Impact of monoclonal antibody treatment on hospitalization and mortality among non-hospitalized adults with SARS-CoV-2 infection
et al., medRxiv, doi:10.1101/2021.03.25.21254322, Mar 2021
25th treatment shown to reduce risk in
May 2021, now with p = 0.00049 from 22 studies, recognized in 11 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
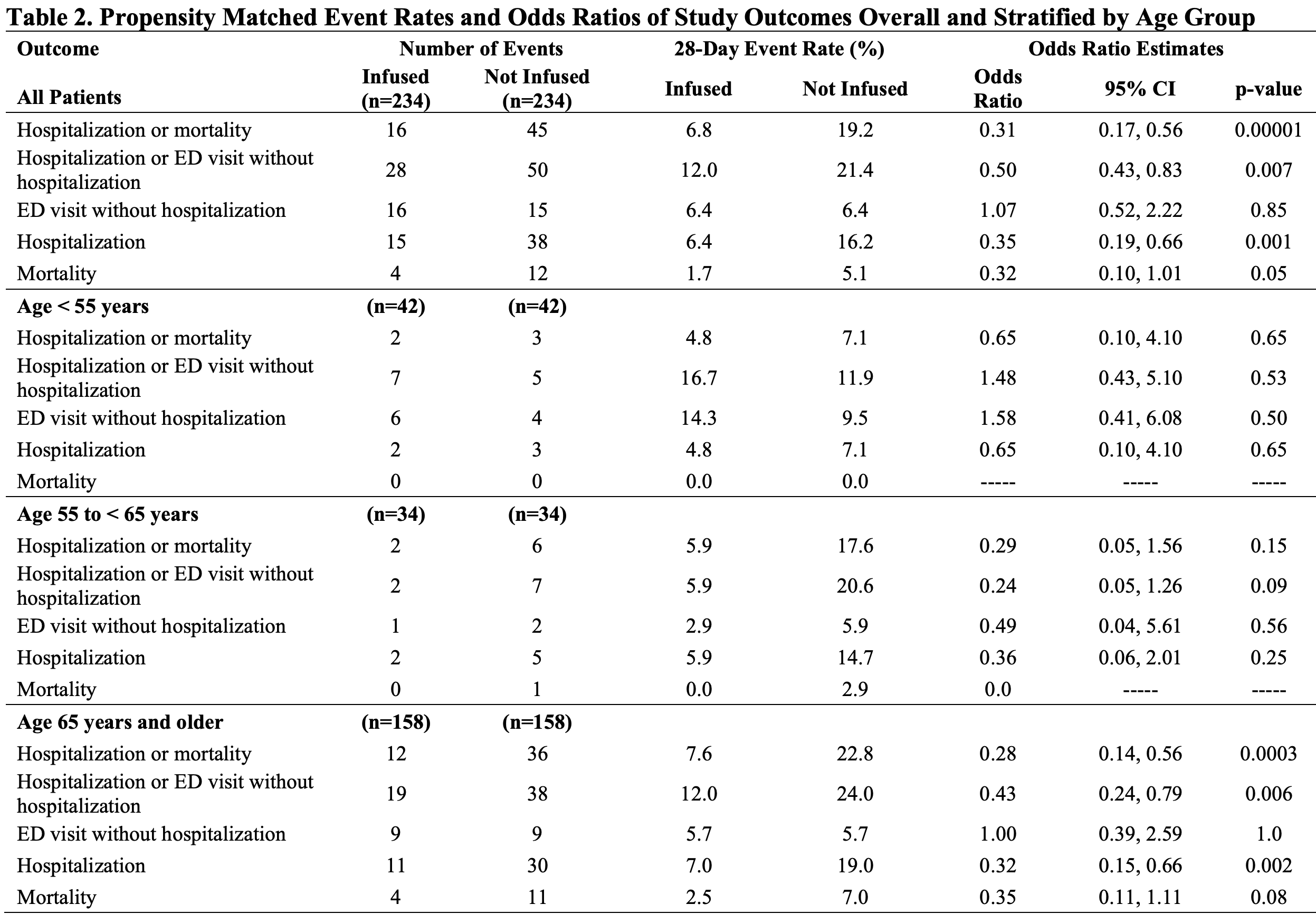
Retrospective 234 patients receiving bamlanivimab and 234 matched controls, showing lower hospitalization and mortality with treatment. Greater benefit was seen with administration within 4 days of their positive COVID-19 test.
Confounding by treatment propensity. This study analyzes a population
where only a fraction of eligible patients received the treatment. Patients
receiving treatment may be more likely to follow other recommendations, more
likely to receive additional care, and more likely to use additional
treatments that are not tracked in the data (e.g., nasal/oral hygiene1,2, vitamin D3, etc.) — either because the physician
recommending bamlanivimab/etesevimab also recommended them, or
because the patient seeking out bamlanivimab/etesevimab is more
likely to be familiar with the efficacy of additional treatments and more
likely to take the time to use them.
Therefore, these kind of studies may
overestimate efficacy.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments9.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 66.8% lower, RR 0.33, p = 0.05, treatment 4 of 234 (1.7%), control 12 of 234 (5.1%), NNT 29, odds ratio converted to relative risk.
|
|
risk of death/hospitalization, 64.3% lower, RR 0.36, p < 0.001, treatment 16 of 234 (6.8%), control 45 of 234 (19.2%), NNT 8.1, odds ratio converted to relative risk, primary outcome.
|
|
risk of hospitalization, 60.7% lower, RR 0.39, p = 0.001, treatment 15 of 234 (6.4%), control 39 of 234 (16.7%), NNT 9.8, odds ratio converted to relative risk.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
4.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
5.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
6.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
7.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
Bariola et al., 30 Mar 2021, retrospective, USA, preprint, 22 authors.
Impact of monoclonal antibody treatment on hospitalization and mortality among non-hospitalized adults with SARS-CoV-2 infection
doi:10.1101/2021.03.25.21254322
Background: Monoclonal antibody (mAb) treatment may prevent complications of COVID-19. We sought to quantify the impact of bamlanivimab monotherapy on hospitalizations and mortality, as well as Emergency Department (ED) visits without hospitalization, among outpatients at high risk of COVID-19 complications.
Methods: We compared patients receiving mAb to patients who met criteria but did not receive mAb from December 2020 through March 2021. The study population selection used propensity scores to match 1:1 by likelihood to receive mAb. The primary outcome was hospitalization or all-cause mortality within 28 days; the secondary outcome was hospitalization or ED visit without hospitalization within 28 days. Odds ratios (OR) calculation used logistic regression modeling including propensity score and mAb receipt predictors.
Results: The study population included 234 patients receiving mAb and 234 matched comparator patients not receiving mAb. Patients receiving mAb were less likely to experience hospitalization or mortality (OR 0.31, 95% confidence interval [95%CI] 0.17-0.56, p=0.00001) and hospitalization or ED visit without hospitalization (OR 0.50, 95%CI 0.43-0.83, p=0.007). The impact of mAb was more pronounced in prevention of hospitalization (among all age groups, OR 0.35, 95%CI 0.19-0.66, p=0.001) than mortality or ED visit without hospitalization, and most strongly associated with patients age 65 years and older (primary outcome OR 0.28, 95%CI 0.14-0.56, p=0.0003).
Conclusions : Bamlanivimab monotherapy was associated with reduction in the composite outcome of hospitalizations and mortality in patients with mild-moderate COVID-19. The benefit may be strongest in preventing hospitalization in patients ages 65 years or older. . CoV016; Eli Lilly), casirivimab 1,200mg (REGN10933; Regeneron), imdevimab 1,200mg (REGN10987). Several clinical trials currently evaluate mAbs for prevention or treatment of COVID-19; however, real-world data are limited, and the role of mAbs for patients with COVID-19 remains controversial. 3, 5 Use of mAb therapy is low in the United States despite widespread drug availability due to lack of robust efficacy data, operational challenges with outpatient infusions, and patient access issues. 6 Our health system established a mAb program in November 2020 to decrease COVID-19-related complications for patients with mild-moderate illness and expand access to care for underserved patients with COVID-19. Initially, only bamlanivimab monotherapy was available; our evaluation and distribution process has been described elsewhere. 7 This study quantifies the impact of bamlanivimab monotherapy on hospitalizations, mortality, and Emergency Department (ED) visits among outpatients at high risk of progressing to severe COVID-19. We also explored whether patient age, body mass index, and timing of infusions relative to initial diagnosis had any association with response to therapy.
METHODS
Study Setting .
Conflict of Interest Disclosure: None of the authors received any payments or influence from a third-party source for the work presented, and none report any potential conflicts of interest.
SUPPLEMENTAL MATERIALS
References
Ault, Rollout of COVID Monoclonal Antibodies Lacked Unified Plan: Expert Panel
Austin, An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies, Multivariate Behav Res. May, doi:10.1080/00273171.2011.568786
Bajaj, Gadi, Spihlman, Wu, Choi et al., Aging, Immunity, and COVID-19: How Age Influences the Host Immune Response to Coronavirus Infections?, Front Physiol, doi:10.3389/fphys.2020.571416
Bariola, Mccreary, Khadem, Snyder, Wadas et al., Establishing a Distribution Network for COVID-19 Monoclonal Antibody Therapy Across a Large Health System During a Global Pandemic, Open Forum Infect Dis
Benchimol, Smeeth, Guttmann, The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement, PLoS Med, doi:10.1371/journal.pmed.1001885
Chen, Nirula, Heller, SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2029849
Cohen1, Nirula2, Mulligan3, Novak4, Marovich5 et al., ). Bamlanivimab prevents COVID-19 morbidity and mortality in nursinghome setting
Gottlieb, Nirula, Chen, Effect of Bamlanivimab as Monotherapy or in Combination With Etesevimab on Viral Load in Patients With Mild to Moderate COVID-19: A Randomized Clinical Trial, Jama, doi:10.1001/jama.2021.0202
Jones, Brown-Augsburger, Corbett, LY-CoV555, a rapidly isolated potent neutralizing antibody, provides protection in a non-human primate model of SARS-CoV-2 infection, bioRxiv, doi:10.1101/2020.09.30.318972
Lim, Subramaniam, Reddy, Case Fatality Rates for Patients with COVID-19 Requiring Invasive Mechanical Ventilation. A Meta-analysis, Am J Respir Crit Care Med. Jan, doi:10.1164/rccm.202006-2405OC
Liu, Wei, Zhang, V2 and 501Y.V3 variants of SARS-CoV-2 lose binding to Bamlanivimab in vitro, bioRxiv, doi:10.1101/2021.02.16.43130519
Reitz, Marroquin, Zenati, Association Between Preoperative Metformin Exposure and Postoperative Outcomes in Adults With Type 2 Diabetes, JAMA Surg. Jun, doi:10.1001/jamasurg.2020.0416
Richardson, Hirsch, Narasimhan, Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area, JAMA, doi:10.1001/jama.2020.6775
Rosenbaum, Db, The central role of the propensity score in observational studies for causal effects, Biometrika, doi:10.1093/biomet/70.1.41
Tartof, Qian, Hong, Obesity and Mortality Among Patients Diagnosed With COVID-19: Results From an Integrated Health Care Organization, Ann Intern Med. Nov, doi:10.7326/m20-3742
Webb, Buckel, Vento, Real-World Effectiveness and Tolerability of Monoclonal Antibodies for Ambulatory Patients with Early COVID-19, doi:10.1101/2021.03.15.21253646
Weinreich, Sivapalasingam, Norton, REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2035002
DOI record:
{
"DOI": "10.1101/2021.03.25.21254322",
"URL": "http://dx.doi.org/10.1101/2021.03.25.21254322",
"abstract": "<jats:title>ABSTRACT</jats:title><jats:sec><jats:title>Background</jats:title><jats:p>Monoclonal antibody (mAb) treatment may prevent complications of COVID-19. We sought to quantify the impact of bamlanivimab monotherapy on hospitalizations and mortality, as well as Emergency Department (ED) visits without hospitalization, among outpatients at high risk of COVID-19 complications.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>We compared patients receiving mAb to patients who met criteria but did not receive mAb from December 2020 through March 2021. The study population selection used propensity scores to match 1:1 by likelihood to receive mAb. The primary outcome was hospitalization or all-cause mortality within 28 days; the secondary outcome was hospitalization or ED visit without hospitalization within 28 days. Odds ratios (OR) calculation used logistic regression modeling including propensity score and mAb receipt predictors.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>The study population included 234 patients receiving mAb and 234 matched comparator patients not receiving mAb. Patients receiving mAb were less likely to experience hospitalization or mortality (OR 0.31, 95% confidence interval [95%CI] 0.17-0.56, p=0.00001) and hospitalization or ED visit without hospitalization (OR 0.50, 95%CI 0.43-0.83, p=0.007). The impact of mAb was more pronounced in prevention of hospitalization (among all age groups, OR 0.35, 95%CI 0.19-0.66, p=0.001) than mortality or ED visit without hospitalization, and most strongly associated with patients age 65 years and older (primary outcome OR 0.28, 95%CI 0.14-0.56, p=0.0003).</jats:p></jats:sec><jats:sec><jats:title>Conclusions</jats:title><jats:p>Bamlanivimab monotherapy was associated with reduction in the composite outcome of hospitalizations and mortality in patients with mild-moderate COVID-19. The benefit may be strongest in preventing hospitalization in patients ages 65 years or older.</jats:p></jats:sec>",
"accepted": {
"date-parts": [
[
2021,
3,
30
]
]
},
"author": [
{
"affiliation": [],
"family": "Bariola",
"given": "J. Ryan",
"sequence": "first"
},
{
"affiliation": [],
"family": "McCreary",
"given": "Erin K.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wadas",
"given": "Richard J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kip",
"given": "Kevin E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Marroquin",
"given": "Oscar C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Minnier",
"given": "Tami",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Koscumb",
"given": "Stephen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Collins",
"given": "Kevin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schmidhofer",
"given": "Mark",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shovel",
"given": "Judith A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wisniewski",
"given": "Mary Kay",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sullivan",
"given": "Colleen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yealy",
"given": "Donald M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nace",
"given": "David A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Huang",
"given": "David T.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Haidar",
"given": "Ghady",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Khadem",
"given": "Tina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Linstrum",
"given": "Kelsey",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Seymour",
"given": "Christopher W.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Montgomery",
"given": "Stephanie K.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Angus",
"given": "Derek C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Snyder",
"given": "Graham M.",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
3,
30
]
],
"date-time": "2021-03-30T15:40:11Z",
"timestamp": 1617118811000
},
"deposited": {
"date-parts": [
[
2021,
12,
15
]
],
"date-time": "2021-12-15T15:07:14Z",
"timestamp": 1639580834000
},
"group-title": "Infectious Diseases (except HIV/AIDS)",
"indexed": {
"date-parts": [
[
2024,
3,
1
]
],
"date-time": "2024-03-01T23:49:26Z",
"timestamp": 1709336966122
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 3,
"issued": {
"date-parts": [
[
2021,
3,
30
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2021.03.25.21254322",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2021,
3,
30
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2021,
3,
30
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference": [
{
"DOI": "10.1101/2020.09.30.318972",
"doi-asserted-by": "publisher",
"key": "2021040110400618000_2021.03.25.21254322v1.1"
},
{
"key": "2021040110400618000_2021.03.25.21254322v1.2",
"unstructured": "Myron S. Cohen 1, Ajay Nirula 2, Mark Mulligan 3, Richard Novak 4, Mary Marovich 5, Alexander Stemer 6, Andrew C. Adams 2, Andrew E. Schade 2, Jack Knorr 2, Jay L. Tuttle 2, Janelle Sabo 2, Paul Klekotka 2, Lei Shen 2, Daniel M. Skovronsky 2, for BLAZE-2 study team (Lilly/NIAID/CoVPN). Bamlanivimab prevents COVID-19 morbidity and mortality in nursing-home setting. Oral abstract #121. Presented at Virtual CROI 2021."
},
{
"DOI": "10.1001/jama.2021.0202",
"doi-asserted-by": "publisher",
"key": "2021040110400618000_2021.03.25.21254322v1.3"
},
{
"DOI": "10.1056/NEJMoa2035002",
"doi-asserted-by": "publisher",
"key": "2021040110400618000_2021.03.25.21254322v1.4"
},
{
"DOI": "10.1101/2021.03.15.21253646",
"doi-asserted-by": "crossref",
"key": "2021040110400618000_2021.03.25.21254322v1.5",
"unstructured": "Webb BJ , Buckel W , Vento T , et al. Real-World Effectiveness and Tolerability of Monoclonal Antibodies for Ambulatory Patients with Early COVID-19. 3/17/2021 2021;doi: https://doi.org/10.1101/2021.03.15.21253646"
},
{
"key": "2021040110400618000_2021.03.25.21254322v1.6",
"unstructured": "Ault A. Rollout of COVID Monoclonal Antibodies Lacked Unified Plan: Expert Panel. Accessed 23 Mar 2021. Available at: https://www.medscape.com/viewarticle/945223."
},
{
"DOI": "10.1093/ofid/ofab151",
"doi-asserted-by": "crossref",
"key": "2021040110400618000_2021.03.25.21254322v1.7",
"unstructured": "Bariola JR , McCreary EK , Khadem T , Snyder GM , Wadas RJ , Nace DA , White DB , Yealy DM , Schmidhofer M. Establishing a Distribution Network for COVID-19 Monoclonal Antibody Therapy Across a Large Health System During a Global Pandemic. Open Forum Infect Dis. 2021. In press."
},
{
"DOI": "10.1001/jamasurg.2020.0416",
"doi-asserted-by": "publisher",
"key": "2021040110400618000_2021.03.25.21254322v1.8"
},
{
"key": "2021040110400618000_2021.03.25.21254322v1.9",
"unstructured": "FDA News Release. Coronavirus (COVID-19) update: FDA authorizes monoclonal antibody for treatment of COVID-19. Published November 9, 2020. Accessed November 13, 2020. https://bit.ly/2HesBBs."
},
{
"DOI": "10.1080/00273171.2011.568786",
"doi-asserted-by": "publisher",
"key": "2021040110400618000_2021.03.25.21254322v1.10"
},
{
"DOI": "10.1093/biomet/70.1.41",
"doi-asserted-by": "publisher",
"key": "2021040110400618000_2021.03.25.21254322v1.11"
},
{
"DOI": "10.1164/rccm.202006-2405OC",
"doi-asserted-by": "publisher",
"key": "2021040110400618000_2021.03.25.21254322v1.12"
},
{
"DOI": "10.1001/jama.2020.6775",
"doi-asserted-by": "publisher",
"key": "2021040110400618000_2021.03.25.21254322v1.13"
},
{
"DOI": "10.3389/fphys.2020.571416",
"doi-asserted-by": "publisher",
"key": "2021040110400618000_2021.03.25.21254322v1.14"
},
{
"DOI": "10.7326/m20-3742",
"doi-asserted-by": "publisher",
"key": "2021040110400618000_2021.03.25.21254322v1.15"
},
{
"DOI": "10.1371/journal.pmed.1001885",
"doi-asserted-by": "publisher",
"key": "2021040110400618000_2021.03.25.21254322v1.16"
},
{
"DOI": "10.1056/NEJMoa2029849",
"doi-asserted-by": "publisher",
"key": "2021040110400618000_2021.03.25.21254322v1.17"
},
{
"DOI": "10.1101/2021.02.16.431305",
"doi-asserted-by": "publisher",
"key": "2021040110400618000_2021.03.25.21254322v1.18"
},
{
"key": "2021040110400618000_2021.03.25.21254322v1.19",
"unstructured": "US Department of Health and Human Services. Update on COVID-19 variants and impact on bamlanivimab distribution. Available from: https://www.phe.gov/emergency/events/COVID19/investigation-MCM/Bamlanivimab/Pages/default.aspx. Accessed March 24th, 2021."
}
],
"reference-count": 19,
"references-count": 19,
"relation": {
"is-preprint-of": [
{
"asserted-by": "subject",
"id": "10.1093/ofid/ofab254",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2021.03.25.21254322"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "Impact of monoclonal antibody treatment on hospitalization and mortality among non-hospitalized adults with SARS-CoV-2 infection",
"type": "posted-content"
}
