huang, Hsu, Chuang et al., Clinical efficacy of nirmatrelvir plus ritonavir in patients with COVID-19 and preexisting cardiovascular diseases, Expert Rev. Anti-Infect. Ther, doi:10.1080/14787210.2023.2284367
Lui, Guaraldi, Drug treatment of COVID-19 infection, Curr. Opin. Pulm. Med, doi:10.1097/MCP.0000000000000953
Ma, Hu, Townsend, Lagarias, Marty et al., Disulfiram, Carmofur, PX-12, Tideglusib, and Shikonin Are Nonspecific Promiscuous SARS-CoV-2 Main Protease Inhibitors, ACS Pharmacol. Transl. Sci, doi:10.1021/acsptsci.0c00130
Markov, Ghafari, Beer, Lythgoe, Simmonds et al., The evolution of SARS-CoV-2, Nat. Rev. Microbiol, doi:10.1038/s41579-023-00878-2
Mengist, Dilnessa, Jin, Structural Basis of Potential Inhibitors Targeting SARS-CoV-2 Main Protease, Front. Chem, doi:10.3389/fchem.2021.622898
Mori, Capasso, Carta, Donald, Supuran, A deadly spillover: SARS-CoV-2 outbreak, Expert Opin. Ther. Pat, doi:10.1080/13543776.2020.1760838
Narayanan, Narwal, Majowicz, Varricchio, Toner et al., Identification of SARS-CoV-2 inhibitors targeting Mpro and PLpro using in-cell-protease assay, Commun. Biol, doi:10.1038/s42003-022-03090-9
Ng, Correia, Seagal, Degoey, Schrimpf et al., Antiviral Drug Discovery for the Treatment of COVID-19 Infections, Viruses, doi:10.3390/v14050961
Niknam, Jafari, Golchin, Danesh Pouya, Nemati et al., Potential therapeutic options for COVID-19: An update on current evidence, Eur. J. Med. Res, doi:10.1186/s40001-021-00626-3
Nocentini, Capasso, Supuran, Perspectives on the design and discovery of alpha-ketoamide inhibitors for the treatment of novel coronavirus: Where do we stand and where do we go?, Expert Opin. Drug Discov, doi:10.1080/17460441.2022.2052847
Pilote, Simard, Drolet, Remdesivir, VEKLURY) for Treating COVID-19: Guinea Pig Ex Vivo and In Vivo Cardiac Electrophysiological Effects, J. Cardiovasc. Pharmacol, doi:10.1097/FJC.0000000000001321
Ravaghi, Naidoo, Mataria, Khalil, Hospitals early challenges and interventions combatting COVID-19 in the Eastern Mediterranean Region, PLoS ONE, doi:10.1371/journal.pone.0268386
Ravi, Eerike, Konda, Bisoi, Raj et al., Efficacy and Safety of Anti-SARS-CoV-2 Monoclonal Antibodies: An Updated Review. Monoclon. Antibodies Immunodiagn, Immunother, doi:10.1089/mab.2022.0036
Razali, Subbiah, Budiman, Technical Data of Heterologous Expression and Purification of SARS-CoV-2 Proteases Using Escherichia coli System, Data, doi:10.3390/data6090099
Sacco, Ma, Lagarias, Gao, Townsend et al., Structure and inhibition of the SARS-CoV-2 main protease reveal strategy for developing dual inhibitors against M pro and cathepsin L, Sci. Adv, doi:10.1126/sciadv.abe0751
Sardar, Abdul-Khaliq, Ingar, Amaidia, Mansour, COVID-19 lockdown: A protective measure or exacerbator of health inequalities? A comparison between the United Kingdom and India.' a commentary on "the socio-economic implications of the coronavirus and COVID-19 pandemic: A review, Int. J. Surg, doi:10.1016/j.ijsu.2020.09.044
Scherf, Matschke, Rieger, Stock market reactions to COVID-19 lockdown: A global analysis, Financ. Res. Lett, doi:10.1016/j.frl.2021.102245
Sharun, Tiwari, Dhama, Dhama, Dexamethasone to combat cytokine storm in COVID-19: Clinical trials and preliminary evidence, Int. J. Surg, doi:10.1016/j.ijsu.2020.08.038
Tanini, Capperucci, Synthesis and Applications of Organic Selenols, Adv. Synth. Catal, doi:10.1002/adsc.202101147
Tanini, Carradori, Capperucci, Lupori, Zara et al., Chalcogenides-incorporating carbonic anhydrase inhibitors concomitantly reverted oxaliplatin-induced neuropathy and enhanced antiproliferative action, Eur. J. Med. Chem, doi:10.1016/j.ejmech.2021.113793
Tanini, Scarpelli, Ermini, Capperucci, Seleno-Michael Reaction of Stable Functionalised Alkyl Selenols: A Versatile Tool for the Synthesis of Acyclic and Cyclic Unsymmetrical Alkyl and Vinyl Selenides, Adv. Synth. Catal, doi:10.1002/adsc.201900168
Tanini, Tiberi, Gellini, Salvi, Capperucci, A Straightforward Access to Stable β-Functionalized Alkyl Selenols, Adv. Synth. Catal, doi:10.1002/adsc.201800602
Thakkar, Agarwal, Ranaweera, Ishiguro, Conda-Sheridan et al., De novo design of a stapled peptide targeting SARS-CoV-2 spike protein receptor-binding domain, RSC Med. Chem, doi:10.1039/D3MD00222E
Ullrich, Nitsche, The SARS-CoV-2 main protease as drug target, Bioorganic Med. Chem. Lett, doi:10.1016/j.bmcl.2020.127377
Wang, Yang, Yang, Hou, Tsai et al., Structural basis of SARS-CoV-2 main protease inhibition by a broad-spectrum anti-coronaviral drug, Am. J. Cancer Res
Waugh, An overview of enzymatic reagents for the removal of affinity tags, Protein Expr. Purif, doi:10.1016/j.pep.2011.08.005
Wynter-Adams, Thomas-Brown, COVID-19 vaccine hesitancy in a developing country: Prevalence, explanatory factors and implications for the future, Public Health, doi:10.1016/j.puhe.2023.01.031
Yadav, Chaudhary, Jain, Chaudhary, Khanra et al., Role of Structural and Non-Structural Proteins and Therapeutic Targets of SARS-CoV-2 for COVID-19, Cells, doi:10.3390/cells10040821
Yang, Ju, Wang, Jia, Wang et al., Proxalutamide for the treatment of COVID-19 rebound following Paxlovid treatment: Report of four cases and review of the literature, J. Clin. Lab. Anal, doi:10.1002/jcla.24880
Zhang, Lin, Sun, Curth, Drosten et al., Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved alpha-ketoamide inhibitors, Science, doi:10.1126/science.abb3405
Zhao, Fang, Zhang, Zhang, Zhao et al., Crystal structure of SARS-CoV-2 main protease in complex with protease inhibitor PF-07321332, Protein Cell, doi:10.1007/s13238-021-00883-2
DOI record:
{
"DOI": "10.3390/ijms25020971",
"ISSN": [
"1422-0067"
],
"URL": "http://dx.doi.org/10.3390/ijms25020971",
"abstract": "<jats:p>The implementation of innovative approaches is crucial in an ongoing endeavor to mitigate the impact of COVID-19 pandemic. The present study examines the strategic application of the SARS-CoV-2 Main Protease (Mpro) as a prospective instrument in the repertoire to combat the virus. The cloning, expression, and purification of Mpro, which plays a critical role in the viral life cycle, through heterologous expression in Escherichia coli in a completely soluble form produced an active enzyme. The hydrolysis of a specific substrate peptide comprising a six-amino-acid sequence (TSAVLQ) linked to a p-nitroaniline (pNA) fragment together with the use of a fluorogenic substrate allowed us to determine effective inhibitors incorporating selenium moieties, such as benzoselenoates and carbamoselenoates. The new inhibitors revealed their potential to proficiently inhibit Mpro with IC50-s in the low micromolar range. Our study contributes to the development of a new class of protease inhibitors targeting Mpro, ultimately strengthening the antiviral arsenal against COVID-19 and possibly, related coronaviruses.</jats:p>",
"alternative-id": [
"ijms25020971"
],
"author": [
{
"affiliation": [
{
"name": "Department of Biology, Agriculture and Food Sciences, National Research Council (CNR), Institute of Biosciences and Bioresources, 80131 Naples, Italy"
}
],
"family": "De Luca",
"given": "Viviana",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-1470-7192",
"affiliation": [
{
"name": "Neurofarba Department, Pharmaceutical and Nutraceutical Section, Laboratory of Molecular Modeling Cheminformatics & QSAR, University of Florence, Via Ugo Schiff 6, Sesto Fiorentino, 50019 Florence, Italy"
}
],
"authenticated-orcid": false,
"family": "Angeli",
"given": "Andrea",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3342-702X",
"affiliation": [
{
"name": "Neurofarba Department, Pharmaceutical and Nutraceutical Section, Laboratory of Molecular Modeling Cheminformatics & QSAR, University of Florence, Via Ugo Schiff 6, Sesto Fiorentino, 50019 Florence, Italy"
}
],
"authenticated-orcid": false,
"family": "Nocentini",
"given": "Alessio",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9137-2509",
"affiliation": [
{
"name": "Neurofarba Department, Pharmaceutical and Nutraceutical Section, Laboratory of Molecular Modeling Cheminformatics & QSAR, University of Florence, Via Ugo Schiff 6, Sesto Fiorentino, 50019 Florence, Italy"
}
],
"authenticated-orcid": false,
"family": "Gratteri",
"given": "Paola",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Chemistry “Ugo Schiff”, University of Florence, Via Della Lastruccia 3-13, Sesto Fiorentino, 50019 Florence, Italy"
}
],
"family": "Pratesi",
"given": "Silvia",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8930-3566",
"affiliation": [
{
"name": "Department of Chemistry “Ugo Schiff”, University of Florence, Via Della Lastruccia 3-13, Sesto Fiorentino, 50019 Florence, Italy"
}
],
"authenticated-orcid": false,
"family": "Tanini",
"given": "Damiano",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1842-494X",
"affiliation": [
{
"name": "Department of Biology, Agriculture and Food Sciences, National Research Council (CNR), Institute of Biosciences and Bioresources, 80131 Naples, Italy"
}
],
"authenticated-orcid": false,
"family": "Carginale",
"given": "Vincenzo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Chemistry “Ugo Schiff”, University of Florence, Via Della Lastruccia 3-13, Sesto Fiorentino, 50019 Florence, Italy"
}
],
"family": "Capperucci",
"given": "Antonella",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4262-0323",
"affiliation": [
{
"name": "Neurofarba Department, Pharmaceutical and Nutraceutical Section, Laboratory of Molecular Modeling Cheminformatics & QSAR, University of Florence, Via Ugo Schiff 6, Sesto Fiorentino, 50019 Florence, Italy"
}
],
"authenticated-orcid": false,
"family": "Supuran",
"given": "Claudiu T.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3314-2411",
"affiliation": [
{
"name": "Department of Biology, Agriculture and Food Sciences, National Research Council (CNR), Institute of Biosciences and Bioresources, 80131 Naples, Italy"
}
],
"authenticated-orcid": false,
"family": "Capasso",
"given": "Clemente",
"sequence": "additional"
}
],
"container-title": "International Journal of Molecular Sciences",
"container-title-short": "IJMS",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
1,
12
]
],
"date-time": "2024-01-12T12:47:16Z",
"timestamp": 1705063636000
},
"deposited": {
"date-parts": [
[
2024,
1,
12
]
],
"date-time": "2024-01-12T13:12:11Z",
"timestamp": 1705065131000
},
"funder": [
{
"name": "Italian National Research Council"
}
],
"indexed": {
"date-parts": [
[
2024,
1,
13
]
],
"date-time": "2024-01-13T00:37:52Z",
"timestamp": 1705106272579
},
"is-referenced-by-count": 0,
"issue": "2",
"issued": {
"date-parts": [
[
2024,
1,
12
]
]
},
"journal-issue": {
"issue": "2",
"published-online": {
"date-parts": [
[
2024,
1
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
1,
12
]
],
"date-time": "2024-01-12T00:00:00Z",
"timestamp": 1705017600000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/1422-0067/25/2/971/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "971",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2024,
1,
12
]
]
},
"published-online": {
"date-parts": [
[
2024,
1,
12
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1038/s41579-023-00878-2",
"article-title": "The evolution of SARS-CoV-2",
"author": "Markov",
"doi-asserted-by": "crossref",
"first-page": "361",
"journal-title": "Nat. Rev. Microbiol.",
"key": "ref_1",
"volume": "21",
"year": "2023"
},
{
"DOI": "10.1016/j.ijsu.2021.106048",
"article-title": "A commentary on “The socio-economic implications of the coronavirus pandemic (COVID-19): A review”",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "106048",
"journal-title": "Int. J. Surg.",
"key": "ref_2",
"volume": "95",
"year": "2021"
},
{
"DOI": "10.1016/j.ijsu.2020.09.044",
"article-title": "‘COVID-19 lockdown: A protective measure or exacerbator of health inequalities? A comparison between the United Kingdom and India.’ a commentary on “the socio-economic implications of the coronavirus and COVID-19 pandemic: A review”",
"author": "Sardar",
"doi-asserted-by": "crossref",
"first-page": "189",
"journal-title": "Int. J. Surg.",
"key": "ref_3",
"volume": "83",
"year": "2020"
},
{
"DOI": "10.1016/j.frl.2021.102245",
"article-title": "Stock market reactions to COVID-19 lockdown: A global analysis",
"author": "Scherf",
"doi-asserted-by": "crossref",
"first-page": "102245",
"journal-title": "Financ. Res. Lett.",
"key": "ref_4",
"volume": "45",
"year": "2022"
},
{
"DOI": "10.1016/j.puhip.2020.100061",
"article-title": "Pfizer: The miracle vaccine for COVID-19?",
"author": "Badiani",
"doi-asserted-by": "crossref",
"first-page": "100061",
"journal-title": "Public Health Pract.",
"key": "ref_5",
"volume": "1",
"year": "2020"
},
{
"article-title": "SARS-CoV-2 variant biology: Immune escape, transmission and fitness",
"author": "Carabelli",
"first-page": "162",
"journal-title": "Nat. Rev. Microbiol.",
"key": "ref_6",
"volume": "21",
"year": "2023"
},
{
"DOI": "10.1038/s41392-022-00996-y",
"article-title": "COVID-19 vaccine development: Milestones, lessons and prospects",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "146",
"journal-title": "Signal Transduct. Target. Ther.",
"key": "ref_7",
"volume": "7",
"year": "2022"
},
{
"DOI": "10.1002/cmdc.202200440",
"article-title": "Recent Drug Development and Medicinal Chemistry Approaches for the Treatment of SARS-CoV-2 Infection and COVID-19",
"author": "Ghosh",
"doi-asserted-by": "crossref",
"first-page": "e202200440",
"journal-title": "ChemMedChem",
"key": "ref_8",
"volume": "17",
"year": "2022"
},
{
"DOI": "10.1097/MCP.0000000000000953",
"article-title": "Drug treatment of COVID-19 infection",
"author": "Lui",
"doi-asserted-by": "crossref",
"first-page": "174",
"journal-title": "Curr. Opin. Pulm. Med.",
"key": "ref_9",
"volume": "29",
"year": "2023"
},
{
"DOI": "10.1371/journal.pone.0268386",
"doi-asserted-by": "crossref",
"key": "ref_10",
"unstructured": "Ravaghi, H., Naidoo, V., Mataria, A., and Khalil, M. (2022). Hospitals early challenges and interventions combatting COVID-19 in the Eastern Mediterranean Region. PLoS ONE, 17."
},
{
"DOI": "10.1016/j.ijsu.2020.08.038",
"article-title": "Dexamethasone to combat cytokine storm in COVID-19: Clinical trials and preliminary evidence",
"author": "Sharun",
"doi-asserted-by": "crossref",
"first-page": "179",
"journal-title": "Int. J. Surg.",
"key": "ref_11",
"volume": "82",
"year": "2020"
},
{
"article-title": "Two Years into the COVID-19 Pandemic: Lessons Learned",
"author": "Mendes",
"first-page": "1758",
"journal-title": "ACS Infect. Dis.",
"key": "ref_12",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.3390/futurepharmacol2040036",
"article-title": "Selenium-Based Drug Development for Antioxidant and Anticancer Activity",
"author": "Debnath",
"doi-asserted-by": "crossref",
"first-page": "595",
"journal-title": "Future Pharmacol.",
"key": "ref_13",
"volume": "2",
"year": "2022"
},
{
"DOI": "10.1016/j.drudis.2020.10.014",
"article-title": "The innovative potential of seleniumcontaining agents for fighting cancer and viral infections",
"author": "Ali",
"doi-asserted-by": "crossref",
"first-page": "256",
"journal-title": "Drug Discov. Today",
"key": "ref_14",
"volume": "26",
"year": "2021"
},
{
"DOI": "10.1016/j.drudis.2022.03.020",
"article-title": "Selenium as an emerging versatile player in heterocycles and natural products modification",
"author": "Hou",
"doi-asserted-by": "crossref",
"first-page": "2268",
"journal-title": "Drug Discov. Today",
"key": "ref_15",
"volume": "27",
"year": "2022"
},
{
"DOI": "10.1002/cmdc.202200085",
"article-title": "Selenocarbamates As a Prodrug-Based Approach to Carbonic Anhydrase Inhibition",
"author": "Angeli",
"doi-asserted-by": "crossref",
"first-page": "e202200085",
"journal-title": "ChemMedChem",
"key": "ref_16",
"volume": "17",
"year": "2022"
},
{
"DOI": "10.1016/j.ejmech.2021.113793",
"article-title": "Chalcogenides-incorporating carbonic anhydrase inhibitors concomitantly reverted oxaliplatin-induced neuropathy and enhanced antiproliferative action",
"author": "Tanini",
"doi-asserted-by": "crossref",
"first-page": "113793",
"journal-title": "Eur. J. Med. Chem.",
"key": "ref_17",
"volume": "225",
"year": "2021"
},
{
"DOI": "10.1038/s41586-020-2223-y",
"article-title": "Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors",
"author": "Jin",
"doi-asserted-by": "crossref",
"first-page": "289",
"journal-title": "Nature",
"key": "ref_18",
"volume": "582",
"year": "2020"
},
{
"DOI": "10.1021/acsptsci.0c00130",
"article-title": "Ebselen, Disulfiram, Carmofur, PX-12, Tideglusib, and Shikonin Are Nonspecific Promiscuous SARS-CoV-2 Main Protease Inhibitors",
"author": "Ma",
"doi-asserted-by": "crossref",
"first-page": "1265",
"journal-title": "ACS Pharmacol. Transl. Sci.",
"key": "ref_19",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.1038/s41467-021-23313-7",
"article-title": "Inhibition mechanism of SARS-CoV-2 main protease by ebselen and its derivatives",
"author": "Amporndanai",
"doi-asserted-by": "crossref",
"first-page": "3061",
"journal-title": "Nat. Commun.",
"key": "ref_20",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1111/bph.16063",
"article-title": "Veklury(R) (remdesivir) formulations inhibit initial membrane-coupled events of SARS-CoV-2 infection due to their sulfobutylether-beta-cyclodextrin content",
"author": "Kovacs",
"doi-asserted-by": "crossref",
"first-page": "2064",
"journal-title": "Br. J. Pharmacol.",
"key": "ref_21",
"volume": "180",
"year": "2023"
},
{
"DOI": "10.1097/FJC.0000000000001321",
"article-title": "Remdesivir (VEKLURY) for Treating COVID-19: Guinea Pig Ex Vivo and In Vivo Cardiac Electrophysiological Effects",
"author": "Pilote",
"doi-asserted-by": "crossref",
"first-page": "616",
"journal-title": "J. Cardiovasc. Pharmacol.",
"key": "ref_22",
"volume": "80",
"year": "2022"
},
{
"DOI": "10.1016/j.copbio.2022.102798",
"article-title": "Monoclonal antibody therapies for COVID-19: Lessons learned and implications for the development of future products",
"author": "Kelley",
"doi-asserted-by": "crossref",
"first-page": "102798",
"journal-title": "Curr. Opin. Biotechnol.",
"key": "ref_23",
"volume": "78",
"year": "2022"
},
{
"DOI": "10.1089/mab.2022.0036",
"article-title": "Efficacy and Safety of Anti-SARS-CoV-2 Monoclonal Antibodies: An Updated Review",
"author": "Ravi",
"doi-asserted-by": "crossref",
"first-page": "77",
"journal-title": "Monoclon. Antibodies Immunodiagn. Immunother.",
"key": "ref_24",
"volume": "42",
"year": "2023"
},
{
"article-title": "Nasally Administered Monoclonal Antibody for COVID-19",
"author": "Harris",
"first-page": "1142",
"journal-title": "JAMA-J. Am. Med. Assoc.",
"key": "ref_25",
"volume": "329",
"year": "2023"
},
{
"DOI": "10.7326/M22-1286",
"article-title": "Evolving Real-World Effectiveness of Monoclonal Antibodies for Treatment of COVID-19 A Cohort Study",
"author": "Kip",
"doi-asserted-by": "crossref",
"first-page": "496",
"journal-title": "Ann. Intern. Med.",
"key": "ref_26",
"volume": "176",
"year": "2023"
},
{
"DOI": "10.1016/j.puhe.2023.01.031",
"article-title": "COVID-19 vaccine hesitancy in a developing country: Prevalence, explanatory factors and implications for the future",
"doi-asserted-by": "crossref",
"first-page": "146",
"journal-title": "Public Health",
"key": "ref_27",
"volume": "217",
"year": "2023"
},
{
"DOI": "10.1136/bmj.o2690",
"article-title": "COVID-19: Evusheld protects the most vulnerable patients, analysis shows",
"author": "Iacobucci",
"doi-asserted-by": "crossref",
"first-page": "o2690",
"journal-title": "BMJ-Br. Med. J.",
"key": "ref_28",
"volume": "379",
"year": "2022"
},
{
"DOI": "10.1186/s40001-021-00626-3",
"article-title": "Potential therapeutic options for COVID-19: An update on current evidence",
"author": "Niknam",
"doi-asserted-by": "crossref",
"first-page": "6",
"journal-title": "Eur. J. Med. Res.",
"key": "ref_29",
"volume": "27",
"year": "2022"
},
{
"DOI": "10.3390/v14050961",
"doi-asserted-by": "crossref",
"key": "ref_30",
"unstructured": "Ng, T.I., Correia, I., Seagal, J., DeGoey, D.A., Schrimpf, M.R., Hardee, D.J., Noey, E.L., and Kati, W.M. (2022). Antiviral Drug Discovery for the Treatment of COVID-19 Infections. Viruses, 14."
},
{
"DOI": "10.1038/s41594-021-00651-0",
"article-title": "Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis",
"author": "Kabinger",
"doi-asserted-by": "crossref",
"first-page": "740",
"journal-title": "Nat. Struct. Mol. Biol.",
"key": "ref_31",
"volume": "28",
"year": "2021"
},
{
"DOI": "10.1016/j.jsps.2022.03.002",
"article-title": "The pill of recovery; Molnupiravir for treatment of COVID-19 patients; a systematic review",
"author": "Kamal",
"doi-asserted-by": "crossref",
"first-page": "508",
"journal-title": "Saudi Pharm. J.",
"key": "ref_32",
"volume": "30",
"year": "2022"
},
{
"article-title": "Nirmatrelvir/ritonavir (Paxlovid(R)), a treatment for COVID-19",
"author": "Buxeraud",
"first-page": "10",
"journal-title": "Actual. Pharm.",
"key": "ref_33",
"volume": "61",
"year": "2022"
},
{
"DOI": "10.1002/jcla.24880",
"article-title": "Proxalutamide for the treatment of COVID-19 rebound following Paxlovid treatment: Report of four cases and review of the literature",
"author": "Yang",
"doi-asserted-by": "crossref",
"first-page": "e24880",
"journal-title": "J. Clin. Lab. Anal.",
"key": "ref_34",
"volume": "37",
"year": "2023"
},
{
"DOI": "10.1007/s43440-022-00425-5",
"article-title": "Recent review of COVID-19 management: Diagnosis, treatment and vaccination",
"author": "Chavda",
"doi-asserted-by": "crossref",
"first-page": "1120",
"journal-title": "Pharmacol. Rep.",
"key": "ref_35",
"volume": "74",
"year": "2022"
},
{
"DOI": "10.1016/j.biopha.2023.114367",
"doi-asserted-by": "crossref",
"key": "ref_36",
"unstructured": "Hashemian, S.M.R., Sheida, A., Taghizadieh, M., Memar, M.Y., Hamblin, M.R., Bannazadeh Baghi, H., Sadri Nahand, J., Asemi, Z., and Mirzaei, H. (2023). Paxlovid (Nirmatrelvir/Ritonavir): A new approach to COVID-19 therapy?. Biomed. Pharmacother., 162."
},
{
"DOI": "10.1021/acsomega.2c03881",
"article-title": "Novel Investigational Anti-SARS-CoV-2 Agent Ensitrelvir “S-217622”: A Very Promising Potential Universal Broad-Spectrum Antiviral at the Therapeutic Frontline of Coronavirus Species",
"author": "Eltayb",
"doi-asserted-by": "crossref",
"first-page": "5234",
"journal-title": "ACS Omega",
"key": "ref_37",
"volume": "8",
"year": "2023"
},
{
"DOI": "10.3390/cells10040821",
"doi-asserted-by": "crossref",
"key": "ref_38",
"unstructured": "Yadav, R., Chaudhary, J.K., Jain, N., Chaudhary, P.K., Khanra, S., Dhamija, P., Sharma, A., Kumar, A., and Handu, S. (2021). Role of Structural and Non-Structural Proteins and Therapeutic Targets of SARS-CoV-2 for COVID-19. Cells, 10."
},
{
"DOI": "10.1080/13543776.2020.1760838",
"article-title": "A deadly spillover: SARS-CoV-2 outbreak",
"author": "Mori",
"doi-asserted-by": "crossref",
"first-page": "481",
"journal-title": "Expert Opin. Ther. Pat.",
"key": "ref_39",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1080/13543776.2021.1857726",
"article-title": "Protease inhibitors targeting the main protease and papain-like protease of coronaviruses",
"author": "Capasso",
"doi-asserted-by": "crossref",
"first-page": "309",
"journal-title": "Expert Opin. Ther. Pat.",
"key": "ref_40",
"volume": "31",
"year": "2021"
},
{
"DOI": "10.1080/14756366.2021.1924165",
"article-title": "Is carbonic anhydrase inhibition useful as a complementary therapy of COVID-19 infection?",
"author": "Deniz",
"doi-asserted-by": "crossref",
"first-page": "1230",
"journal-title": "J. Enzym. Inhib. Med. Chem.",
"key": "ref_41",
"volume": "36",
"year": "2021"
},
{
"DOI": "10.3390/biom11040607",
"doi-asserted-by": "crossref",
"key": "ref_42",
"unstructured": "Citarella, A., Scala, A., Piperno, A., and Micale, N. (2021). SARS-CoV-2 Mpro: A Potential Target for Peptidomimetics and Small-Molecule Inhibitors. Biomolecules, 11."
},
{
"DOI": "10.7554/eLife.77433",
"article-title": "Comprehensive fitness landscape of SARS-CoV-2 Mpro reveals insights into viral resistance mechanisms",
"author": "Flynn",
"doi-asserted-by": "crossref",
"first-page": "e77433",
"journal-title": "eLife",
"key": "ref_43",
"volume": "11",
"year": "2022"
},
{
"article-title": "The SARS-CoV-2 main protease (Mpro): Structure, function, and emerging therapies for COVID-19",
"author": "Hu",
"first-page": "e151",
"journal-title": "MedComm (2020)",
"key": "ref_44",
"volume": "3",
"year": "2022"
},
{
"DOI": "10.3389/fchem.2021.622898",
"article-title": "Structural Basis of Potential Inhibitors Targeting SARS-CoV-2 Main Protease",
"author": "Mengist",
"doi-asserted-by": "crossref",
"first-page": "622898",
"journal-title": "Front. Chem.",
"key": "ref_45",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1016/j.pep.2011.08.005",
"article-title": "An overview of enzymatic reagents for the removal of affinity tags",
"author": "Waugh",
"doi-asserted-by": "crossref",
"first-page": "283",
"journal-title": "Protein Expr. Purif.",
"key": "ref_46",
"volume": "80",
"year": "2011"
},
{
"DOI": "10.1126/science.abb3405",
"article-title": "Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved alpha-ketoamide inhibitors",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "409",
"journal-title": "Science",
"key": "ref_47",
"volume": "368",
"year": "2020"
},
{
"DOI": "10.3390/data6090099",
"doi-asserted-by": "crossref",
"key": "ref_48",
"unstructured": "Razali, R., Subbiah, V.K., and Budiman, C. (2021). Technical Data of Heterologous Expression and Purification of SARS-CoV-2 Proteases Using Escherichia coli System. Data, 6."
},
{
"DOI": "10.1126/sciadv.abe0751",
"article-title": "Structure and inhibition of the SARS-CoV-2 main protease reveal strategy for developing dual inhibitors against Mpro and cathepsin L",
"author": "Sacco",
"doi-asserted-by": "crossref",
"first-page": "eabe0751",
"journal-title": "Sci. Adv.",
"key": "ref_49",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1016/j.compbiomed.2020.104117",
"article-title": "Evaluation of acridinedione analogs as potential SARS-CoV-2 main protease inhibitors and their comparison with repurposed anti-viral drugs",
"author": "Bhardwaj",
"doi-asserted-by": "crossref",
"first-page": "104117",
"journal-title": "Comput. Biol. Med.",
"key": "ref_50",
"volume": "128",
"year": "2021"
},
{
"DOI": "10.1016/j.bmcl.2020.127377",
"article-title": "The SARS-CoV-2 main protease as drug target",
"author": "Ullrich",
"doi-asserted-by": "crossref",
"first-page": "127377",
"journal-title": "Bioorganic Med. Chem. Lett.",
"key": "ref_51",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1007/s13238-021-00883-2",
"article-title": "Crystal structure of SARS-CoV-2 main protease in complex with protease inhibitor PF-07321332",
"author": "Zhao",
"doi-asserted-by": "crossref",
"first-page": "689",
"journal-title": "Protein Cell",
"key": "ref_52",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.3389/fphar.2022.1035969",
"article-title": "Review of preclinical data of PF-07304814 and its active metabolite derivatives against SARS-CoV-2 infection",
"author": "Chen",
"doi-asserted-by": "crossref",
"first-page": "1035969",
"journal-title": "Front. Pharmacol.",
"key": "ref_53",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1186/s43556-023-00134-2",
"article-title": "Crystal structures of main protease (Mpro) mutants of SARS-CoV-2 variants bound to PF-07304814",
"author": "Jiang",
"doi-asserted-by": "crossref",
"first-page": "23",
"journal-title": "Mol. Biomed.",
"key": "ref_54",
"volume": "4",
"year": "2023"
},
{
"DOI": "10.1016/j.jmb.2022.167706",
"article-title": "Structural Basis of Main Proteases of Coronavirus Bound to Drug Candidate PF-07304814",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "167706",
"journal-title": "J. Mol. Biol.",
"key": "ref_55",
"volume": "434",
"year": "2022"
},
{
"DOI": "10.3390/idr15060061",
"article-title": "Oral Molnupiravir and Nirmatrelvir/Ritonavir for the Treatment of COVID-19: A Literature Review with a Focus on Real-World Evidence",
"author": "Karniadakis",
"doi-asserted-by": "crossref",
"first-page": "662",
"journal-title": "Infect. Dis. Rep.",
"key": "ref_56",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.1080/14787210.2023.2284367",
"doi-asserted-by": "crossref",
"key": "ref_57",
"unstructured": "Liu, T.H., Wu, J.Y., Huang, P.Y., Hsu, W.H., Chuang, M.H., Tsai, Y.W., Lai, C.C., and Huang, C.Y. (2023). Clinical efficacy of nirmatrelvir plus ritonavir in patients with COVID-19 and preexisting cardiovascular diseases. Expert Rev. Anti-Infect. Ther., 1–8."
},
{
"DOI": "10.1002/adsc.202101147",
"article-title": "Synthesis and Applications of Organic Selenols",
"author": "Tanini",
"doi-asserted-by": "crossref",
"first-page": "5360",
"journal-title": "Adv. Synth. Catal.",
"key": "ref_58",
"volume": "363",
"year": "2021"
},
{
"DOI": "10.1002/adsc.201900168",
"article-title": "Seleno-Michael Reaction of Stable Functionalised Alkyl Selenols: A Versatile Tool for the Synthesis of Acyclic and Cyclic Unsymmetrical Alkyl and Vinyl Selenides",
"author": "Tanini",
"doi-asserted-by": "crossref",
"first-page": "2337",
"journal-title": "Adv. Synth. Catal.",
"key": "ref_59",
"volume": "361",
"year": "2019"
},
{
"DOI": "10.1002/adsc.201800602",
"article-title": "A Straightforward Access to Stable β-Functionalized Alkyl Selenols",
"author": "Tanini",
"doi-asserted-by": "crossref",
"first-page": "3367",
"journal-title": "Adv. Synth. Catal.",
"key": "ref_60",
"volume": "360",
"year": "2018"
},
{
"DOI": "10.1039/C8CC08562E",
"article-title": "Selenols: A new class of carbonic anhydrase inhibitors",
"author": "Angeli",
"doi-asserted-by": "crossref",
"first-page": "648",
"journal-title": "Chem. Commun.",
"key": "ref_61",
"volume": "55",
"year": "2019"
},
{
"DOI": "10.1039/D0CC00995D",
"article-title": "Selenolesterase enzyme activity of carbonic anhydrases",
"author": "Angeli",
"doi-asserted-by": "crossref",
"first-page": "4444",
"journal-title": "Chem. Commun.",
"key": "ref_62",
"volume": "56",
"year": "2020"
},
{
"DOI": "10.1002/adsc.202100611",
"article-title": "Click Reaction of Selenols with Isocyanates: Rapid Access to Selenocarbamates as Peroxide-Switchable Reservoir of Thiol-peroxidase-Like Catalysts",
"author": "Capperucci",
"doi-asserted-by": "crossref",
"first-page": "4256",
"journal-title": "Adv. Synth. Catal.",
"key": "ref_63",
"volume": "363",
"year": "2021"
},
{
"DOI": "10.1021/ja005800o",
"article-title": "Reaction of lithium aluminum hydride with elemental selenium: Its application as a selenating reagent into organic molecules",
"author": "Ishihara",
"doi-asserted-by": "crossref",
"first-page": "8408",
"journal-title": "J. Am. Chem. Soc.",
"key": "ref_64",
"volume": "123",
"year": "2001"
},
{
"DOI": "10.1126/science.abd9909",
"article-title": "De novo design of picomolar SARS-CoV-2 miniprotein inhibitors",
"author": "Cao",
"doi-asserted-by": "crossref",
"first-page": "426",
"journal-title": "Science",
"key": "ref_65",
"volume": "370",
"year": "2020"
},
{
"DOI": "10.1039/D3MD00222E",
"article-title": "De novo design of a stapled peptide targeting SARS-CoV-2 spike protein receptor-binding domain",
"author": "Thakkar",
"doi-asserted-by": "crossref",
"first-page": "1722",
"journal-title": "RSC Med. Chem.",
"key": "ref_66",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.1021/acs.jmedchem.0c01063",
"article-title": "Discovery of Ketone-Based Covalent Inhibitors of Coronavirus 3CL Proteases for the Potential Therapeutic Treatment of COVID-19",
"author": "Hoffman",
"doi-asserted-by": "crossref",
"first-page": "12725",
"journal-title": "J. Med. Chem.",
"key": "ref_67",
"volume": "63",
"year": "2020"
},
{
"DOI": "10.1038/s42003-022-03090-9",
"article-title": "Identification of SARS-CoV-2 inhibitors targeting Mpro and PLpro using in-cell-protease assay",
"author": "Narayanan",
"doi-asserted-by": "crossref",
"first-page": "169",
"journal-title": "Commun. Biol.",
"key": "ref_68",
"volume": "5",
"year": "2022"
},
{
"article-title": "Structural basis of SARS-CoV-2 main protease inhibition by a broad-spectrum anti-coronaviral drug",
"author": "Wang",
"first-page": "2535",
"journal-title": "Am. J. Cancer Res.",
"key": "ref_69",
"volume": "10",
"year": "2020"
},
{
"key": "ref_70",
"unstructured": "Schrödinger Suite Release 2022-4 (2022). (a) Maestro v.13.2; (b) Prime, v.5.5; (c) Epik, v.6.0; (d) Impact, v.9.5; (e) Macromodel v.13.6. (f) Glide, v.9.5, Schrödinger, LLC."
},
{
"DOI": "10.1080/17460441.2022.2052847",
"article-title": "Perspectives on the design and discovery of alpha-ketoamide inhibitors for the treatment of novel coronavirus: Where do we stand and where do we go?",
"author": "Nocentini",
"doi-asserted-by": "crossref",
"first-page": "547",
"journal-title": "Expert Opin. Drug Discov.",
"key": "ref_71",
"volume": "17",
"year": "2022"
},
{
"DOI": "10.1080/14756366.2023.2234665",
"article-title": "Identification of sulphonamide-tethered N-((triazol-4-yl)methyl)isatin derivatives as inhibitors of SARS-CoV-2 main protease",
"author": "ElNaggar",
"doi-asserted-by": "crossref",
"first-page": "2234665",
"journal-title": "J. Enzym. Inhib. Med. Chem.",
"key": "ref_72",
"volume": "38",
"year": "2023"
}
],
"reference-count": 72,
"references-count": 72,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/1422-0067/25/2/971"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Inorganic Chemistry",
"Organic Chemistry",
"Physical and Theoretical Chemistry",
"Computer Science Applications",
"Spectroscopy",
"Molecular Biology",
"General Medicine",
"Catalysis"
],
"subtitle": [],
"title": "Leveraging SARS-CoV-2 Main Protease (Mpro) for COVID-19 Mitigation with Selenium-Based Inhibitors",
"type": "journal-article",
"volume": "25"
}