Inhaled nitric oxide: can it serve as a savior for COVID-19 and related respiratory and cardiovascular diseases?
et al., Frontiers in Microbiology, doi:10.3389/fmicb.2023.1277552, Oct 2023
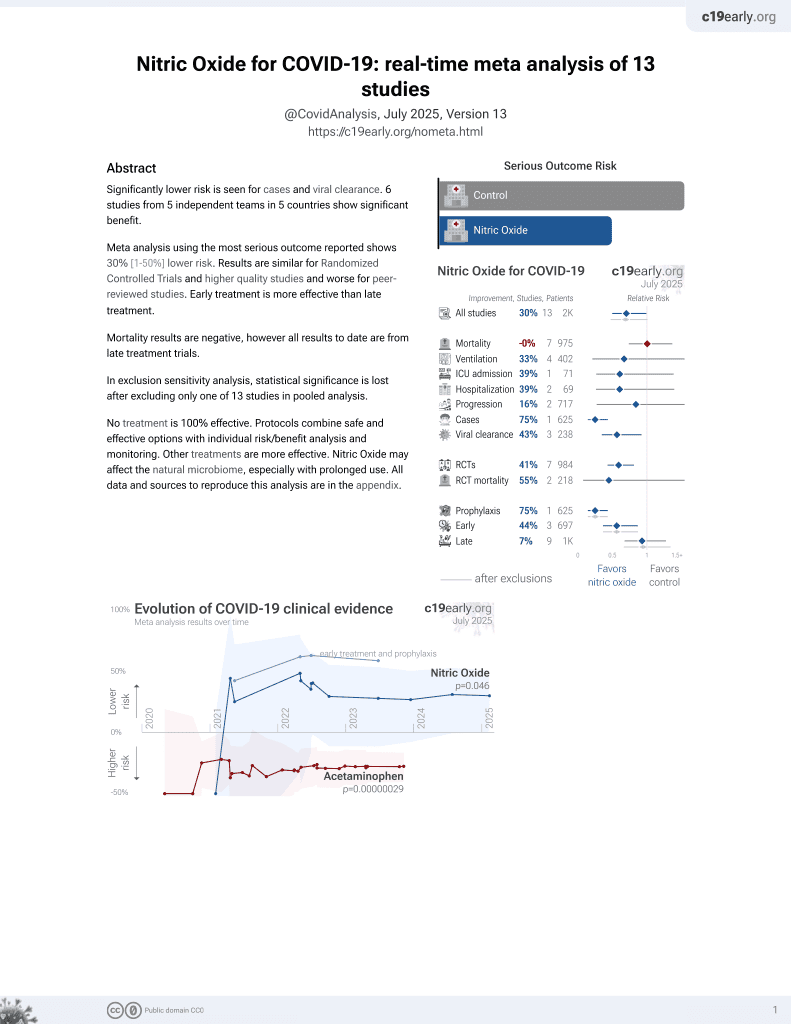
43rd treatment shown to reduce risk in
June 2022, now with p = 0.012 from 12 studies, recognized in 10 countries.
Lower risk for cases and viral clearance.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
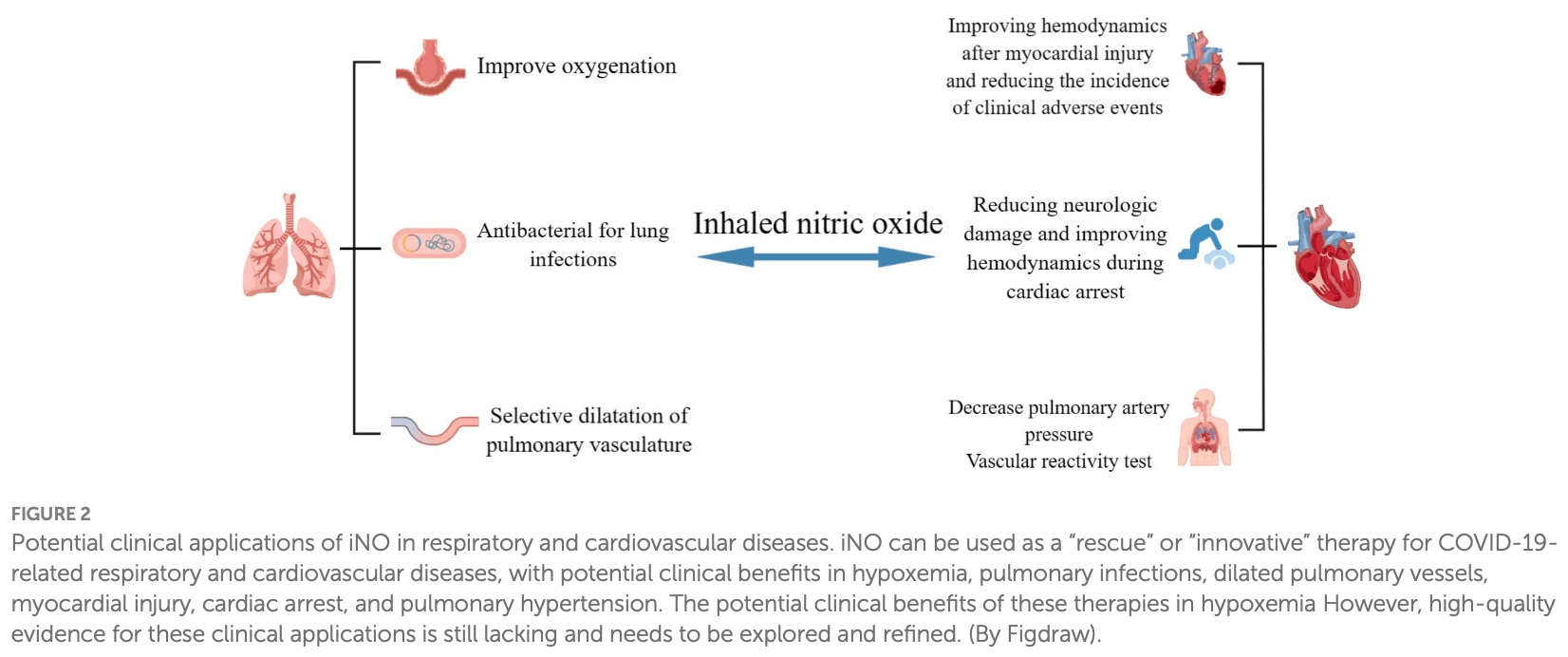
Review of inhaled nitric oxide (iNO) as a therapy for COVID-19 and related respiratory and cardiovascular diseases. Nitric oxide is an important signaling molecule produced by nitric oxide synthases that regulates various physiological processes. SARS-CoV-2 can decrease nitric oxide production through several mechanisms, while iNO selectively dilates the pulmonary vasculature and may have antiviral effects against SARS-CoV-2 by inhibiting viral proteins. Some studies show iNO can improve oxygenation and reduce right heart strain in COVID-19 patients. iNO has also demonstrated benefits in some studies of acute respiratory distress syndrome, lung infections, and pulmonary hypertension.
1.
Wright et al., Nitric Oxide in the Treatment of COVID‐19: Nasal Sprays, Inhalants and Nanoparticles, Biochemistry Research International, doi:10.1155/bri/8846903.
2.
Zhang et al., Saying No to SARS-CoV-2: the potential of nitric oxide in the treatment of COVID-19 pneumonia, Medical Gas Research, doi:10.4103/2045-9912.385414.
3.
Zhao et al., Inhaled nitric oxide: can it serve as a savior for COVID-19 and related respiratory and cardiovascular diseases?, Frontiers in Microbiology, doi:10.3389/fmicb.2023.1277552.
4.
Yamasaki et al., Pleiotropic Functions of Nitric Oxide Produced by Ascorbate for the Prevention and Mitigation of COVID-19: A Revaluation of Pauling’s Vitamin C Therapy, Microorganisms, doi:10.3390/microorganisms11020397.
Zhao et al., 2 Oct 2023, peer-reviewed, 8 authors.
Contact: wangyg1982@jlu.edu.cn.
Inhaled nitric oxide: can it serve as a savior for COVID-19 and related respiratory and cardiovascular diseases?
Frontiers in Microbiology, doi:10.3389/fmicb.2023.1277552
Nitric oxide (NO), as an important gaseous medium, plays a pivotal role in the human body, such as maintaining vascular homeostasis, regulating immuneinflammatory responses, inhibiting platelet aggregation, and inhibiting leukocyte adhesion. In recent years, the rapid prevalence of coronavirus disease 2019 (COVID-19) has greatly affected the daily lives and physical and mental health of people all over the world, and the therapeutic efficacy and resuscitation strategies for critically ill patients need to be further improved and perfected. Inhaled nitric oxide (iNO) is a selective pulmonary vasodilator, and some studies have demonstrated its potential therapeutic use for COVID-19, severe respiratory distress syndrome, pulmonary infections, and pulmonary hypertension. In this article, we describe the biochemistry and basic characteristics of NO and discuss whether iNO can act as a "savior" for COVID-19 and related respiratory and cardiovascular disorders to exert a potent clinical protective effect.
Conflict of interest The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Academy, Pediatrics, Committee on fetus and newborn. Use of inhaled nitric oxide, Pediatrics, doi:10.1542/peds.106.2.344
Adhikari, Burns, Friedrich, Granton, Cook et al., Effect of nitric oxide on oxygenation and mortality in acute lung injury: systematic review and meta-analysis, BMJ, doi:10.1136/bmj.39139.716794.55
Adusumilli, Zhang, Friedman, Friedman, Harnessing nitric oxide for preventing, limiting and treating the severe pulmonary consequences of COVID-19, Nitric Oxide, doi:10.1016/j.niox.2020.07.003
Afshari, Brok, Møller, Wetterslev, Inhaled nitric oxide for acute respiratory distress syndrome and acute lung injury in adults and children: a systematic review with meta-analysis and trial sequential analysis, Anesth. Analg, doi:10.1213/ANE.0b013e31820bd185
Akaberi, Krambrich, Ling, Luni, Hedenstierna et al., Mitigation of the replication of SARS-CoV-2 by nitric oxide in vitro, Redox Biol, doi:10.1016/j.redox.2020.101734
Al Sulaiman, Korayem, Altebainawi, Al Harbi, Alissa et al., Evaluation of inhaled nitric oxide (iNO) treatment for moderate-to-severe ARDS in critically ill patients with COVID-19: a multicenter cohort study, Crit. Care, doi:10.1186/s13054-022-04158-y
Ambrose, Barua, The pathophysiology of cigarette smoking and cardiovascular disease: an update, J. Am. Coll. Cardiol, doi:10.1016/j.jacc.2003.12.047
Archer, Sharp, Weir, Differentiating COVID-19 pneumonia from acute respiratory distress syndrome and high altitude pulmonary edema: therapeutic implications, Circulation, doi:10.1161/circulationaha.120.047915
Arnold, Mittal, Katsuki, Murad, Nitric oxide activates guanylate cyclase and increases guanosine 3′:5′-cyclic monophosphate levels in various tissue preparations, Proc. Natl. Acad. Sci. U. S. A, doi:10.1073/pnas.74.8.3203
Ataei Ataabadi, Golshiri, Jüttner, Krenning, Danser et al., Nitric oxide-cGMP signaling in hypertension: current and future options for pharmacotherapy, Hypertension, doi:10.1161/hypertensionaha.120.15856
Barnes, Brisbois, Clinical use of inhaled nitric oxide: local and systemic applications, Free Radic. Biol. Med, doi:10.1016/j.freeradbiomed.2019.11.029
Berlin, Thomas, Le Faou, Cornuz, COVID-19 and smoking, Nicotine Tob. Res, doi:10.1093/ntr/ntaa059
Betancor, Olaguibel, Sastre, The discrepant role of fractional exhaled nitric oxide in SARS-CoV-2 infection, J. Investig. Allergol. Clin. Immunol, doi:10.18176/jiaci.0842
Betancor, Valverde-Mongue, Gomez-Lopez, Barroso, Ruete et al., Evaluation of fractional exhaled nitric oxide during SARS-CoV-2 infection, J. Investig. Allergol. Clin. Immunol, doi:10.18176/jiaci.0762
Bonizzoli, Lazzeri, Cianchi, Guetti, Fulceri et al., Effects of rescue inhaled nitric oxide on right ventricle and pulmonary circulation in severe COVID-related acute respiratory distress syndrome, J. Crit. Care, doi:10.1016/j.jcrc.2022.153987
Bos, Ware, Acute respiratory distress syndrome: causes, pathophysiology, and phenotypes, Lancet, doi:10.1016/s0140-6736(22)01485-4
Burov, Kletskii, Kurbatov, Lisovin, Fedik, Mechanisms of nitric oxide generation in living systems, Nitric Oxide, doi:10.1016/j.niox.2021.10.003
Cespuglio, Strekalova, Spencer, Román, Reis et al., SARS-CoV-2 infection and sleep disturbances: nitric oxide involvement and therapeutic opportunity, Sleep, doi:10.1093/sleep/zsab009
Chambers, Tunnicliffe, Ayres, Acute inhalation of cigarette smoke increases lower respiratory tract nitric oxide concentrations, Thorax, doi:10.1136/thx.53.8.677
Chen, Klein, Garibaldi, Li, Wu et al., Aging in COVID-19: vulnerability, immunity and intervention, Ageing Res. Rev, doi:10.1016/j.arr.2020.101205
Cinelli, Do, Miley, Silverman, Inducible nitric oxide synthase: regulation, structure, and inhibition, Med. Res. Rev, doi:10.1002/med.21599
Demoncheaux, Higenbottam, Kiely, Wong, Wharton et al., Decreased whole body endogenous nitric oxide production in patients with primary pulmonary hypertension, J. Vasc. Res, doi:10.1159/000083502
Deppisch, Herrmann, Graepler-Mainka, Wirtz, Heyder et al., Gaseous nitric oxide to treat antibiotic resistant bacterial and fungal lung infections in patients with cystic fibrosis: a phase I clinical study, Infection, doi:10.1007/s15010-016-0879-x
Dominic, Ahmad, Bhandari, Pardue, Solorzano et al., Decreased availability of nitric oxide and hydrogen sulfide is a hallmark of COVID-19, Redox Biol, doi:10.1016/j.redox.2021.101982
Fang, Jiang, Su, Shu, Liu et al., The role of NO in COVID-19 and potential therapeutic strategies, Free Radic. Biol. Med, doi:10.1016/j.freeradbiomed.2020.12.008
Feng, Yang, Wen, Liu, Liu et al., Implication of inhaled nitric oxide for the treatment of critically ill COVID-19 patients with pulmonary hypertension, ESC Heart Fail, doi:10.1002/ehf2.13023
Ferrari, Protti, Nitric oxide in COVID-19: too little of a good thing?, EBioMedicine, doi:10.1016/j.ebiom.2022.103925
Flume, Garcia, Wilson, Steed, Dorman, Inhaled nitric oxide for adults with pulmonary non-tuberculous mycobacterial infection, Respir. Med, doi:10.1016/j.rmed.2022.107069
Frostell, Hedenstierna, Nitric oxide and COVID-19: dose, timing and how to administer it might be crucial, Acta Anaesthesiol. Scand, doi:10.1111/aas.13788
Förstermann, Sessa, Nitric oxide synthases: regulation and function, Eur. Heart J, doi:10.1093/eurheartj/ehr304
Garfield, Mcfadyen, Briar, Bleakley, Vlachou et al., Potential for personalised application of inhaled nitric oxide in COVID-19 pneumonia, Br. J. Anaesth, doi:10.1016/j.bja.2020.11.006
Garufi, Carbognin, Orlandi, Tortora, Bria, Smoking habit and hospitalization for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-related pneumonia: the unsolved paradox behind the evidence, Eur. J. Intern. Med, doi:10.1016/j.ejim.2020.04.042
Gebistorf, Karam, Wetterslev, Afshari, Inhaled nitric oxide for acute respiratory distress syndrome (ARDS) in children and adults, Cochrane Database Syst. Rev, doi:10.1002/14651858.CD002787.pub3
Ghaffari, Miller, Mcmullin, Ghahary, Potential application of gaseous nitric oxide as a topical antimicrobial agent, Nitric Oxide, doi:10.1016/j.niox.2005.08.003
Giaid, Saleh, Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension, N. Engl. J. Med, doi:10.1056/nejm199507273330403
Goldbart, Lavie, Lubetzky, Pillar, Landau et al., Inhaled nitric oxide for the treatment of acute bronchiolitis: a multicenter randomized controlled clinical trial to evaluate dose response, Ann. Am. Thorac. Soc, doi:10.1513/AnnalsATS.202103-348OC
Goldberg, Mazur, Kalra, Pulmonary hypertension: diagnosis, imaging techniques, and novel therapies, Cardiovasc Diagn Ther, doi:10.21037/cdt.2017.04.11
Grześk, Witczyńska, Węglarz, Wołowiec, Nowaczyk et al., Soluble guanylyl cyclase activators-promising therapeutic option in the pharmacotherapy of heart failure and pulmonary hypertension, Molecules, doi:10.3390/molecules28020861
Guida, Carriero, Bertolini, Pizzimenti, Heffler et al., Exhaled nitric oxide in asthma: from diagnosis to management, Curr. Opin. Allergy Clin. Immunol, doi:10.1097/aci.0000000000000877
Guimarães, Rossini, Lameu, Implications of SARS-Cov-2 infection on eNOS and iNOS activity: consequences for the respiratory and vascular systems, Nitric Oxide, doi:10.1016/j.niox.2021.04.003
Hedenstierna, Chen, Hedenstierna, Lieberman, Fine, Nitric oxide dosed in short bursts at high concentrations may protect against Covid 19, Nitric Oxide, doi:10.1016/j.niox.2020.06.005
Ishii, Hatano, Maki, Minatsuki, Saito et al., Prognostic value of follow-up vasoreactivity test in pulmonary arterial hypertension, J. Cardiol, doi:10.1016/j.jjcc.2023.01.005
Kaisers, Busch, Deja, Donaubauer, Falke, Selective pulmonary vasodilation in acute respiratory distress syndrome, Crit. Care Med, doi:10.1097/01.Ccm.0000057913.45273.1a
Kamenshchikov, Berra, Carroll, Therapeutic effects of inhaled nitric oxide therapy in COVID-19 patients, Biomedicine, doi:10.3390/biomedicines10020369
Karki, Sharma, Tuladhar, Williams, Zalduondo et al., Synergism of TNF-α and IFN-γ triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes, Cells, doi:10.1016/j.cell.2020.11.025
Kashiwagi, Morita, Yokomizo, Ogawa, Komai et al., Photobiomodulation and nitric oxide signaling, Nitric Oxide, doi:10.1016/j.niox.2022.11.005
Kerget, Araz, Akgün, The role of exhaled nitric oxide (FeNO) in the evaluation of lung parenchymal involvement in COVID-19 patients, Intern. Emerg. Med, doi:10.1007/s11739-022-03035-4
Keshavarz, Kadry, Alobaida, Ahsan, Newer approaches and novel drugs for inhalational therapy for pulmonary arterial hypertension, Expert Opin. Drug Deliv, doi:10.1080/17425247.2020.1729119
Li, Horke, Förstermann, Vascular oxidative stress, nitric oxide and atherosclerosis, Atherosclerosis, doi:10.1016/j.atherosclerosis.2014.09.001
Lior, Yatzkan, Brami, Yogev, Riff et al., Fractional exhaled nitric oxide (FeNO) level as a predictor of COVID-19 disease severity, Nitric Oxide, doi:10.1016/j.niox.2022.05.002
Lisi, Zelikin, Chandrawati, Nitric oxide to fight viral infections, Adv. Sci, doi:10.1002/advs.202003895
Longobardo, Montanari, Shulman, Benhalim, Singer et al., Inhaled nitric oxide minimally improves oxygenation in COVID-19 related acute respiratory distress syndrome, Br. J. Anaesth, doi:10.1016/j.bja.2020.10.011
Lotz, Muellenbach, Meybohm, Mutlak, Lepper et al., Effects of inhaled nitric oxide in COVID-19-induced ARDS-is it worthwhile?, Acta Anaesthesiol. Scand, doi:10.1111/aas.13757
Lugg, Scott, Parekh, Naidu, Thickett, Cigarette smoke exposure and alveolar macrophages: mechanisms for lung disease, Thorax, doi:10.1136/thoraxjnl-2020-216296
Lundberg, Weitzberg, Nitric oxide signaling in health and disease, Cells, doi:10.1016/j.cell.2022.06.010
Ma, Li, Yang, Liu, Zhang et al., Signaling pathways in vascular function and hypertension: molecular mechanisms and therapeutic interventions, Signal Transduct. Target. Ther, doi:10.1038/s41392-023-01430-7
Mac Micking, Xie, Nathan, Nitric oxide and macrophage function, Annu. Rev. Immunol, doi:10.1146/annurev.immunol.15.1.323
Malhotra, Hess, Lewis, Bloch, Waxman et al., Vasoreactivity to inhaled nitric oxide with oxygen predicts long-term survival in pulmonary arterial hypertension, Pulm Circ, doi:10.4103/2045-8932.83449
Mandal, Nitric oxide mediated hypoxia dynamics in COVID-19, Nitric Oxide, doi:10.1016/j.niox.2023.02.002
Maniscalco, Ambrosino, Poto, Fuschillo, Poto et al., Can FeNO be a biomarker in the post-COVID-19 patients monitoring?, Respir. Med, doi:10.1016/j.rmed.2022.106745
Matthay, Aldrich, Gotts, Treatment for severe acute respiratory distress syndrome from COVID-19, Lancet Respir. Med, doi:10.1016/s2213-2600(20)30127-2
Mekontso Dessap, Papazian, Schaller, Nseir, Megarbane et al., Inhaled nitric oxide in patients with acute respiratory distress syndrome caused by COVID-19: treatment modalities, clinical response, and outcomes, Ann. Intensive Care, doi:10.1186/s13613-023-01150-9
Meyer, Gattinoni, Calfee, Acute respiratory distress syndrome, Lancet, doi:10.1016/s0140-6736(21)00439-6
Michaelsen, Ribeiro, Brambate, Ali, Wang et al., A novel pre-clinical strategy to deliver antimicrobial doses of inhaled nitric oxide, PloS One, doi:10.1371/journal.pone.0258368
Miller, Hergott, Rohan, Arsenault-Mehta, Döring et al., Inhaled nitric oxide decreases the bacterial load in a rat model of Pseudomonas aeruginosa pneumonia, J. Cyst. Fibros, doi:10.1016/j.jcf.2013.01.008
Miller, Megson, Recent developments in nitric oxide donor drugs, Br. J. Pharmacol, doi:10.1038/sj.bjp.0707224
Miller, Miller, Mcmullin, Regev, Serghides et al., A phase I clinical study of inhaled nitric oxide in healthy adults, J. Cyst. Fibros, doi:10.1016/j.jcf.2012.01.003
Montani, Chaumais, Guignabert, Günther, Girerd et al., Targeted therapies in pulmonary arterial hypertension, Pharmacol. Ther, doi:10.1016/j.pharmthera.2013.10.002
Montiel, Lobysheva, Gérard, Vermeersch, Perez-Morga et al., Oxidative stress-induced endothelial dysfunction and decreased vascular nitric oxide in COVID-19 patients, EBioMedicine, doi:10.1016/j.ebiom.2022.103893
Mortaz, Malkmohammad, Jamaati, Naghan, Hashemian et al., Silent hypoxia: higher NO in red blood cells of COVID-19 patients, BMC Pulm. Med, doi:10.1186/s12890-020-01310-8
Murugesan, Saxena, Dileep, Adrish, Hanania, Update on the role of FeNO in asthma management, Diagnostics, doi:10.3390/diagnostics13081428
Nakane, Esaki, Ueda, Honda, Okabayashi, Inhaled nitric oxide improves pulmonary hypertension and organ functions after adult heart valve surgeries, Gen. Thorac. Cardiovasc. Surg, doi:10.1007/s11748-021-01651-z
Nasyrova, Moskaleva, Vaiman, Shnayder, Blatt et al., Genetic factors of nitric Oxide's system in Psychoneurologic disorders, Int. J. Mol. Sci, doi:10.3390/ijms21051604
Nathan, Flaherty, Glassberg, Raghu, Swigris et al., A randomized, double-blind, placebo-controlled study of pulsed, inhaled nitric oxide in subjects at risk of pulmonary hypertension associated with pulmonary fibrosis, Chest, doi:10.1016/j.chest.2020.02.016
Nogueira, Minnion, Clark, Dyson, Tanus-Santos et al., On the origin of nitrosylated hemoglobin in COVID-19: endothelial NO capture or redox conversion of nitrite?: experimental results and a cautionary note on challenges in translational research, Redox Biol, doi:10.1016/j.redox.2022.102362
Papadopoulos, Papadopoulou, Aw, Live to die another day: novel insights may explain the pathophysiology behind smoker's paradox in SARS-CoV-2 infection, Mol. Cell. Biochem, doi:10.1007/s11010-023-04681-8
Parikh, Wilson, Weinberg, Gavin, Murphy et al., Inhaled nitric oxide treatment in spontaneously breathing COVID-19 patients, Ther. Adv. Respir. Dis, doi:10.1177/1753466620933510
Redaelli, Magliocca, Malhotra, Ristagno, Citerio et al., Nitric oxide: clinical applications in critically ill patients, Nitric Oxide, doi:10.1016/j.niox.2022.01.007
Redaelli, Pozzi, Giani, Magliocca, Fumagalli et al., Inhaled nitric oxide in acute respiratory distress syndrome subsets: rationale and clinical applications, J. Aerosol Med. Pulm. Drug Deliv, doi:10.1089/jamp.2022.0058
Roberts, Polaner, Lang, Zapol, Inhaled nitric oxide in persistent pulmonary hypertension of the newborn, Lancet, doi:10.1016/0140-6736(92)92686-a
Rousseaud, Prot, Loriere, Katz, Ramirez-Gil et al., Gaseous nitric oxide failed to inhibit the replication cycle of SARS-CoV-2 in vitro, Nitric Oxide, doi:10.1016/j.niox.2023.01.004
Rupani, Kent, Using fractional exhaled nitric oxide measurement in clinical asthma management, Chest, doi:10.1016/j.chest.2021.10.015
Russo, Barale, Melchionda, Penna, Pagliaro, Platelets and Cardioprotection: the role of nitric oxide and carbon oxide, Int. J. Mol. Sci, doi:10.3390/ijms24076107
Safaee Fakhr, Di Fenza, Gianni, Wiegand, Miyazaki et al., Inhaled high dose nitric oxide is a safe and effective respiratory treatment in spontaneous breathing hospitalized patients with COVID-19 pneumonia, Nitric Oxide, doi:10.1016/j.niox.2021.08.003
Safaee Fakhr, Wiegand, Pinciroli, Gianni, Morais et al., High concentrations of nitric oxide inhalation therapy in pregnant patients with severe coronavirus disease 2019 (COVID-19), Obstet. Gynecol, doi:10.1097/aog.0000000000004128
Santacroce, Di Domenico, Montagnani, Jirillo, Antibiotic resistance and microbiota response, Curr. Pharm. Des, doi:10.2174/1381612829666221219093450
Sardo, Osawa, Finco, Gomes Galas, De Almeida et al., Nitric oxide in cardiac surgery: a Meta-analysis of randomized controlled trials, J. Cardiothorac. Vasc. Anesth, doi:10.1053/j.jvca.2018.02.003
Sharma, Patel, Post-translational regulation of neuronal nitric oxide synthase: implications for sympathoexcitatory states, Expert Opin. Ther. Targets, doi:10.1080/14728222.2017.1265505
Shei, Baranauskas, More questions than answers for the use of inhaled nitric oxide in COVID-19, Nitric Oxide, doi:10.1016/j.niox.2022.05.001
Signori, Magliocca, Hayashida, Graw, Malhotra et al., Inhaled nitric oxide: role in the pathophysiology of cardio-cerebrovascular and respiratory diseases, Intensive Care Med. Exp, doi:10.1186/s40635-022-00455-6
Sokol, Konduri, Van Meurs, Inhaled nitric oxide therapy for pulmonary disorders of the term and preterm infant, Semin. Perinatol, doi:10.1053/j.semperi.2016.05.007
Sorbo, Michaelsen, Ali, Wang, Ribeiro et al., High doses of inhaled nitric oxide as an innovative antimicrobial strategy for lung infections, Biomedicine, doi:10.3390/biomedicines10071525
Soundararajan, Dharmarajan, Samji, Regulation of pleiotropic physiological roles of nitric oxide signaling, Cell. Signal, doi:10.1016/j.cellsig.2022.110496
Stasch, Pacher, Evgenov, Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease, Circulation, doi:10.1161/circulationaha.110.981738
Stefano, Esch, Kream, Potential Immunoregulatory and antiviral/SARS-CoV-2 activities of nitric oxide, Med. Sci. Monit, doi:10.12659/msm.925679
Stratton, Tang, Lu, Pathogenesis-directed therapy of 2019 novel coronavirus disease, J. Med. Virol, doi:10.1002/jmv.26610
Tooba, Almoushref, Tonelli, Is there value in repeating inhaled nitric oxide Vasoreactivity tests in patients with pulmonary arterial hypertension?, Lung, doi:10.1007/s00408-019-00318-0
Triposkiadis, Xanthopoulos, Skoularigis, Starling, Therapeutic augmentation of NO-sGC-cGMP signalling: lessons learned from pulmonary arterial hypertension and heart failure, Heart Fail. Rev, doi:10.1007/s10741-022-10239-5
Usman, Siddiqi, Khan, Patel, Shahid et al., Is there a smoker's paradox in COVID-19?, BMJ Evid Based Med, doi:10.1136/bmjebm-2020-111492
Wang, Yu, Ochani, Amella, Tanovic et al., Nicotinic acetylcholine receptor alpha 7 subunit is an essential regulator of inflammation, Nature, doi:10.1038/nature01339
Wiegand, Traeger, Nguyen, Rouillard, Fischbach et al., Antimicrobial effects of nitric oxide in murine models of Klebsiella pneumonia, Redox Biol, doi:10.1016/j.redox.2020.101826
Winchester, John, Jabbar, John, Clinical efficacy of nitric oxide nasal spray (NONS) for the treatment of mild COVID-19 infection, J. Infect, doi:10.1016/j.jinf.2021.05.009
Yao, Zou, Cui, Quan, Gao et al., Recent advances in strategies to combat bacterial drug resistance: antimicrobial materials and drug delivery systems, Pharmaceutics, doi:10.3390/pharmaceutics15041188
Yong, Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments, Infect. Dis, doi:10.1080/23744235.2021.1924397
Yuki, Fujiogi, Koutsogiannaki, COVID-19 pathophysiology: a review, Clin. Immunol, doi:10.1016/j.clim.2020.108427
Zhao, None, Frontiers in Microbiology
Zhao, None, Frontiers in Microbiology
Zhao, Vanhoutte, Leung, Vascular nitric oxide: Beyond eNOS, J. Pharmacol. Sci, doi:10.1016/j.jphs.2015.09.002
Zolty, Pulmonary arterial hypertension specific therapy: the old and the new, Pharmacol. Ther, doi:10.1016/j.pharmthera.2020.107576
DOI record:
{
"DOI": "10.3389/fmicb.2023.1277552",
"ISSN": [
"1664-302X"
],
"URL": "http://dx.doi.org/10.3389/fmicb.2023.1277552",
"abstract": "<jats:p>Nitric oxide (NO), as an important gaseous medium, plays a pivotal role in the human body, such as maintaining vascular homeostasis, regulating immune-inflammatory responses, inhibiting platelet aggregation, and inhibiting leukocyte adhesion. In recent years, the rapid prevalence of coronavirus disease 2019 (COVID-19) has greatly affected the daily lives and physical and mental health of people all over the world, and the therapeutic efficacy and resuscitation strategies for critically ill patients need to be further improved and perfected. Inhaled nitric oxide (iNO) is a selective pulmonary vasodilator, and some studies have demonstrated its potential therapeutic use for COVID-19, severe respiratory distress syndrome, pulmonary infections, and pulmonary hypertension. In this article, we describe the biochemistry and basic characteristics of NO and discuss whether iNO can act as a “savior” for COVID-19 and related respiratory and cardiovascular disorders to exert a potent clinical protective effect.</jats:p>",
"alternative-id": [
"10.3389/fmicb.2023.1277552"
],
"author": [
{
"affiliation": [],
"family": "Zhao",
"given": "Yifan",
"sequence": "first"
},
{
"affiliation": [],
"family": "Li",
"given": "Cheng",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Shuai",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cheng",
"given": "Jiayu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liu",
"given": "Yucheng",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Han",
"given": "Xiaorong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Yinghui",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Yonggang",
"sequence": "additional"
}
],
"container-title": "Frontiers in Microbiology",
"container-title-short": "Front. Microbiol.",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"frontiersin.org"
]
},
"created": {
"date-parts": [
[
2023,
10,
3
]
],
"date-time": "2023-10-03T04:33:35Z",
"timestamp": 1696307615000
},
"deposited": {
"date-parts": [
[
2023,
10,
3
]
],
"date-time": "2023-10-03T04:33:38Z",
"timestamp": 1696307618000
},
"indexed": {
"date-parts": [
[
2023,
10,
4
]
],
"date-time": "2023-10-04T11:45:00Z",
"timestamp": 1696419900773
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
10,
2
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
10,
2
]
],
"date-time": "2023-10-02T00:00:00Z",
"timestamp": 1696204800000
}
}
],
"link": [
{
"URL": "https://www.frontiersin.org/articles/10.3389/fmicb.2023.1277552/full",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1965",
"original-title": [],
"prefix": "10.3389",
"published": {
"date-parts": [
[
2023,
10,
2
]
]
},
"published-online": {
"date-parts": [
[
2023,
10,
2
]
]
},
"publisher": "Frontiers Media SA",
"reference": [
{
"DOI": "10.1136/bmj.39139.716794.55",
"article-title": "Effect of nitric oxide on oxygenation and mortality in acute lung injury: systematic review and meta-analysis",
"author": "Adhikari",
"doi-asserted-by": "publisher",
"first-page": "779",
"journal-title": "BMJ",
"key": "ref1",
"volume": "334",
"year": "2007"
},
{
"DOI": "10.1016/j.niox.2020.07.003",
"article-title": "Harnessing nitric oxide for preventing, limiting and treating the severe pulmonary consequences of COVID-19",
"author": "Adusumilli",
"doi-asserted-by": "publisher",
"first-page": "4",
"journal-title": "Nitric Oxide",
"key": "ref2",
"volume": "103",
"year": "2020"
},
{
"DOI": "10.1213/ANE.0b013e31820bd185",
"article-title": "Inhaled nitric oxide for acute respiratory distress syndrome and acute lung injury in adults and children: a systematic review with meta-analysis and trial sequential analysis",
"author": "Afshari",
"doi-asserted-by": "publisher",
"first-page": "1411",
"journal-title": "Anesth. Analg.",
"key": "ref3",
"volume": "112",
"year": "2011"
},
{
"DOI": "10.1016/j.redox.2020.101734",
"article-title": "Mitigation of the replication of SARS-CoV-2 by nitric oxide in vitro",
"author": "Akaberi",
"doi-asserted-by": "publisher",
"first-page": "101734",
"journal-title": "Redox Biol.",
"key": "ref4",
"volume": "37",
"year": "2020"
},
{
"DOI": "10.1186/s13054-022-04158-y",
"article-title": "Evaluation of inhaled nitric oxide (iNO) treatment for moderate-to-severe ARDS in critically ill patients with COVID-19: a multicenter cohort study",
"author": "Al Sulaiman",
"doi-asserted-by": "publisher",
"first-page": "304",
"journal-title": "Crit. Care",
"key": "ref5",
"volume": "26",
"year": "2022"
},
{
"DOI": "10.1016/j.jacc.2003.12.047",
"article-title": "The pathophysiology of cigarette smoking and cardiovascular disease: an update",
"author": "Ambrose",
"doi-asserted-by": "publisher",
"first-page": "1731",
"journal-title": "J. Am. Coll. Cardiol.",
"key": "ref6",
"volume": "43",
"year": "2004"
},
{
"DOI": "10.1542/peds.106.2.344",
"article-title": "Committee on fetus and newborn. Use of inhaled nitric oxide",
"doi-asserted-by": "publisher",
"first-page": "344",
"journal-title": "Pediatrics",
"key": "ref7",
"volume": "106",
"year": "2000"
},
{
"DOI": "10.1161/circulationaha.120.047915",
"article-title": "Differentiating COVID-19 pneumonia from acute respiratory distress syndrome and high altitude pulmonary edema: therapeutic implications",
"author": "Archer",
"doi-asserted-by": "publisher",
"first-page": "101",
"journal-title": "Circulation",
"key": "ref8",
"volume": "142",
"year": "2020"
},
{
"DOI": "10.1073/pnas.74.8.3203",
"article-title": "Nitric oxide activates guanylate cyclase and increases guanosine 3′:5′-cyclic monophosphate levels in various tissue preparations",
"author": "Arnold",
"doi-asserted-by": "publisher",
"first-page": "3203",
"journal-title": "Proc. Natl. Acad. Sci. U. S. A.",
"key": "ref9",
"volume": "74",
"year": "1977"
},
{
"DOI": "10.1161/hypertensionaha.120.15856",
"article-title": "Nitric oxide-cGMP signaling in hypertension: current and future options for pharmacotherapy",
"author": "Ataei Ataabadi",
"doi-asserted-by": "publisher",
"first-page": "1055",
"journal-title": "Hypertension",
"key": "ref10",
"volume": "76",
"year": "2020"
},
{
"DOI": "10.1016/j.freeradbiomed.2019.11.029",
"article-title": "Clinical use of inhaled nitric oxide: local and systemic applications",
"author": "Barnes",
"doi-asserted-by": "publisher",
"first-page": "422",
"journal-title": "Free Radic. Biol. Med.",
"key": "ref11",
"volume": "152",
"year": "2020"
},
{
"DOI": "10.1093/ntr/ntaa059",
"article-title": "COVID-19 and smoking",
"author": "Berlin",
"doi-asserted-by": "publisher",
"first-page": "1650",
"journal-title": "Nicotine Tob. Res.",
"key": "ref12",
"volume": "22",
"year": "2020"
},
{
"DOI": "10.18176/jiaci.0842",
"article-title": "The discrepant role of fractional exhaled nitric oxide in SARS-CoV-2 infection",
"author": "Betancor",
"doi-asserted-by": "publisher",
"first-page": "417",
"journal-title": "J. Investig. Allergol. Clin. Immunol.",
"key": "ref13",
"volume": "32",
"year": ""
},
{
"DOI": "10.18176/jiaci.0762",
"article-title": "Evaluation of fractional exhaled nitric oxide during SARS-CoV-2 infection",
"author": "Betancor",
"doi-asserted-by": "publisher",
"first-page": "301",
"journal-title": "J. Investig. Allergol. Clin. Immunol.",
"key": "ref14",
"volume": "32",
"year": ""
},
{
"DOI": "10.1016/j.jcrc.2022.153987",
"article-title": "Effects of rescue inhaled nitric oxide on right ventricle and pulmonary circulation in severe COVID-related acute respiratory distress syndrome",
"author": "Bonizzoli",
"doi-asserted-by": "publisher",
"first-page": "153987",
"journal-title": "J. Crit. Care",
"key": "ref15",
"volume": "72",
"year": "2022"
},
{
"DOI": "10.1016/s0140-6736(22)01485-4",
"article-title": "Acute respiratory distress syndrome: causes, pathophysiology, and phenotypes",
"author": "Bos",
"doi-asserted-by": "publisher",
"first-page": "1145",
"journal-title": "Lancet",
"key": "ref16",
"volume": "400",
"year": "2022"
},
{
"DOI": "10.1016/j.niox.2021.10.003",
"article-title": "Mechanisms of nitric oxide generation in living systems",
"author": "Burov",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Nitric Oxide",
"key": "ref17",
"volume": "118",
"year": "2022"
},
{
"DOI": "10.1093/sleep/zsab009",
"article-title": "SARS-CoV-2 infection and sleep disturbances: nitric oxide involvement and therapeutic opportunity",
"author": "Cespuglio",
"doi-asserted-by": "publisher",
"first-page": "zsab009",
"journal-title": "Sleep",
"key": "ref18",
"volume": "44",
"year": "2021"
},
{
"DOI": "10.1136/thx.53.8.677",
"article-title": "Acute inhalation of cigarette smoke increases lower respiratory tract nitric oxide concentrations",
"author": "Chambers",
"doi-asserted-by": "publisher",
"first-page": "677",
"journal-title": "Thorax",
"key": "ref19",
"volume": "53",
"year": "1998"
},
{
"DOI": "10.1016/j.arr.2020.101205",
"article-title": "Aging in COVID-19: vulnerability, immunity and intervention",
"author": "Chen",
"doi-asserted-by": "publisher",
"first-page": "101205",
"journal-title": "Ageing Res. Rev.",
"key": "ref20",
"volume": "65",
"year": "2021"
},
{
"DOI": "10.1002/med.21599",
"article-title": "Inducible nitric oxide synthase: regulation, structure, and inhibition",
"author": "Cinelli",
"doi-asserted-by": "publisher",
"first-page": "158",
"journal-title": "Med. Res. Rev.",
"key": "ref21",
"volume": "40",
"year": "2020"
},
{
"DOI": "10.1159/000083502",
"article-title": "Decreased whole body endogenous nitric oxide production in patients with primary pulmonary hypertension",
"author": "Demoncheaux",
"doi-asserted-by": "publisher",
"first-page": "133",
"journal-title": "J. Vasc. Res.",
"key": "ref22",
"volume": "42",
"year": "2005"
},
{
"DOI": "10.1007/s15010-016-0879-x",
"article-title": "Gaseous nitric oxide to treat antibiotic resistant bacterial and fungal lung infections in patients with cystic fibrosis: a phase I clinical study",
"author": "Deppisch",
"doi-asserted-by": "publisher",
"first-page": "513",
"journal-title": "Infection",
"key": "ref23",
"volume": "44",
"year": "2016"
},
{
"DOI": "10.1016/j.redox.2021.101982",
"article-title": "Decreased availability of nitric oxide and hydrogen sulfide is a hallmark of COVID-19",
"author": "Dominic",
"doi-asserted-by": "publisher",
"first-page": "101982",
"journal-title": "Redox Biol.",
"key": "ref24",
"volume": "43",
"year": "2021"
},
{
"DOI": "10.1016/j.freeradbiomed.2020.12.008",
"article-title": "The role of NO in COVID-19 and potential therapeutic strategies",
"author": "Fang",
"doi-asserted-by": "publisher",
"first-page": "153",
"journal-title": "Free Radic. Biol. Med.",
"key": "ref25",
"volume": "163",
"year": "2021"
},
{
"DOI": "10.1002/ehf2.13023",
"article-title": "Implication of inhaled nitric oxide for the treatment of critically ill COVID-19 patients with pulmonary hypertension",
"author": "Feng",
"doi-asserted-by": "publisher",
"first-page": "714",
"journal-title": "ESC Heart Fail",
"key": "ref26",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1016/j.ebiom.2022.103925",
"article-title": "Nitric oxide in COVID-19: too little of a good thing?",
"author": "Ferrari",
"doi-asserted-by": "publisher",
"first-page": "103925",
"journal-title": "EBioMedicine",
"key": "ref27",
"volume": "77",
"year": "2022"
},
{
"DOI": "10.1016/j.rmed.2022.107069",
"article-title": "Inhaled nitric oxide for adults with pulmonary non-tuberculous mycobacterial infection",
"author": "Flume",
"doi-asserted-by": "publisher",
"first-page": "107069",
"journal-title": "Respir. Med.",
"key": "ref28",
"volume": "206",
"year": "2023"
},
{
"DOI": "10.1093/eurheartj/ehr304",
"article-title": "Nitric oxide synthases: regulation and function",
"author": "Förstermann",
"doi-asserted-by": "publisher",
"first-page": "829",
"journal-title": "Eur. Heart J.",
"key": "ref29",
"volume": "33",
"year": "2012"
},
{
"DOI": "10.1111/aas.13788",
"article-title": "Nitric oxide and COVID-19: dose, timing and how to administer it might be crucial",
"author": "Frostell",
"doi-asserted-by": "publisher",
"first-page": "576",
"journal-title": "Acta Anaesthesiol. Scand.",
"key": "ref30",
"volume": "65",
"year": "2021"
},
{
"DOI": "10.1016/j.bja.2020.11.006",
"article-title": "Potential for personalised application of inhaled nitric oxide in COVID-19 pneumonia",
"author": "Garfield",
"doi-asserted-by": "publisher",
"first-page": "e72",
"journal-title": "Br. J. Anaesth.",
"key": "ref31",
"volume": "126",
"year": "2021"
},
{
"DOI": "10.1016/j.ejim.2020.04.042",
"article-title": "Smoking habit and hospitalization for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-related pneumonia: the unsolved paradox behind the evidence",
"author": "Garufi",
"doi-asserted-by": "publisher",
"first-page": "121",
"journal-title": "Eur. J. Intern. Med.",
"key": "ref32",
"volume": "77",
"year": "2020"
},
{
"DOI": "10.1002/14651858.CD002787.pub3",
"article-title": "Inhaled nitric oxide for acute respiratory distress syndrome (ARDS) in children and adults",
"author": "Gebistorf",
"doi-asserted-by": "publisher",
"first-page": "CD002787",
"journal-title": "Cochrane Database Syst. Rev.",
"key": "ref33",
"volume": "2018",
"year": "2016"
},
{
"DOI": "10.1016/j.niox.2005.08.003",
"article-title": "Potential application of gaseous nitric oxide as a topical antimicrobial agent",
"author": "Ghaffari",
"doi-asserted-by": "publisher",
"first-page": "21",
"journal-title": "Nitric Oxide",
"key": "ref34",
"volume": "14",
"year": "2006"
},
{
"DOI": "10.1056/nejm199507273330403",
"article-title": "Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension",
"author": "Giaid",
"doi-asserted-by": "publisher",
"first-page": "214",
"journal-title": "N. Engl. J. Med.",
"key": "ref35",
"volume": "333",
"year": "1995"
},
{
"DOI": "10.1513/AnnalsATS.202103-348OC",
"article-title": "Inhaled nitric oxide for the treatment of acute bronchiolitis: a multicenter randomized controlled clinical trial to evaluate dose response",
"author": "Goldbart",
"doi-asserted-by": "publisher",
"first-page": "236",
"journal-title": "Ann. Am. Thorac. Soc.",
"key": "ref36",
"volume": "20",
"year": "2023"
},
{
"DOI": "10.21037/cdt.2017.04.11",
"article-title": "Pulmonary hypertension: diagnosis, imaging techniques, and novel therapies",
"author": "Goldberg",
"doi-asserted-by": "publisher",
"first-page": "405",
"journal-title": "Cardiovasc Diagn Ther",
"key": "ref37",
"volume": "7",
"year": "2017"
},
{
"DOI": "10.3390/molecules28020861",
"article-title": "Soluble guanylyl cyclase activators-promising therapeutic option in the pharmacotherapy of heart failure and pulmonary hypertension",
"author": "Grześk",
"doi-asserted-by": "publisher",
"first-page": "861",
"journal-title": "Molecules",
"key": "ref38",
"volume": "28",
"year": "2023"
},
{
"DOI": "10.1097/aci.0000000000000877",
"article-title": "Exhaled nitric oxide in asthma: from diagnosis to management",
"author": "Guida",
"doi-asserted-by": "publisher",
"first-page": "29",
"journal-title": "Curr. Opin. Allergy Clin. Immunol.",
"key": "ref39",
"volume": "23",
"year": "2023"
},
{
"DOI": "10.1016/j.niox.2021.04.003",
"article-title": "Implications of SARS-Cov-2 infection on eNOS and iNOS activity: consequences for the respiratory and vascular systems",
"author": "Guimarães",
"doi-asserted-by": "publisher",
"first-page": "64",
"journal-title": "Nitric Oxide",
"key": "ref40",
"year": "2021"
},
{
"DOI": "10.1016/j.niox.2020.06.005",
"article-title": "Nitric oxide dosed in short bursts at high concentrations may protect against Covid 19",
"author": "Hedenstierna",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Nitric Oxide",
"key": "ref41",
"volume": "103",
"year": "2020"
},
{
"DOI": "10.1016/j.jjcc.2023.01.005",
"article-title": "Prognostic value of follow-up vasoreactivity test in pulmonary arterial hypertension",
"author": "Ishii",
"doi-asserted-by": "publisher",
"first-page": "69",
"journal-title": "J. Cardiol.",
"key": "ref42",
"volume": "82",
"year": "2023"
},
{
"DOI": "10.1097/01.Ccm.0000057913.45273.1a",
"article-title": "Selective pulmonary vasodilation in acute respiratory distress syndrome",
"author": "Kaisers",
"doi-asserted-by": "publisher",
"first-page": "S337",
"journal-title": "Crit. Care Med.",
"key": "ref43",
"volume": "31",
"year": "2003"
},
{
"DOI": "10.3390/biomedicines10020369",
"article-title": "Therapeutic effects of inhaled nitric oxide therapy in COVID-19 patients",
"author": "Kamenshchikov",
"doi-asserted-by": "publisher",
"first-page": "20369",
"journal-title": "Biomedicine",
"key": "ref44",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1016/j.cell.2020.11.025",
"article-title": "Synergism of TNF-α and IFN-γ triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes",
"author": "Karki",
"doi-asserted-by": "publisher",
"first-page": "149",
"journal-title": "Cells",
"key": "ref45",
"volume": "184",
"year": "2021"
},
{
"DOI": "10.1016/j.niox.2022.11.005",
"article-title": "Photobiomodulation and nitric oxide signaling",
"author": "Kashiwagi",
"doi-asserted-by": "publisher",
"first-page": "58",
"journal-title": "Nitric Oxide",
"key": "ref46",
"volume": "130",
"year": "2023"
},
{
"DOI": "10.1007/s11739-022-03035-4",
"article-title": "The role of exhaled nitric oxide (FeNO) in the evaluation of lung parenchymal involvement in COVID-19 patients",
"author": "Kerget",
"doi-asserted-by": "publisher",
"first-page": "1951",
"journal-title": "Intern. Emerg. Med.",
"key": "ref47",
"volume": "17",
"year": "2022"
},
{
"DOI": "10.1080/17425247.2020.1729119",
"article-title": "Newer approaches and novel drugs for inhalational therapy for pulmonary arterial hypertension",
"author": "Keshavarz",
"doi-asserted-by": "publisher",
"first-page": "439",
"journal-title": "Expert Opin. Drug Deliv.",
"key": "ref48",
"volume": "17",
"year": "2020"
},
{
"DOI": "10.1016/j.atherosclerosis.2014.09.001",
"article-title": "Vascular oxidative stress, nitric oxide and atherosclerosis",
"author": "Li",
"doi-asserted-by": "publisher",
"first-page": "208",
"journal-title": "Atherosclerosis",
"key": "ref49",
"volume": "237",
"year": "2014"
},
{
"DOI": "10.1016/j.niox.2022.05.002",
"article-title": "Fractional exhaled nitric oxide (FeNO) level as a predictor of COVID-19 disease severity",
"author": "Lior",
"doi-asserted-by": "publisher",
"first-page": "68",
"journal-title": "Nitric Oxide",
"key": "ref50",
"volume": "124",
"year": "2022"
},
{
"DOI": "10.1002/advs.202003895",
"article-title": "Nitric oxide to fight viral infections",
"author": "Lisi",
"doi-asserted-by": "publisher",
"first-page": "2003895",
"journal-title": "Adv. Sci.",
"key": "ref51",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1016/j.bja.2020.10.011",
"article-title": "Inhaled nitric oxide minimally improves oxygenation in COVID-19 related acute respiratory distress syndrome",
"author": "Longobardo",
"doi-asserted-by": "publisher",
"first-page": "e44",
"journal-title": "Br. J. Anaesth.",
"key": "ref52",
"volume": "126",
"year": "2021"
},
{
"DOI": "10.1111/aas.13757",
"article-title": "Effects of inhaled nitric oxide in COVID-19-induced ARDS—is it worthwhile?",
"author": "Lotz",
"doi-asserted-by": "publisher",
"first-page": "629",
"journal-title": "Acta Anaesthesiol. Scand.",
"key": "ref53",
"volume": "65",
"year": "2021"
},
{
"DOI": "10.1136/thoraxjnl-2020-216296",
"article-title": "Cigarette smoke exposure and alveolar macrophages: mechanisms for lung disease",
"author": "Lugg",
"doi-asserted-by": "publisher",
"first-page": "94",
"journal-title": "Thorax",
"key": "ref54",
"volume": "77",
"year": "2022"
},
{
"DOI": "10.1016/j.cell.2022.06.010",
"article-title": "Nitric oxide signaling in health and disease",
"author": "Lundberg",
"doi-asserted-by": "publisher",
"first-page": "2853",
"journal-title": "Cells",
"key": "ref55",
"volume": "185",
"year": "2022"
},
{
"DOI": "10.1038/s41392-023-01430-7",
"article-title": "Signaling pathways in vascular function and hypertension: molecular mechanisms and therapeutic interventions",
"author": "Ma",
"doi-asserted-by": "publisher",
"first-page": "168",
"journal-title": "Signal Transduct. Target. Ther.",
"key": "ref56",
"volume": "8",
"year": "2023"
},
{
"DOI": "10.1146/annurev.immunol.15.1.323",
"article-title": "Nitric oxide and macrophage function",
"author": "Mac Micking",
"doi-asserted-by": "publisher",
"first-page": "323",
"journal-title": "Annu. Rev. Immunol.",
"key": "ref57",
"volume": "15",
"year": "1997"
},
{
"DOI": "10.4103/2045-8932.83449",
"article-title": "Vasoreactivity to inhaled nitric oxide with oxygen predicts long-term survival in pulmonary arterial hypertension",
"author": "Malhotra",
"doi-asserted-by": "publisher",
"first-page": "250",
"journal-title": "Pulm Circ",
"key": "ref58",
"volume": "1",
"year": "2011"
},
{
"DOI": "10.1016/j.niox.2023.02.002",
"article-title": "Nitric oxide mediated hypoxia dynamics in COVID-19",
"author": "Mandal",
"doi-asserted-by": "publisher",
"first-page": "18",
"journal-title": "Nitric Oxide",
"key": "ref59",
"volume": "133",
"year": "2023"
},
{
"DOI": "10.1016/j.rmed.2022.106745",
"article-title": "Can FeNO be a biomarker in the post-COVID-19 patients monitoring?",
"author": "Maniscalco",
"doi-asserted-by": "publisher",
"first-page": "106745",
"journal-title": "Respir. Med.",
"key": "ref60",
"volume": "193",
"year": "2022"
},
{
"DOI": "10.1016/s2213-2600(20)30127-2",
"article-title": "Treatment for severe acute respiratory distress syndrome from COVID-19",
"author": "Matthay",
"doi-asserted-by": "publisher",
"first-page": "433",
"journal-title": "Lancet Respir. Med.",
"key": "ref61",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1186/s13613-023-01150-9",
"article-title": "Inhaled nitric oxide in patients with acute respiratory distress syndrome caused by COVID-19: treatment modalities, clinical response, and outcomes",
"author": "Mekontso Dessap",
"doi-asserted-by": "publisher",
"first-page": "57",
"journal-title": "Ann. Intensive Care",
"key": "ref62",
"volume": "13",
"year": "2023"
},
{
"DOI": "10.1016/s0140-6736(21)00439-6",
"article-title": "Acute respiratory distress syndrome",
"author": "Meyer",
"doi-asserted-by": "publisher",
"first-page": "622",
"journal-title": "Lancet",
"key": "ref63",
"volume": "398",
"year": "2021"
},
{
"DOI": "10.1371/journal.pone.0258368",
"article-title": "A novel pre-clinical strategy to deliver antimicrobial doses of inhaled nitric oxide",
"author": "Michaelsen",
"doi-asserted-by": "publisher",
"first-page": "e0258368",
"journal-title": "PloS One",
"key": "ref64",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1016/j.jcf.2013.01.008",
"article-title": "Inhaled nitric oxide decreases the bacterial load in a rat model of Pseudomonas aeruginosa pneumonia",
"author": "Miller",
"doi-asserted-by": "publisher",
"first-page": "817",
"journal-title": "J. Cyst. Fibros.",
"key": "ref65",
"volume": "12",
"year": "2013"
},
{
"DOI": "10.1038/sj.bjp.0707224",
"article-title": "Recent developments in nitric oxide donor drugs",
"author": "Miller",
"doi-asserted-by": "publisher",
"first-page": "305",
"journal-title": "Br. J. Pharmacol.",
"key": "ref66",
"volume": "151",
"year": "2007"
},
{
"DOI": "10.1016/j.jcf.2012.01.003",
"article-title": "A phase I clinical study of inhaled nitric oxide in healthy adults",
"author": "Miller",
"doi-asserted-by": "publisher",
"first-page": "324",
"journal-title": "J. Cyst. Fibros.",
"key": "ref67",
"volume": "11",
"year": "2012"
},
{
"DOI": "10.1016/j.pharmthera.2013.10.002",
"article-title": "Targeted therapies in pulmonary arterial hypertension",
"author": "Montani",
"doi-asserted-by": "publisher",
"first-page": "172",
"journal-title": "Pharmacol. Ther.",
"key": "ref68",
"volume": "141",
"year": "2014"
},
{
"DOI": "10.1016/j.ebiom.2022.103893",
"article-title": "Oxidative stress-induced endothelial dysfunction and decreased vascular nitric oxide in COVID-19 patients",
"author": "Montiel",
"doi-asserted-by": "publisher",
"first-page": "103893",
"journal-title": "EBioMedicine",
"key": "ref69",
"volume": "77",
"year": "2022"
},
{
"DOI": "10.1186/s12890-020-01310-8",
"article-title": "Silent hypoxia: higher NO in red blood cells of COVID-19 patients",
"author": "Mortaz",
"doi-asserted-by": "publisher",
"first-page": "269",
"journal-title": "BMC Pulm. Med.",
"key": "ref70",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.3390/diagnostics13081428",
"article-title": "Update on the role of FeNO in asthma management",
"author": "Murugesan",
"doi-asserted-by": "publisher",
"first-page": "1428",
"journal-title": "Diagnostics",
"key": "ref71",
"volume": "13",
"year": "2023"
},
{
"DOI": "10.1007/s11748-021-01651-z",
"article-title": "Inhaled nitric oxide improves pulmonary hypertension and organ functions after adult heart valve surgeries",
"author": "Nakane",
"doi-asserted-by": "publisher",
"first-page": "1519",
"journal-title": "Gen. Thorac. Cardiovasc. Surg.",
"key": "ref72",
"volume": "69",
"year": "2021"
},
{
"DOI": "10.3390/ijms21051604",
"article-title": "Genetic factors of nitric Oxide's system in Psychoneurologic disorders",
"author": "Nasyrova",
"doi-asserted-by": "publisher",
"first-page": "604",
"journal-title": "Int. J. Mol. Sci.",
"key": "ref73",
"volume": "21",
"year": "2020"
},
{
"DOI": "10.1016/j.chest.2020.02.016",
"article-title": "A randomized, double-blind, placebo-controlled study of pulsed, inhaled nitric oxide in subjects at risk of pulmonary hypertension associated with pulmonary fibrosis",
"author": "Nathan",
"doi-asserted-by": "publisher",
"first-page": "637",
"journal-title": "Chest",
"key": "ref74",
"volume": "158",
"year": "2020"
},
{
"DOI": "10.1016/j.redox.2022.102362",
"article-title": "On the origin of nitrosylated hemoglobin in COVID-19: endothelial NO capture or redox conversion of nitrite?: experimental results and a cautionary note on challenges in translational research",
"author": "Nogueira",
"doi-asserted-by": "publisher",
"first-page": "102362",
"journal-title": "Redox Biol.",
"key": "ref75",
"volume": "54",
"year": "2022"
},
{
"DOI": "10.1007/s11010-023-04681-8",
"article-title": "Live to die another day: novel insights may explain the pathophysiology behind smoker's paradox in SARS-CoV-2 infection",
"author": "Papadopoulos",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Mol. Cell. Biochem.",
"key": "ref76",
"year": "2023"
},
{
"DOI": "10.1177/1753466620933510",
"article-title": "Inhaled nitric oxide treatment in spontaneously breathing COVID-19 patients",
"author": "Parikh",
"doi-asserted-by": "publisher",
"first-page": "1753466620933510",
"journal-title": "Ther. Adv. Respir. Dis.",
"key": "ref77",
"volume": "14",
"year": "2020"
},
{
"DOI": "10.1016/j.niox.2022.01.007",
"article-title": "Nitric oxide: clinical applications in critically ill patients",
"author": "Redaelli",
"doi-asserted-by": "publisher",
"first-page": "20",
"journal-title": "Nitric Oxide",
"key": "ref78",
"volume": "121",
"year": "2022"
},
{
"DOI": "10.1089/jamp.2022.0058",
"article-title": "Inhaled nitric oxide in acute respiratory distress syndrome subsets: rationale and clinical applications",
"author": "Redaelli",
"doi-asserted-by": "publisher",
"first-page": "112",
"journal-title": "J. Aerosol Med. Pulm. Drug Deliv.",
"key": "ref79",
"volume": "36",
"year": "2023"
},
{
"DOI": "10.1016/0140-6736(92)92686-a",
"article-title": "Inhaled nitric oxide in persistent pulmonary hypertension of the newborn",
"author": "Roberts",
"doi-asserted-by": "publisher",
"first-page": "818",
"journal-title": "Lancet",
"key": "ref80",
"volume": "340",
"year": "1992"
},
{
"DOI": "10.1016/j.niox.2023.01.004",
"article-title": "Gaseous nitric oxide failed to inhibit the replication cycle of SARS-CoV-2 in vitro",
"author": "Rousseaud",
"doi-asserted-by": "publisher",
"first-page": "27",
"journal-title": "Nitric Oxide",
"key": "ref81",
"volume": "132",
"year": "2023"
},
{
"DOI": "10.1016/j.chest.2021.10.015",
"article-title": "Using fractional exhaled nitric oxide measurement in clinical asthma management",
"author": "Rupani",
"doi-asserted-by": "publisher",
"first-page": "906",
"journal-title": "Chest",
"key": "ref82",
"volume": "161",
"year": "2022"
},
{
"DOI": "10.3390/ijms24076107",
"article-title": "Platelets and Cardioprotection: the role of nitric oxide and carbon oxide",
"author": "Russo",
"doi-asserted-by": "publisher",
"first-page": "6107",
"journal-title": "Int. J. Mol. Sci.",
"key": "ref83",
"volume": "24",
"year": "2023"
},
{
"DOI": "10.1016/j.niox.2021.08.003",
"article-title": "Inhaled high dose nitric oxide is a safe and effective respiratory treatment in spontaneous breathing hospitalized patients with COVID-19 pneumonia",
"author": "Safaee Fakhr",
"doi-asserted-by": "publisher",
"first-page": "7",
"journal-title": "Nitric Oxide",
"key": "ref84",
"volume": "116",
"year": "2021"
},
{
"DOI": "10.1097/aog.0000000000004128",
"article-title": "High concentrations of nitric oxide inhalation therapy in pregnant patients with severe coronavirus disease 2019 (COVID-19)",
"author": "Safaee Fakhr",
"doi-asserted-by": "publisher",
"first-page": "1109",
"journal-title": "Obstet. Gynecol.",
"key": "ref85",
"volume": "136",
"year": "2020"
},
{
"DOI": "10.2174/1381612829666221219093450",
"article-title": "Antibiotic resistance and microbiota response",
"author": "Santacroce",
"doi-asserted-by": "publisher",
"first-page": "356",
"journal-title": "Curr. Pharm. Des.",
"key": "ref86",
"volume": "29",
"year": "2023"
},
{
"DOI": "10.1053/j.jvca.2018.02.003",
"article-title": "Nitric oxide in cardiac surgery: a Meta-analysis of randomized controlled trials",
"author": "Sardo",
"doi-asserted-by": "publisher",
"first-page": "2512",
"journal-title": "J. Cardiothorac. Vasc. Anesth.",
"key": "ref87",
"volume": "32",
"year": "2018"
},
{
"DOI": "10.1080/14728222.2017.1265505",
"article-title": "Post-translational regulation of neuronal nitric oxide synthase: implications for sympathoexcitatory states",
"author": "Sharma",
"doi-asserted-by": "publisher",
"first-page": "11",
"journal-title": "Expert Opin. Ther. Targets",
"key": "ref88",
"volume": "21",
"year": "2017"
},
{
"DOI": "10.1016/j.niox.2022.05.001",
"article-title": "More questions than answers for the use of inhaled nitric oxide in COVID-19",
"author": "Shei",
"doi-asserted-by": "publisher",
"first-page": "39",
"journal-title": "Nitric Oxide",
"key": "ref89",
"volume": "124",
"year": "2022"
},
{
"DOI": "10.1186/s40635-022-00455-6",
"article-title": "Inhaled nitric oxide: role in the pathophysiology of cardio-cerebrovascular and respiratory diseases",
"author": "Signori",
"doi-asserted-by": "publisher",
"first-page": "28",
"journal-title": "Intensive Care Med. Exp.",
"key": "ref90",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1053/j.semperi.2016.05.007",
"article-title": "Inhaled nitric oxide therapy for pulmonary disorders of the term and preterm infant",
"author": "Sokol",
"doi-asserted-by": "publisher",
"first-page": "356",
"journal-title": "Semin. Perinatol.",
"key": "ref91",
"volume": "40",
"year": "2016"
},
{
"DOI": "10.3390/biomedicines10071525",
"article-title": "High doses of inhaled nitric oxide as an innovative antimicrobial strategy for lung infections",
"author": "Sorbo",
"doi-asserted-by": "publisher",
"first-page": "1525",
"journal-title": "Biomedicine",
"key": "ref92",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1016/j.cellsig.2022.110496",
"article-title": "Regulation of pleiotropic physiological roles of nitric oxide signaling",
"author": "Soundararajan",
"doi-asserted-by": "publisher",
"first-page": "110496",
"journal-title": "Cell. Signal.",
"key": "ref93",
"volume": "101",
"year": "2023"
},
{
"DOI": "10.1161/circulationaha.110.981738",
"article-title": "Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease",
"author": "Stasch",
"doi-asserted-by": "publisher",
"first-page": "2263",
"journal-title": "Circulation",
"key": "ref94",
"volume": "123",
"year": "2011"
},
{
"DOI": "10.12659/msm.925679",
"article-title": "Potential Immunoregulatory and antiviral/SARS-CoV-2 activities of nitric oxide",
"author": "Stefano",
"doi-asserted-by": "publisher",
"first-page": "e925679",
"journal-title": "Med. Sci. Monit.",
"key": "ref95",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1002/jmv.26610",
"article-title": "Pathogenesis-directed therapy of 2019 novel coronavirus disease",
"author": "Stratton",
"doi-asserted-by": "publisher",
"first-page": "1320",
"journal-title": "J. Med. Virol.",
"key": "ref96",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.1007/s00408-019-00318-0",
"article-title": "Is there value in repeating inhaled nitric oxide Vasoreactivity tests in patients with pulmonary arterial hypertension?",
"author": "Tooba",
"doi-asserted-by": "publisher",
"first-page": "87",
"journal-title": "Lung",
"key": "ref97",
"volume": "198",
"year": "2020"
},
{
"DOI": "10.1007/s10741-022-10239-5",
"article-title": "Therapeutic augmentation of NO-sGC-cGMP signalling: lessons learned from pulmonary arterial hypertension and heart failure",
"author": "Triposkiadis",
"doi-asserted-by": "publisher",
"first-page": "1991",
"journal-title": "Heart Fail. Rev.",
"key": "ref98",
"volume": "27",
"year": "2022"
},
{
"DOI": "10.1136/bmjebm-2020-111492",
"article-title": "Is there a smoker's paradox in COVID-19?",
"author": "Usman",
"doi-asserted-by": "publisher",
"first-page": "279",
"journal-title": "BMJ Evid Based Med",
"key": "ref99",
"volume": "26",
"year": "2021"
},
{
"DOI": "10.1038/nature01339",
"article-title": "Nicotinic acetylcholine receptor alpha 7 subunit is an essential regulator of inflammation",
"author": "Wang",
"doi-asserted-by": "publisher",
"first-page": "384",
"journal-title": "Nature",
"key": "ref100",
"volume": "421",
"year": "2003"
},
{
"DOI": "10.1016/j.redox.2020.101826",
"article-title": "Antimicrobial effects of nitric oxide in murine models of Klebsiella pneumonia",
"author": "Wiegand",
"doi-asserted-by": "publisher",
"first-page": "101826",
"journal-title": "Redox Biol.",
"key": "ref101",
"volume": "39",
"year": "2021"
},
{
"DOI": "10.1016/j.jinf.2021.05.009",
"article-title": "Clinical efficacy of nitric oxide nasal spray (NONS) for the treatment of mild COVID-19 infection",
"author": "Winchester",
"doi-asserted-by": "publisher",
"first-page": "237",
"journal-title": "J. Infect.",
"key": "ref102",
"volume": "83",
"year": "2021"
},
{
"DOI": "10.3390/pharmaceutics15041188",
"article-title": "Recent advances in strategies to combat bacterial drug resistance: antimicrobial materials and drug delivery systems",
"author": "Yao",
"doi-asserted-by": "publisher",
"first-page": "1188",
"journal-title": "Pharmaceutics",
"key": "ref103",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.1080/23744235.2021.1924397",
"article-title": "Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments",
"author": "Yong",
"doi-asserted-by": "publisher",
"first-page": "737",
"journal-title": "Infect. Dis.",
"key": "ref104",
"volume": "53",
"year": "2021"
},
{
"DOI": "10.1016/j.clim.2020.108427",
"article-title": "COVID-19 pathophysiology: a review",
"author": "Yuki",
"doi-asserted-by": "publisher",
"first-page": "108427",
"journal-title": "Clin. Immunol.",
"key": "ref105",
"volume": "215",
"year": "2020"
},
{
"DOI": "10.1016/j.jphs.2015.09.002",
"article-title": "Vascular nitric oxide: Beyond eNOS",
"author": "Zhao",
"doi-asserted-by": "publisher",
"first-page": "83",
"journal-title": "J. Pharmacol. Sci.",
"key": "ref106",
"volume": "129",
"year": "2015"
},
{
"DOI": "10.1016/j.pharmthera.2020.107576",
"article-title": "Pulmonary arterial hypertension specific therapy: the old and the new",
"author": "Zolty",
"doi-asserted-by": "publisher",
"first-page": "107576",
"journal-title": "Pharmacol. Ther.",
"key": "ref107",
"volume": "214",
"year": "2020"
}
],
"reference-count": 107,
"references-count": 107,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.frontiersin.org/articles/10.3389/fmicb.2023.1277552/full"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Microbiology (medical)",
"Microbiology"
],
"subtitle": [],
"title": "Inhaled nitric oxide: can it serve as a savior for COVID-19 and related respiratory and cardiovascular diseases?",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.3389/crossmark-policy",
"volume": "14"
}