Oral Sabizabulin for High-Risk, Hospitalized Adults with Covid-19: Interim Analysis
et al., NEJM Evidence, doi:10.1056/EVIDoa2200145, Jul 2022
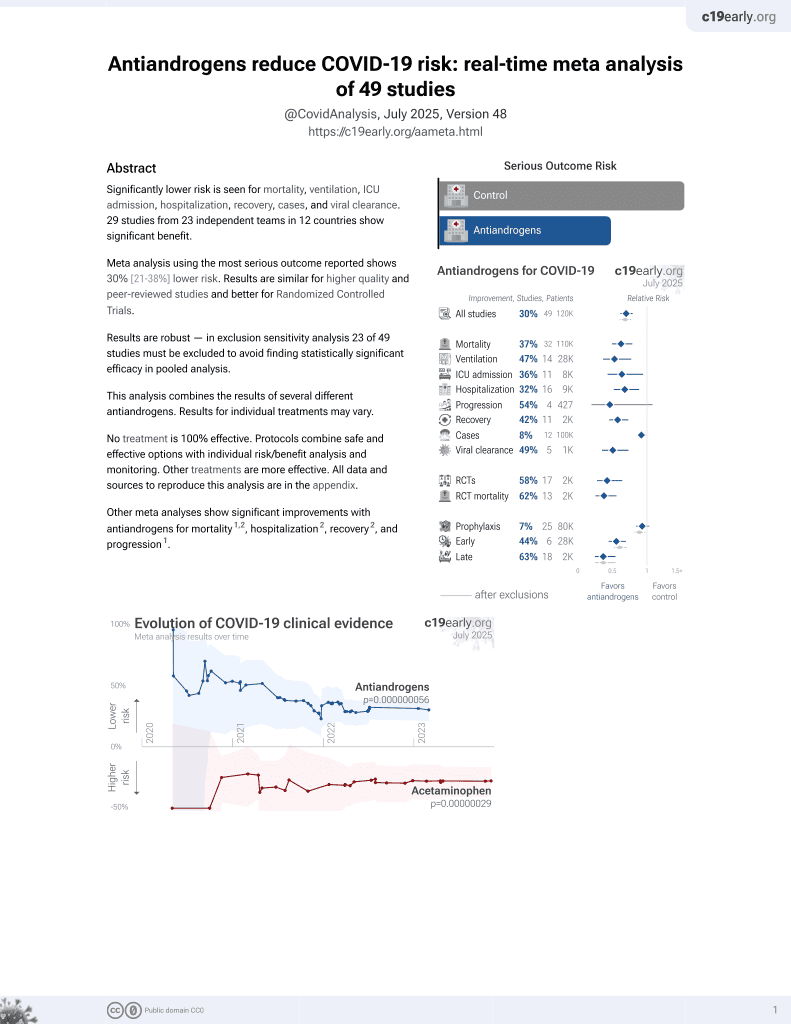
7th treatment shown to reduce risk in
September 2020, now with p = 0.000000056 from 49 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
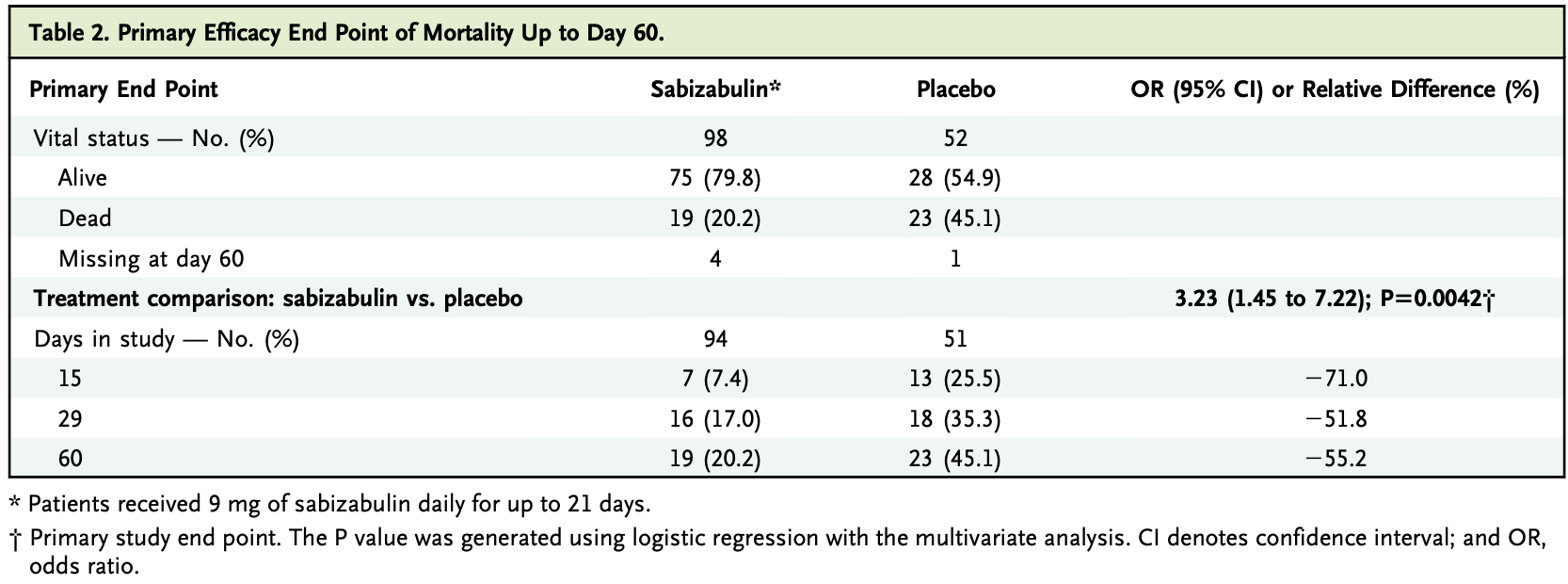
RCT with 98 hospitalized moderate/severe patients treated with sabizabulin and 52 control patients, showing lower mortality with treatment. Sabizabulin 9mg for up to 21 days. For more discussion see1-3.
|
risk of death, 55.2% lower, RR 0.45, p = 0.002, treatment 19 of 94 (20.2%), control 23 of 51 (45.1%), NNT 4.0.
|
|
ventilation time, 49.5% lower, relative time 0.51, p = 0.001, treatment 98, control 52.
|
|
ICU time, 43.5% lower, relative time 0.56, p = 0.001, treatment 98, control 52.
|
|
hospitalization time, 26.0% lower, relative time 0.74, p = 0.03, treatment 98, control 52.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Barnette et al., 6 Jul 2022, Double Blind Randomized Controlled Trial, placebo-controlled, multiple countries, peer-reviewed, 12 authors, study period 18 May, 2021 - 31 January, 2022.
Contact: gbarnette@verupharma.com.
Oral Sabizabulin for High-Risk, Hospitalized Adults with Covid-19: Interim Analysis
NEJM Evidence, doi:10.1056/evidoa2200145
BACKGROUND Sabizabulin is an oral, novel microtubule disruptor that has dual antiviral and anti-inflammatory activities in preclinical models. METHODS A randomized, multicenter placebo-controlled phase 3 clinical trial was conducted with hospitalized patients with moderate to severe Covid-19 who were at high risk for acute respiratory distress syndrome (ARDS) and death. Patients were randomly assigned (2:1) to 9 mg of oral sabizabulin or placebo daily (up to 21 days). The primary end point was all-cause mortality up to day 60. Key secondary end points were days in the intensive care unit (ICU), days on mechanical ventilation, and days in the hospital. RESULTS A total of 204 patients were randomly assigned to treatment: 134 to sabizabulin and 70 to placebo. Baseline characteristics were similar. Sabizabulin superiority was demonstrated by a planned interim analysis for the first 150 randomized patients. Sabizabulin treatment resulted in a 24.9 percentage point absolute reduction and a 55.2% relative reduction in deaths compared with placebo (odds ratio, 3.23; 95% CI confidence interval, 1.45 to 7.22; P50.0042). The mortality rate was 20.2% (19 of 94) for sabizabulin versus 45.1% (23 of 51) for placebo. For the key secondary end points, sabizabulin treatment resulted in a 43% relative reduction in ICU days (P50.0013), a 49% relative reduction in days on mechanical ventilation (P50.0013), and a 26% relative reduction in days in the hospital (P50.0277) versus placebo. Adverse and serious adverse events were lower in the sabizabulin group compared with the placebo group. CONCLUSIONS Sabizabulin treatment resulted in a 24.9% absolute reduction in deaths compared with placebo in hospitalized patients with moderate to severe Covid-19 at high risk for ARDS and death, with a lower incidence of adverse and serious adverse events compared with placebo. (Funded by Veru, Inc.; ClinicalTrials.gov number, NCT04842747.)
Author Affiliations 1 Veru, Inc., Miami 2 HonorHealth Research Institute, Scottsdale, AZ 3 Memorial Hermann, Memorial City Medical Center, Houston
References
Arribas, Bhagani, Lobo, Randomized trial of molnupiravir or placebo in patients hospitalized with Covid-19, NEJM Evid, doi:10.1056/EVIDoa2100044
Beigel, Tomashek, Dodd, Remdesivir for the treatment of Covid-19 -final report, N Engl J Med, doi:10.1056/NEJMoa2007764
Berlin, Gulick, Martinez, Severe Covid-19, N Engl J Med, doi:10.1056/NEJMcp2009575
Bernal, Da Silva, Musungaie, Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients, N Engl J Med, doi:10.1056/NEJMoa2116044
Bhatia, Zemans, Jeyaseelan, Role of chemokines in the pathogenesis of acute lung injury, Am J Respir Cell Mol Biol, doi:10.1165/rcmb.2011-0392TR
Chen, Ahn, Wang, Discovery of novel 2-aryl-4-benzoylimidazole (ABI-III) analogues targeting tubulin polymerization as antiproliferative agents, J Med Chem, doi:10.1021/jm300564b
Chopra, Flanders, Malley, Malani, Prescott, Sixty-day outcomes among patients hospitalized with COVID-19, Ann Intern Med, doi:10.7326/M20-5661
Criglar, Hu, Crawford, A novel form of rotavirus NSP2 and phosphorylation-dependent NSP2-NPS5 interactions are associated with viroplasm assembly, J Virol, doi:10.1128/JVI.03022-13
Dodd, Follmann, Wang, Endpoints for randomized controlled clinical trials for COVID-19 treatments, Clin Trials, doi:10.1177/1740774520939938
D€ Ohner, Nagel, Sodeik, Viral stop-and-go along microtubules: taking a ride with dynein and kinesins, Trends Microbiol, doi:10.1016/j.tim.2005.05.010
Eichwald, Arnoldi, Laimbacher, Rotavirus viroplasm fusion and perinuclear localization are dynamic processes requiring stabilized microtubules, PLoS One, doi:10.1371/journal.pone.0047947
French, Hulse, Nguyen, Impact of hospital strain on excess deaths during the COVID-19 pandemic -United States, July 2020-July 2021, Am J Transplant, doi:10.1111/ajt.16645
Fung, Liu, Human coronavirus: host-pathogen interaction, Annu Rev Microbiol, doi:10.1146/annurev-micro-020518-115759
Gordon, Skolnick, Barnette, Phase II study of oral sabizabulin for the treatment of SARS-CoV-2 in hospitalised patients at high-risk for ARDS, ECCMID
Guimarães, Quirk, Furtado, Tofacitinib in patients hospitalized with Covid-19 pneumonia, N Engl J Med, doi:10.1056/NEJMoa2101643
Gupta, Gonzalez-Rojas, Juarez, Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab, N Engl J Med, doi:10.1056/NEJMoa2107934
Hammond, Leister-Tebbe, Gardner, Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2118542
Holm, A simple sequentially rejective multiple test procedure, Scand J Stat
Horby, Lim, Emberson, Dexamethasone in hospitalized patients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2021436
Huang, Wang, Li, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet, doi:10.1016/S0140-6736(20)30183-5
Kalil, Patterson, Mehta, Baricitinib plus remdesivir for hospitalized adults with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2031994
Li, Lu, Chen, Orally bioavailable tubulin antagonists for paclitaxel-refractory cancer, Pharm Res, doi:10.1007/s11095-012-0814-5
Malik, Kanneganti, Inflammasome activation and assembly at a glance, J Cell Sci, doi:10.1242/jcs.207365
Naghavi, Walsh, Microtubule regulation and function during virus infection, J Virol, doi:10.1128/JVI.00538-17
R€ Udiger, Mayrhofer, Ma-Lauer, Tubulins interact with porcine and human S proteins of the genus Alphacoronavirus and support successful assembly and release of infectious viral particles, Virology, doi:10.1016/j.virol.2016.07.022
Salama, Han, Yau, Tocilizumab in patients hospitalized with Covid-19 pneumonia, N Engl J Med, doi:10.1056/NEJMoa2030340
Tenforde, Kim, Lindsell, Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate healthcare systems network -United States, MMWR Morb Mortal Wkly Rep
Veru, VERU-111 suppresses key cytokines responsible for severe acute respiratory distress syndrome in COVID-19
Verweyen, Holzinger, Weinhage, Synergistic signaling of TLR and IFN alpha/beta facilitates escape of IL-18 expression from endotoxin tolerance, Am J Respir Crit Care Med, doi:10.1164/rccm.201903-0659OC
Wang, Arnst, Wang, Structure-guided design, synthesis, and biological evaluation of (2-(1H-indol-3-yl)-1H-imidazol-4-yl)(3,4,5-trimethoxyphenyl) methanone (ABI-231) analogues targeting the colchicine binding site in tubulin, J Med Chem, doi:10.1021/acs.jmedchem.9b00706
Worldometer, Coronavirus cases
Zahid, Li, Kombe, Tao, Pharmacological inhibitors of NLRP3 inflammasome, Front Immunol, doi:10.3389/fimmu.2019.02538
DOI record:
{
"DOI": "10.1056/evidoa2200145",
"ISSN": [
"2766-5526"
],
"URL": "http://dx.doi.org/10.1056/EVIDoa2200145",
"alternative-id": [
"10.1056/EVIDoa2200145"
],
"author": [
{
"affiliation": [
{
"name": "Veru, Inc., Miami"
}
],
"family": "Barnette",
"given": "K. Gary",
"sequence": "first"
},
{
"affiliation": [
{
"name": "HonorHealth Research Institute, Scottsdale, AZ"
}
],
"family": "Gordon",
"given": "Michael S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Veru, Inc., Miami"
}
],
"family": "Rodriguez",
"given": "Domingo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Veru, Inc., Miami"
}
],
"family": "Bird",
"given": "T. Gary",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Memorial Hermann, Memorial City Medical Center, Houston"
}
],
"family": "Skolnick",
"given": "Alan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Methodist Hospital, St. Louis Park, MN"
}
],
"family": "Schnaus",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Regions Hospital, St. Paul, MN"
}
],
"family": "Skarda",
"given": "Paula K.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Fundação Faculdade Regional de Medicina, São José do Rio Preto, Brazil"
}
],
"family": "Lobo",
"given": "Suzana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectologia, Hospital de Clínicas de Porto Alegre, Centro de Pesquisa Clínica, Porto Alegre, Brazil"
}
],
"family": "Sprinz",
"given": "Eduardo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University Multiprofile Hospital for Active Treatment, Zagora, Bulgaria"
}
],
"family": "Arabadzhiev",
"given": "Georgi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University Multiprofile Hospital for Active Treatment, Sofia, Bulgaria"
}
],
"family": "Kalaydzhiev",
"given": "Petar",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Veru, Inc., Miami"
}
],
"family": "Steiner",
"given": "Mitchell",
"sequence": "additional"
}
],
"container-title": "NEJM Evidence",
"container-title-short": "NEJM Evidence",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
7,
6
]
],
"date-time": "2022-07-06T11:00:07Z",
"timestamp": 1657105207000
},
"deposited": {
"date-parts": [
[
2022,
7,
6
]
],
"date-time": "2022-07-06T11:00:10Z",
"timestamp": 1657105210000
},
"indexed": {
"date-parts": [
[
2022,
7,
6
]
],
"date-time": "2022-07-06T11:41:18Z",
"timestamp": 1657107678771
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
7,
6
]
]
},
"language": "en",
"member": "150",
"original-title": [],
"prefix": "10.1056",
"published": {
"date-parts": [
[
2022,
7,
6
]
]
},
"published-online": {
"date-parts": [
[
2022,
7,
6
]
]
},
"publisher": "Massachusetts Medical Society",
"reference": [
{
"key": "e_1_3_4_2_2",
"unstructured": "Worldometer. Coronavirus cases. 2022 (https://www.worldometers.info/coronavirus)."
},
{
"DOI": "10.1056/NEJMoa2107934",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_3_2"
},
{
"DOI": "10.1056/NEJMoa2116044",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_4_2"
},
{
"DOI": "10.1056/NEJMoa2118542",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_5_2"
},
{
"DOI": "10.1056/NEJMoa2007764",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_6_2"
},
{
"DOI": "10.1056/NEJMoa2021436",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_7_2"
},
{
"DOI": "10.1056/NEJMoa2030340",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_8_2"
},
{
"DOI": "10.1056/NEJMoa2101643",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_9_2"
},
{
"DOI": "10.1056/NEJMoa2031994",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_10_2"
},
{
"key": "e_1_3_4_11_2",
"unstructured": "National Institutes of Health. Coronavirus disease 2019 (COVID-19) treatment guidelines. 2022 (https://www.covid19treatmentguidelines.nih.gov)."
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_12_2"
},
{
"DOI": "10.1056/NEJMcp2009575",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_13_2"
},
{
"DOI": "10.1111/ajt.16645",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_14_2"
},
{
"DOI": "10.1021/jm300564b",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_15_2"
},
{
"DOI": "10.1007/s11095-012-0814-5",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_16_2"
},
{
"DOI": "10.1016/j.virol.2016.07.022",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_17_2"
},
{
"DOI": "10.1146/annurev-micro-020518-115759",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_18_2"
},
{
"DOI": "10.1128/JVI.00538-17",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_19_2"
},
{
"DOI": "10.1016/j.tim.2005.05.010",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_20_2"
},
{
"DOI": "10.1371/journal.pone.0047947",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_21_2"
},
{
"DOI": "10.1128/JVI.03022-13",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_22_2"
},
{
"DOI": "10.1164/rccm.201903-0659OC",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_23_2"
},
{
"DOI": "10.3389/fimmu.2019.02538",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_24_2"
},
{
"DOI": "10.1242/jcs.207365",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_25_2"
},
{
"DOI": "10.1165/rcmb.2011-0392TR",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_26_2"
},
{
"key": "e_1_3_4_27_2",
"unstructured": "Veru Inc. VERU-111 suppresses key cytokines responsible for severe acute respiratory distress syndrome in COVID-19. August 4 2020 (https://verupharma.com/news/veru-111-suppresses-key-cytokines-responsible-for-severe-acute-respiratory-distress-syndrome-in-covid-19/)."
},
{
"key": "e_1_3_4_28_2",
"unstructured": "Gordon M Skolnick A Barnette KG et al. Phase II study of oral sabizabulin for the treatment of SARS-CoV-2 in hospitalised patients at high-risk for ARDS. ECCMID. April 23 2022. (https://online.eccmid.org/media-4157-phase-ii-study-of-oral-sabizabulin-for-the-treatment-of-sars-cov-2-in-hospitalised-patient)."
},
{
"DOI": "10.1177/1740774520939938",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_29_2"
},
{
"article-title": "A simple sequentially rejective multiple test procedure",
"first-page": "65",
"journal-title": "Scand J Stat",
"key": "e_1_3_4_30_2",
"unstructured": "Holm SA. A simple sequentially rejective multiple test procedure. Scand J Stat 1979;6:65–70.",
"volume": "6",
"year": "1979"
},
{
"article-title": "Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate healthcare systems network — United States, March July 2021",
"first-page": "1294",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "e_1_3_4_31_2",
"unstructured": "Tenforde MW, Kim SS, Lindsell CJ, et al. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate healthcare systems network — United States, March July 2021. MMWR Morb Mortal Wkly Rep 2021;70:1294–1299.",
"volume": "70",
"year": "2021"
},
{
"DOI": "10.1056/EVIDoa2100044",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_32_2"
},
{
"DOI": "10.1021/acs.jmedchem.9b00706",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_33_2"
},
{
"DOI": "10.7326/M20-5661",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_34_2"
}
],
"reference-count": 33,
"references-count": 33,
"relation": {},
"resource": {
"primary": {
"URL": "https://evidence.nejm.org/doi/10.1056/EVIDoa2200145"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"title": "Oral Sabizabulin for High-Risk, Hospitalized Adults with Covid-19: Interim Analysis",
"type": "journal-article"
}