The Transfer of Molnupiravir and Nirmatrelvir Across the Human Placenta and Prediction of Drug Safety in Pregnancy
, M., Electronic Thesis and Dissertation Repository, 10427, Sep 2024
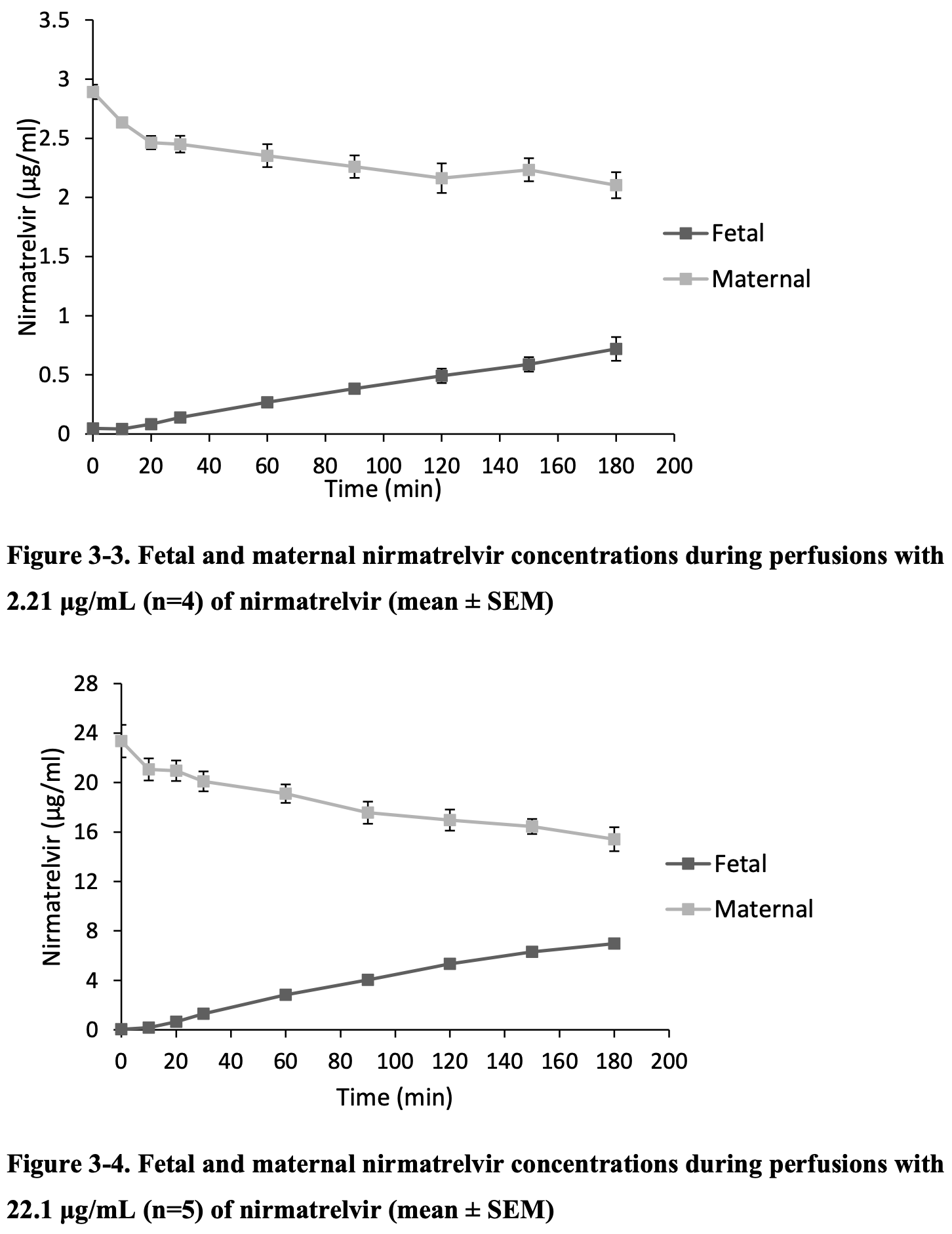
Ex vivo study showing placental transfer of molnupiravir (NHC) and nirmatrelvir in human placentas. Author used a placental perfusion model to assess drug transfer across the human placental barrier. Both compounds readily crossed the placental barrier at therapeutic and supratherapeutic concentrations, and were detectable on the fetal side within 10-20 minutes. The study suggests passive transfer as the main mechanism for both drugs, though efflux transporters may play a role for nirmatrelvir at therapeutic concentrations.
Study covers paxlovid and molnupiravir.
Zabek et al., 27 Sep 2024, preprint, 1 author.
Ex vivo studies are an important part of preclinical research, however results may be very different in vivo.
The Transfer of Molnupiravir and Nirmatrelvir Across the Human The Transfer of Molnupiravir and Nirmatrelvir Across the Human Placenta and Prediction of Drug Safety in Pregnancy Placenta and Prediction of Drug Safety in Pregnancy
Molnupiravir and nirmatrelvir are effective COVID-19 therapeutics, however there is limited data on their safety during pregnancy. The objective is to quantify the placental transfer of nirmatrelvir and -D-N4-hydroxycytidine (NHC), the metabolite of molnupiravir found in plasma. A systematic review on the pharmacokinetics of nirmatrelvir found no data in pregnant subjects, though several studies reported parameters in non-pregnant individuals such as CL, VD, and fu. Using the ex vivo human placental perfusion model, nirmatrelvir showed a fetal-maternal concentration ratio (F:M) of 0.34 at therapeutic doses (2.21 µg/mL) and 0.46 (22.1 µg/mL) at supratherapeutic doses. Adjusted F:M for non-placental physiological factors ranged from 0.26-0.35 (2.21 µg/mL) and 0.38-0.51 (22.1 µg/mL). For NHC, the F:M was (2.97µg/mL) and (29.7 µg/mL). Results suggest that therapeutic and supratherapeutic doses of nirmatrelvir and NHC can passively cross the placental barrier. This data can be considered to create better safety recommendations regarding their use in pregnancy.
List of Appendices 3 The placental transfer of nirmatrelvir using the ex vivo human placental perfusion model Magdalene Zabek 1, 4, 5 , Eddie Chan 4, 5 , Facundo Garcia-Bournissen 1, 3, 4, 5 and Janine Viability calculations were completed for all perfusions and presented in Table 3 -2. Fetal arterial pressure was under 80mmHg and was constant throughout the experiment, as well as the fetal volume loss for all experiments was < 4 mL/hr. The mean maternal and fetal flow rate was 12 mL/min and 3 mL/min, respectively. pH levels in maternal and fetal perfusates were maintained within physiological ranges. Calculated viability parameters indicated preserved placental viability, reflecting normal physiological processes.
Chapter 4 4 Molnupiravir use in pregnancy: a transplacental study Magdalene Zabek 1,4,5 , Eddie Chan 4, 5 , Facundo Garcia-Bournissen 1,3,4,5 and Janine
Appendices
Appendix A. Viability and integrity assessment of placental perfusions Throughout the experiment, several parameters are measured to ensure the viability and integrity of the placenta. Fetal volume loss (>4ml/hr) and fetal arterial inflow pressure (>80mmHg) were monitored. A volume loss or pressure any greater indicates trauma in the placental tissue and the perfusion will seize. In addition, samples were taken from maternal and fetal circuits (1.6ml) at specific timepoints to measure perfusate pH, glucose consumption, lactate production, oxygen transfer, net oxygen..
References
Abdelnabi, Foo, Jochmans, The oral protease inhibitor (PF-07321332) protects Syrian hamsters against infection with SARS-CoV-2 variants of concern, Nat Commun, doi:10.1038/s41467-022-28354-0
Afrouzian, Role of the efflux transporters BCRP and MRP1 in human placental bio-disposition of pravastatin, Biochem. Pharmacol
Ahmad, Sameen, Dar, Jallu, Shora et al., Do SARS-CoV-2-Infected Pregnant Women Have Adverse Pregnancy Outcomes as Compared to Non-Infected Pregnant Women?, Int J Womens Health, doi:10.2147/IJWH.S375739
Ahmad, Sameen, Dar, Jallu, Shora et al., Do SARS-CoV-2-Infected Pregnant Women Have Adverse Pregnancy Outcomes as Compared to Non-Infected Pregnant Women?, Int J Womens Health, doi:10.2147/IJWH.S375739
Al-Enazy, Ali, Albekairi, El-Tawil, Rytting, Placental control of drug delivery, Adv. Drug Deliv. Rev
Allegaert, Cefazolin pharmacokinetics in maternal plasma and amniotic fluid during pregnancy, Am. J. Obstet. Gynecol
Alwan, Chambers, Identifying Human Teratogens: An Update, J. Pediatr. Genet
Antonopoulou, Sapountzaki, Rova, Christakopoulos, Inhibition of the main protease of SARS-CoV-2 (Mpro) by repurposing/designing drug-like substances and utilizing nature's toolbox of bioactive compounds, Comput Struct Biotechnol J, doi:10.1016/j.csbj.2022.03.009
Arumugasaamy, Rock, Kuo, Bale, Fisher, Microphysiological systems of the placental barrier, Adv Drug Deliv Rev, doi:10.1016/j.addr.2020.08.010
Arutyunova, The Effect of Deuteration and Homologation of the Lactam Ring of Nirmatrelvir on Its Biochemical Properties and Oxidative Metabolism, ACS Bio Med Chem Au
Assali, Prystowsky, Studies on autonomic blockade. I. comparision between the effects of tetraethylammonium chloride (TEAC) and high selective spinal anesthesia on blood pressure of normal and toxemic pregnancy 1, J. Clin. Invest
Atkinson, Greenwood, Sibley, Glazier, Fairbairn, Role of MDR1 and MRP1 in trophoblast cells, elucidated using retroviral gene transfer, Am. J. Physiol.-Cell Physiol
Atmar, Finch, New Perspectives on Antimicrobial Agents: Molnupiravir and Nirmatrelvir/Ritonavir for Treatment of COVID-19, Antimicrob Agents Chemother, doi:10.1128/aac.02404-21
Avram, Pharmacokinetic studies in pregnancy, Semin. Perinatol
Bakos, Interactions of the Anti-SARS-CoV-2 Agents Molnupiravir and Nirmatrelvir/Paxlovid with Human Drug Transporters, Int. J. Mol. Sci
Bakos, Temesszentandrasi-Ambrus, Ozvegy-Laczka, Gaborik, Sarkadi et al., Interactions of the Anti-SARS-CoV-2 Agents Molnupiravir and Nirmatrelvir/Paxlovid with Human Drug Transporters, Int J Mol Sci, doi:10.3390/ijms241411237
Balhara, Kumar, Unadkat, Predicting Human Fetal Drug Exposure Through Maternal-Fetal PBPK Modeling and In Vitro or Ex Vivo Studies, J. Clin. Pharmacol
Balykova, Selezneva, Gorshenina, Modern directed antiviral COVID-19 therapy: results of multicenter clincial effectivness and safety study of fixed nirmatrevlir+ritonavir combination, Farmatsiya Farmakol, doi:10.19163/2307-9266-2022-10-4-371-386
Bapat, Pinto, Lubetsky, Berger, Koren, Rivaroxaban transfer across the dually perfused isolated human placental cotyledon, Am. J. Obstet. Gynecol
Barta, Lactate transport at the uteroplacental unit-A theoretical study, doi:10.1101/2020.10.23.351841
Barta, Lactate transport at the uteroplacental unit-A theoretical study, doi:10.1101/2020.10.23.351841
Bastian, Chen, Zhang, Dose-adjusted plasma concentrations of sublingual buprenorphine are lower during than after pregnancy, Am J Obstet Gynecol, doi:10.1016/j.ajog.2016.09.095
Berman, Erenburg, Beloosesky, Eyal, Kovo, Placental disposition of cannabidiol: An ex vivo perfusion study, Epilepsia
Bernal, Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients, N. Engl. J. Med
Betz, Fane, Human Chorionic Gonadotropin
Betz, Fane, Human Chorionic Gonadotropin
Blackburn, Pharmacokinetic Changes in the Pregnant Woman, J. Perinat. Neonatal Nurs
Bloom, Dias, Bawdon, Gilstrap, The maternal-fetal transfer of lamivudine in the ex vivo human placenta, Am J Obstet Gynecol, doi:10.1016/S0002-9378(97)70487-3
Bode, In Vitro Models for Studying Trophoblast Transcellular Transport, Methods Mol. Med
Boettcher, Metz, Maternal and neonatal outcomes following SARS-CoV-2 infection, Semin. Fetal. Neonatal Med
Boss, Chamley, James, Placental formation in early pregnancy: how is the centre of the placenta made?, Hum. Reprod. Update
Burd, Jones, Simmons, Makowski, Meschia et al., Placental production and foetal utilisation of lactate and pyruvate, Nature, doi:10.1038/254710a0
Bérard, New evidence for concern over the risk of birth defects from medications for nausea and vomitting of pregnancy, J. Clin. Epidemiol
Bérard, The French Pregnancy Cohort: Medication use during pregnancy in the French population, PloS One
Calis, Vojtech, Hladik, Gravett, A review of ex vivo placental perfusion models: an underutilized but promising method to study maternal-fetal interactions, J. Matern. Fetal Neonatal Med
Camus, Increased Expression of MDR1 mRNAs and P-glycoprotein in Placentas from HIV-1 Infected Women, Placenta
Caritis, Bastian, Zhang, An Evidence-Based Recommendation to Increase the Dosing Frequency of Buprenorphine During Pregnancy, Am J Obstet Gynecol, doi:10.1016/j.ajog.2017.06.029
Carlson, Pre-Delta, Delta, and Omicron Periods of the Coronavirus Disease 2019 (COVID-19) Pandemic and Health Outcomes During Delivery Hospitalization, Obstet. Gynecol
Carlson, Simeone, Ellington, Pre-Delta, Delta, and Omicron Periods of the Coronavirus Disease 2019 (COVID-19) Pandemic and Health Outcomes During Delivery Hospitalization, Obstet Gynecol, doi:10.1097/AOG.0000000000005449
Carlson, Simeone, Ellington, Pre-Delta, Delta, and Omicron Periods of the Coronavirus Disease 2019 (COVID-19) Pandemic and Health Outcomes During Delivery Hospitalization, Obstet Gynecol, doi:10.1097/AOG.0000000000005449
Cascella, Rajnik, Aleem, Dulebohn, Di Napoli et al., Evaluation, and Treatment of Coronavirus (COVID-19)
Catlin, Bowman, Campion, Reproductive and developmental safety of nirmatrelvir (PF-07321332), an oral SARS-CoV-2 Mpro inhibitor in animal models, Reprod Toxicol Elmsford N, doi:10.1016/j.reprotox.2022.01.006
Catlin, Reproductive and developmental safety of nirmatrelvir (PF-07321332), an oral SARS-CoV-2 Mpro inhibitor in animal models, Reprod. Toxicol. Elmsford N
Ceccaldi, Functional Role of P-Glycoprotein and Binding Protein Effect on the Placental Transfer of Lopinavir/Ritonavir in the Ex Vivo Human Perfusion Model, Obstet. Gynecol. Int
Ceccaldi, Gavard, Mandelbrot, Functional Role of P-Glycoprotein and Binding Protein Effect on the Placental Transfer of Lopinavir/Ritonavir in the Ex Vivo Human Perfusion Model, Obstet Gynecol Int, doi:10.1155/2009/726593
Celewicz, Celewicz, Michalczyk, Pregnancy as a Risk Factor of Severe COVID-19, J Clin Med, doi:10.3390/jcm10225458
Celewicz, Celewicz, Michalczyk, Pregnancy as a Risk Factor of Severe COVID-19, J Clin Med, doi:10.3390/jcm10225458
Celewicz, Celewicz, Michalczyk, Pregnancy as a Risk Factor of Severe COVID-19, J Clin Med, doi:10.3390/jcm10225458
Celewicz, Pregnancy as a Risk Factor of Severe COVID-19, J. Clin. Med
Cerveny, Equilibrative Nucleoside Transporter 1 (ENT1, SLC29A1) Facilitates Transfer of the Antiretroviral Drug Abacavir across the Placenta, Drug Metab. Dispos. Biol. Fate Chem
Cerveny, Ptackova, Ceckova, Equilibrative Nucleoside Transporter 1 (ENT1, SLC29A1) Facilitates Transfer of the Antiretroviral Drug Abacavir across the Placenta, Drug Metab Dispos Biol Fate Chem, doi:10.1124/dmd.118.083329
Challier, Schneider, Dancis, In vitro perfusion of human placenta: V. Oxygen consumption, American Journal of Obstetrics & Gynecology, doi:10.1016/0002-9378(76)90287-8
Chan, Singh, Cox, Shi, Damle et al., Dosing recommendation of nirmatrelvir/ritonavir using an integrated population pharmacokinetic analysis, CPT Pharmacomet Syst Pharmacol, doi:10.1002/psp4.13039
Chang, A Newly Engineered A549 Cell Line Expressing ACE2 and TMPRSS2 Is Highly Permissive to SARS-CoV-2, Including the Delta and Omicron Variants, Viruses
Chang, Peng, Lee, Transfer and biotransformation of the COVID-19 prodrug molnupiravir and its metabolite β-D-N4-hydroxycytidine across the blood-placenta barrier, eBioMedicine, doi:10.1016/j.ebiom.2023.104748
Chang, Transfer and biotransformation of the COVID-19 prodrug molnupiravir and its metabolite β-D-N4-hydroxycytidine across the blood-placenta barrier, eBioMedicine
Chaphekar, Caritis, Venkataramanan, Model-Informed Dose Optimization in Pregnancy, J. Clin. Pharmacol
Chen, Leong, Lee, Chowbay, Pharmacokinetic and pharmacogenomic considerations in managing use of nirmatrelvir-ritonavir and molnupiravir and dermatological treatments, Ann Acad Med Singapore, doi:10.47102/annals-acadmedsg.2022430
Chen, Li, Huang, Discovery of novel bicyclic[3.3.0]proline peptidyl alpha-ketoamides as potent 3CL-protease inhibitors for SARS-CoV-2, Bioorg Med Chem Lett, doi:10.1016/j.bmcl.2023.129324
Cheung, Lafayette, Renal Physiology of Pregnancy, Adv. Chronic Kidney Dis
Chuang, Su, Chou, Transplacental passage of nirmatrelvir in pregnant women with COVID-19, Int J Gynecol Obstet, doi:10.1002/ijgo.15147
Chuang, Su, Chou, Transplacental passage of nirmatrelvir in pregnant women with COVID-19, Int J Gynecol Obstet, doi:10.1002/ijgo.151473.6
Chuang, Transplacental passage of nirmatrelvir in pregnant women with COVID-19, Int. J. Gynecol. Obstet
Cindrova-Davies, Oxidative stress, gene expression, and protein changes induced in the human placenta during labor, Am. J. Pathol
Clark, Newmark, Wisner, Stika, Avram, Lithium Pharmacokinetics in the Perinatal Patient With Bipolar Disorder, J. Clin. Pharmacol
Colbers, Greupink, Litjens, Burger, Russel, Physiologically Based Modelling of Darunavir/Ritonavir Pharmacokinetics During Pregnancy, Clin Pharmacokinet, doi:10.1007/s40262-015-0325-8
Conings, Amant, Annaert, Van Calsteren, Integration and validation of the ex vivo human placenta perfusion model, J. Pharmacol. Toxicol. Methods
Costantine, Physiologic and pharmacokinetic changes in pregnancy, Front. Pharmacol
Cox, Van Eyck, Pawlak, Effects of itraconazole and carbamazepine on the pharmacokinetics of nirmatrelvir/ritonavir in healthy adults, Br J Clin Pharmacol, doi:10.1111/bcp.15788
D'angelo, An Enhanced Dissolving Cyclosporin-A Inhalable Powder Efficiently Reduces SARS-CoV-2 Infection In Vitro, Pharmaceutics
Dashraath, Coronavirus disease 2019 (COVID-19) pandemic and pregnancy, Am. J. Obstet. Gynecol
Dashraath, Wong, Lim, Coronavirus disease 2019 (COVID-19) pandemic and pregnancy, Am J Obstet Gynecol, doi:10.1016/j.ajog.2020.03.021
Denti, Population Pharmacokinetics of Rifampin in Pregnant Women with Tuberculosis and HIV Coinfection in Soweto, South Africa, Antimicrob. Agents Chemother
Dong, Huang, Ling, Li, Yu et al., High concentrations of nirmatrelvir/ritonavir in critically ill patients receiving continuous renal replacement therapy, Int J Antimicrob Agents, doi:10.1016/j.ijantimicag.2023.106997
Dusza, Experimental human placental models for studying uptake, transport and toxicity of micro-and nanoplastics, Sci. Total Environ
Earhart, Transplacental transfer and metabolism of bupropion, J. Matern. Fetal Neonatal Med
Economides, Johnson, Mackenzie, Does amniotic fluid analysis reflect acid-base balance in fetal blood?, Am. J. Obstet. Gynecol
Elango, Sundaramoorthy, Subramanian, Lella, Radhakrishnan, Molnupiravir and Combination of Nirmatrelvir and Ritonavir (PaxlovidTM) -Oral Anti-viral Drugs in COVID-19: A Systematic Review, J Commun Dis, doi:10.24321/0019.5138.202310
Elkomy, Pharmacokinetics of Prophylactic Cefazolin in Parturients Undergoing Cesarean Delivery, Antimicrob. Agents Chemother
Elzinga, Placenta-on-a-Chip as an In Vitro Approach to Evaluate the Physiological and Structural Characteristics of the Human Placental Barrier upon Drug Exposure: A Systematic Review, J. Clin. Med
Eng, Dantonio, Kadar, Disposition of Nirmatrelvir, an Orally Bioavailable Inhibitor of SARS-CoV-2 3C-Like Protease, across Animals and Humans, Drug Metab Dispos Biol Fate Chem, doi:10.1124/dmd.121.000801
Eng, Dantonio, Kadar, Disposition of Nirmatrelvir, an Orally Bioavailable Inhibitor of SARS-CoV-2 3C-Like Protease, across Animals and Humans, Drug Metab Dispos Biol Fate Chem, doi:10.1124/dmd.121.000801
Ernst, Identification of side effects of COVID-19 drug candidates on embryogenesis using an integrated zebrafish screening platform, Sci. Rep
Errasti-Murugarren, Díaz, Godoy, Riquelme, Pastor-Anglada, Expression and Distribution of Nucleoside Transporter Proteins in the Human Syncytiotrophoblast, Mol. Pharmacol
Favre, Maternal and perinatal outcomes following pre-Delta, Delta, and Omicron SARS-CoV-2 variants infection among unvaccinated pregnant women in France and Switzerland: a prospective cohort study using the COVI-PREG registry, Lancet Reg. Health Eur
Feghali, Venkataramanan, Caritis, Pharmacokinetics of drugs in pregnancy, Semin. Perinatol
Feinshtein, Nitrofurantoin transport by placental choriocarcinoma JAr cells: involvement of BCRP, OATP2B1 and other MDR transporters, Arch. Gynecol. Obstet
Feng, Combining 'Bottom-Up' and 'Top-Down' Methods to Assess Ethnic Difference in Clearance: Bitopertin as an Example, Clin. Pharmacokinet
Fischer, Eron, Holman, Molnupiravir, an Oral Antiviral Treatment for COVID-19, MedRxiv Prepr Serv Health Sci, doi:10.1101/2021.06.17.21258639
Fischer, Eron, Holman, Molnupiravir, an Oral Antiviral Treatment for COVID-19, MedRxiv Prepr Serv Health Sci, doi:10.1101/2021.06.17.21258639
Forestier, De Renty, Peytavin, Dohin, Farinotti et al., Maternalfetal transfer of saquinavir studied in the ex vivo placental perfusion model, Am J Obstet Gynecol, doi:10.1067/mob.2001.113319
Freriksen, Assessment of Maternal and Fetal Dolutegravir Exposure by Integrating Ex Vivo Placental Perfusion Data and Physiologically-Based Pharmacokinetic Modeling, Clin. Pharmacol. Ther
Freriksen, Schalkwijk, Colbers, Assessment of Maternal and Fetal Dolutegravir Exposure by Integrating Ex Vivo Placental Perfusion Data and Physiologically-Based Pharmacokinetic Modeling, Clin Pharmacol Ther, doi:10.1002/cpt.1748
Friis-Hansen, Body water metabolism in early infancy, Acta Paediatr
Furukawa, Tsuji, Sugiyama, Morphology and physiology of rat placenta for toxicological evaluation, J Toxicol Pathol, doi:10.1293/tox.2018-0042
Garland, Abildskov, Kiu, Daniel, Stark, The contribution of fetal metbolism to the disposition of morphine, Drug Metab. Dispos
Garland, Pharmacology of drug transfer across the placenta, Obstet Gynecol Clin North Am, doi:10.1016/S0889-8545(05)70356-9
Garneau, Analysis of Clinical Outcomes of Pregnant Patients Treated With Nirmatrelvir and Ritonavir for Acute SARS-CoV-2 Infection, JAMA Netw. Open
Garneau, Jones-Beatty, Ufua, Analysis of Clinical Outcomes of Pregnant Patients Treated With Nirmatrelvir and Ritonavir for Acute SARS-CoV-2 Infection, JAMA Netw Open, doi:10.1001/jamanetworkopen.2022.44141
Garneau, Jones-Beatty, Ufua, Analysis of Clinical Outcomes of Pregnant Patients Treated With Nirmatrelvir and Ritonavir for Acute SARS-CoV-2 Infection, JAMA Netw Open, doi:10.1001/jamanetworkopen.2022.44141
Gedeon, Anger, Piquette-Miller, Koren, Breast cancer resistance protein: mediating the trans-placental transfer of glyburide across the human placenta, Placenta
Gil, Saura, Forestier, Farinotti, P-glycoprotein expression of the human placenta during pregnancy, Placenta
Githaka, Molnupiravir Does Not Induce Mutagenesis in Host Lung Cells during SARS-CoV-2 Treatment, Bioinforma Biol Insights, doi:10.1177/11779322221085077
Githaka, Molnupiravir Does Not Induce Mutagenesis in Host Lung Cells during SARS-CoV-2 Treatment, Bioinforma. Biol. Insights
Gordon, Tchesnokov, Schinazi, Götte, Molnupiravir promotes SARS-CoV-2 mutagenesis via the RNA template, J Biol Chem, doi:10.1016/j.jbc.2021.100770
Gordon, Tchesnokov, Schinazi, Götte, Molnupiravir promotes SARS-CoV-2 mutagenesis via the RNA template, J. Biol. Chem
Greenfield, Eng, Yang, Species differences in plasma protein binding of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) main protease inhibitor nirmatrelvir, Xenobiotica Fate Foreign Compd Biol Syst, doi:10.1080/00498254.2023.2183158
Greenfield, Eng, Yang, Species differences in plasma protein binding of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) main protease inhibitor nirmatrelvir, Xenobiotica Fate Foreign Compd Biol Syst, doi:10.1080/00498254.2023.2183158
Griffiths, Campbell, Placental structure, function and drug transfer, Contin. Educ. Anaesth. Crit. Care Pain
Hakkola, Pelkonen, Pasanen, Raunio, Xenobiotic-metabolizing cytochrome P450 enzymes in the human feto-placental unit: role in intrauterine toxicity, Crit. Rev. Toxicol
Hammond, Leister-Tebbe, Gardner, Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2118542
Hammond, Leister-Tebbe, Gardner, Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2118542
Hammond, Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19, N. Engl. J. Med
Han, Gao, Mao, An update on expression and function of p-gp/abcb1 and bcrp/abcg2 in the placenta and fetus, Expert Opin Drug Metab Toxicol, doi:10.1080/17425255.2018.1499726
Haque, Mahar, Hussain, Sloane, Pharmacokinetic interaction between verapamil and ritonavir-boosted nirmatrelvir: implications for the management of COVID-19 in patients with hypertension, BMJ Case Rep
Hemauer, Role of transporter-mediated efflux in the placental biodisposition of bupropion and its metabolite, OH-bupropion, Biochem. Pharmacol
Herrick, Bordoni, Embryology, Placenta. in StatPearls
Hill, Abramson, The significance of plasma protein binding on the fetal/maternal distribution of drugs at steady-state, Clin Pharmacokinet, doi:10.2165/00003088-198814030-00004
Hill, Abramson, The significance of plasma protein binding on the fetal/maternal distribution of drugs at steady-state, Clin. Pharmacokinet
Hu, Guo, Zhou, Shi, Characteristics of SARS-CoV-2 and COVID-19, Nat. Rev. Microbiol
Hu, Lin, Xu, Structural Basis for the Inhibition of Coronaviral Main Proteases by a Benzothiazole-Based Inhibitor, Viruses, doi:10.3390/v14092075
Hung, Lee, Chiu, Oral Nirmatrelvir/Ritonavir Therapy for COVID-19: The Dawn in the Dark?, Antibiot Basel Switz, doi:10.3390/antibiotics11020220
Hung, Oral Nirmatrelvir/Ritonavir Therapy for COVID-19: The Dawn in the Dark?, Antibiot. Basel Switz
Hutson, CSPT 2011 -Prediction of Placental Drug Transfer Using the Human Placental Perfusion Model, J Popul Ther Clin Pharmacol
Hutson, CSPT 2011 -Prediction of Placental Drug Transfer Using the Human Placental Perfusion Model, J Popul Ther Clin Pharmacol
Hutson, CSPT 2011 -Prediction of Placental Drug Transfer Using the Human Placental Perfusion Model, J. Popul. Ther. Clin. Pharmacol
Hutson, Lubetsky, Walfisch, Ballios, Garcia-Bournissen et al., The transfer of 6-mercaptopurine in the dually perfused human placenta, Reprod Toxicol, doi:10.1016/j.reprotox.2011.08.008
Hutson, The transfer of 6-mercaptopurine in the dually perfused human placenta, Reprod. Toxicol
Jaini, Lin, Di, Sagawa, PBPK Modeling of PAXLOVIDTM: Incorporating Rotamer Conversion Kinetics to Advanced Dissolution and Absorption Model, J Pharm Sci, doi:10.1016/j.xphs.2023.09.028
Jamieson, Rasmussen, An update on COVID-19 and pregnancy, Am J Obstet Gynecol, doi:10.1016/j.ajog.2021.08.054
Jamieson, Rasmussen, An update on COVID-19 and pregnancy, Am J Obstet Gynecol, doi:10.1016/j.ajog.2021.08.054
Jamieson, Rasmussen, An update on COVID-19 and pregnancy, Am J Obstet Gynecol, doi:10.1016/j.ajog.2021.08.054
Jamieson, Rasmussen, An update on COVID-19 and pregnancy, Am. J. Obstet. Gynecol
Jeong, Altered drug metabolism during pregnancy: Hormonal regulation of drugmetabolizing enzymes, Expert Opin. Drug Metab. Toxicol
Jiraskova, Cerveny, Karbanova, Ptackova, Staud, Expression of Concentrative Nucleoside Transporters ( SLC28A ) in the Human Placenta: Effects of Gestation Age and Prototype Differentiation-Affecting Agents, Mol Pharm, doi:10.1021/acs.molpharmaceut.8b00238
Jiraskova, Cerveny, Karbanova, Ptackova, Staud, Expression of Concentrative Nucleoside Transporters ( SLC28A ) in the Human Placenta: Effects of Gestation Age and Prototype Differentiation-Affecting Agents, Mol. Pharm
Johnson, Effects of Fetal pH on Local Anesthetic Transfer across the Human Placenta, Anesthesiology
Joyce, Hu, Wang, The history, mechanism, and perspectives of nirmatrelvir (PF-07321332): an orally bioavailable main protease inhibitor used in combination with ritonavir to reduce COVID-19-related hospitalizations, Med Chem Res, doi:10.1007/s00044-022-02951-6
Juchau, Faustman-Watts, Pharmacokinetic Considerations in the Maternal-Placental-Fetal Unit, Clin. Obstet. Gynecol
Kabinger, Stiller, Schmitzová, Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis, Nat Struct Mol Biol, doi:10.1038/s41594-021-00651-0
Kabinger, emergency use authorization for lagevrio TM (molnupiravir) capsules, Nat. Struct. Mol. Biol
Karbanova, Cerveny, Ceckova, Role of nucleoside transporters in transplacental pharmacokinetics of nucleoside reverse transcriptase inhibitors zidovudine and emtricitabine, Placenta, doi:10.1016/j.placenta.2017.10.011
Karttunen, Criteria and challenges of the human placental perfusion -Data from a large series of perfusions, Toxicol. In Vitro
Karttunen, Myllynen, Prochazka, Pelkonen, Segerbäck et al., Placental transfer and DNA binding of benzo(a)pyrene in human placental perfusion, Toxicol Lett, doi:10.1016/j.toxlet.2010.04.028
Karttunen, Placental transfer and DNA binding of benzo(a)pyrene in human placental perfusion, Toxicol. Lett
Kesson, Acyclovir for the prevention and treatment of varicella zoster in children, adolescents and pregnancy, J. Paediatr. Child Health
Khalil, Change in the Incidence of Stillbirth and Preterm Delivery During the COVID-19 Pandemic, JAMA
Khalil, Von Dadelszen, Draycott, Ugwumadu, 'brien et al., Change in the Incidence of Stillbirth and Preterm Delivery During the COVID-19 Pandemic, JAMA, doi:10.1001/jama.2020.12746
Kim, Scialli, Thalidomide: The Tragedy of Birth Defects and the Effective Treatment of Disease, Toxicol. Sci
Koren, Ornoy, The role of the placenta in drug transport and fetal drug exposure, Expert Rev. Clin. Pharmacol
Koren, Pariente, Pregnancy-Associated Changes in Pharmacokinetics and their Clinical Implications, Pharm. Res
Kraemer, Klein, Lubetsky, Koren, Perfusion studies of glyburide transfer across the human placenta: implications for fetal safety, Am J Obstet Gynecol, doi:10.1016/j.ajog.2005.12.005
Krauer, Dayer, Anner, Changes in serum albumin and α 1 -acid glycoprotein concentrations during pregnancy: an analysis of fetal-maternal pairs, BJOG Int. J. Obstet. Gynaecol
Kurosawa, Chiba, Noguchi, Nishimura, Tomi, Development of a Pharmacokinetic Model of Transplacental Transfer of Metformin to Predict In Vivo Fetal Exposure, Drug Metab Dispos, doi:10.1124/dmd.120.000127
Lafont, Blez, Bildan, Nirmatrelvir and Ritonavir combination in COVID-19 patients with advanced chronic kidney disease, Clin Infect Dis Off Publ Infect Dis Soc Am, doi:10.1093/cid/ciad785
Lamb, Nirmatrelvir Plus Ritonavir: First Approval, Drugs
Lamb, Nirmatrelvir Plus Ritonavir: First Approval, Drugs, doi:10.1007/s40265-022-01692-5
Lambot, Evidence for a clathrin-mediated recycling of albumin in human term placenta, Biol. Reprod
Li, Lin, Zhou, Structural Basis of the Main Proteases of Coronavirus Bound to Drug Candidate PF-07321332, J Virol, doi:10.1128/jvi.02013-21
Liebes, Mendoza, Wilson, Dancis, Transfer of Zidovudine (AZT) by Human Placenta, J. Infect. Dis
Lin, Cassidy, Li, Prahl, Golan et al., Nirmatrelvir-Ritonavir (Paxlovid) for Mild Coronavirus Disease 2019 (COVID-19) in Pregnancy and Lactation, Obstet Gynecol, doi:10.1097/AOG.0000000000005152
Lin, Clinical outcomes of nirmatrelvir-ritonavir use in pregnant women during the Omicron wave of the coronavirus disease 2019 pandemic, J. Infect. Public Health
Lin, Liang, Chuang, Tseng, Tsai et al., Clinical outcomes of nirmatrelvir-ritonavir use in pregnant women during the Omicron wave of the coronavirus disease 2019 pandemic, J Infect Public Health, doi:10.1016/j.jiph.2023.10.007
Lin, Liang, Chuang, Tseng, Tsai et al., Clinical outcomes of nirmatrelvir-ritonavir use in pregnant women during the Omicron wave of the coronavirus disease 2019 pandemic, J Infect Public Health, doi:10.1016/j.jiph.2023.10.007
Lingscheid, Kinzig, Kruger, Pharmacokinetics of Nirmatrelvir and Ritonavir in COVID-19 Patients with End-Stage Renal Disease on Intermittent Hemodialysis, Antimicrob Agents Chemother, doi:10.1128/aac.01229-22
Link-Gelles, Early Estimates of Updated 2023-2024 (Monovalent XBB.1.5) COVID-19 Vaccine Effectiveness Against Symptomatic SARS-CoV-2 Infection Attributable to Co-Circulating Omicron Variants Among Immunocompetent Adults -Increasing Community Access to Testing Program, United States, September 2023-January 2024, MMWR Morb. Mortal. Wkly. Rep
Liu, Liu, Contributions of Drug Transporters to Blood-Placental Barrier, doi:10.1007/978-981-13-7647-4_11
Liu, Physiologically-based pharmacokinetic modeling of remdesivir and its metabolites in pregnant women with COVID-19, CPT Pharmacomet. Syst. Pharmacol
Liu, Yang, Yang, Jing, Zhao et al., Clinical characteristics and pharmacokinetics of PAXLOVID in COVID-19 patients with hematological tumor, Med Rev, doi:10.1515/mr-2023-0068
Liu, Zhu, Cao, Simultaneous determination of nirmatrelvir and ritonavir in human plasma using LC-MS/MS and its pharmacokinetic application in healthy Chinese volunteers, Biomed Chromatogr, doi:10.1002/bmc.5456
Loebstein, Lalkin, Koren, Pharmacokinetic Changes During Pregnancy and Their Clinical Relevance, Clin Pharmacokinet, doi:10.2165/00003088-199733050-00002
Loza, Farias, Gavin, Wagner, Hammer et al., Short-term Pregnancy Outcomes After Nirmatrelvir-Ritonavir Treatment for Mild-to-Moderate Coronavirus Disease 2019 (COVID-19), Obstet Gynecol, doi:10.1097/AOG.0000000000004900
Loza, Farias, Gavin, Wagner, Hammer et al., Short-term Pregnancy Outcomes After Nirmatrelvir-Ritonavir Treatment for Mild-to-Moderate Coronavirus Disease 2019 (COVID-19), Obstet Gynecol, doi:10.1097/AOG.0000000000004900
Loza, Short-term Pregnancy Outcomes After Nirmatrelvir-Ritonavir Treatment for Mild-to-Moderate Coronavirus Disease 2019 (COVID-19), Obstet. Gynecol
Lu, Cai, Hao, Nirmatrelvir/Ritonavir for hemodialysis patients with COVID-19, Front Pharmacol, doi:10.3389/fphar.2023.1161897
Luxford, Kellaway, Pharmacokinetics of digoxin in pregnancy, Eur. J. Clin. Pharmacol
Lye, Effect of oxygen on multidrug resistance in the first trimester human placenta, Placenta
Lye, Impact of Bacterial and Viral Challenge on Multidrug Resistance in First-and Third-Trimester Human Placenta, Am. J. Pathol
Ma, Yang, Jiang, Multiple SLC and ABC Transporters Contribute to the Placental Transfer of Entecavir, Drug Metab Dispos Biol Fate Chem, doi:10.1124/dmd.116.073304
Magawa, COVID-19 during pregnancy could potentially affect placental function, J. Matern.-Fetal Neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obstet
Maltepe, Bakardjiev, Fisher, The placenta: transcriptional, epigenetic, and physiological integration during development, J Clin Invest, doi:10.1172/JCI41211
Maltepe, Fisher, Placenta: The Forgotten Organ, Annu. Rev. Cell Dev. Biol
Mao, BCRP/ABCG2 in the Placenta: Expression, Function and Regulation, Pharm. Res
Mao, Chen, These concentrations were chosen since 2.97 µg/mL was the Cmax observed in healthy non pregnant participants during phase 1 clinical trials 8 . At the end of each successful experiment, the perfused lobule was weighed, doi:10.1515/mr-2022-0025(1Oxygentransfer=
Marikawa, Alarcon, An active metabolite of the anti-COVID-19 drug molnupiravir impairs mouse preimplantation embryos at clinically relevant concentrations, Reprod. Toxicol
Mason, ATP-Binding Cassette Transporter Expression in Human Placenta as a Function of Pregnancy Condition, Drug Metab. Dispos
Mathias, Maggio-Price, Lai, Gupta, Unadkat, Changes in Pharmacokinetics of Anti-HIV Protease Inhibitors during Pregnancy: The Role of CYP3A and P-glycoprotein, J. Pharmacol. Exp. Ther
Mathiesen, Placental transfer of pesticides studied in human placental perfusion, Basic Clin. Pharmacol. Toxicol
Mathiesen, Quality assessment of a placental perfusion protocol, Reprod. Toxicol. Elmsford N
Meyer Zu Schwabedissen, Variable expression of MRP2 (ABCC2) in human placenta: influence of gestational age and cellular differentiation, Drug Metab. Dispos. Biol. Fate Chem
Miller, Jessee, Barrish, Gilbert, Manson, Pharmacokinetic studies of enalaprilat in the in vitro perfused human placental lobule system, Teratology
Miller, Mcgrath, Zorn, Ekins, Wright et al., Remdesivir and EIDD-1931 Interact with Human Equilibrative Nucleoside Transporters 1 and 2: Implications for Reaching SARS-CoV-2 Viral Sanctuary Sites, Mol Pharmacol, doi:10.1124/molpharm.121.000333
Miller, Remdesivir and EIDD-1931 Interact with Human Equilibrative Nucleoside Transporters 1 and 2: Implications for Reaching SARS-CoV-2 Viral Sanctuary Sites, Mol. Pharmacol
Miller, Wier, Maulik, Di Sant' Agnese, Human Placenta in vitro: Characterization during 12 h of Dual Perfusion
Miller, Wier, Maulik, Di Sant' Agnese, Human Placenta in vitro: Characterization during 12 h of Dual Perfusion, Contributions to Gynecology and Obstetrics, doi:10.1159/000410672
Miranda, Mckinzie, Dobrovolsky, Revollo, Evaluation of the mutagenic effects of Molnupiravir and N4-hydroxycytidine in bacterial and mammalian cells by HiFi sequencing, Environ Mol Mutagen, doi:10.1002/em.22510
Miranda, Mckinzie, Dobrovolsky, Revollo, Evaluation of the mutagenic effects of Molnupiravir and N4-hydroxycytidine in bacterial and mammalian cells by HiFi sequencing, Environ. Mol. Mutagen
Mitchell, Medication use during pregnancy, with particular focus on prescription drugs: 1976-2008, Am. J. Obstet. Gynecol
Momper, Nikanjam, Best, Mirochnick, Capparelli et al., Dolutegravir Plasma Protein Binding and Unbound Concentrations During Pregnancy and Postpartum, J Acquir Immune Defic Syndr
Morgan, Drug disposition in mother and foetus, Clin Exp Pharmacol Physiol, doi:10.1111/j.1440-1681.1997.tb02707.x
Mose, Meta-analysis of data from human ex vivo placental perfusion studies on genotoxic and immunotoxic agents within the integrated European project NewGeneris, Placenta
Myllynen, Immonen, Kummu, Vähäkangas, Developmental expression of drug metabolizing enzymes and transporter proteins in human placenta and fetal tissues, Expert Opin. Drug Metab. Toxicol
Najjar-Debbiny, Gronich, Weber, Effectiveness of Paxlovid in Reducing Severe Coronavirus Disease 2019 and Mortality in High-Risk Patients, Clin Infect Dis Off Publ Infect Dis Soc Am, doi:10.1093/cid/ciac443
Nau, Clinical pharmacokinetics in pregnancy and perinatology. II. Penicillins, Dev. Pharmacol. Ther
Neumanova, Cerveny, Ceckova, Staud, Interactions of tenofovir and tenofovir disoproxil fumarate with drug efflux transporters ABCB1, ABCG2, and ABCC2; role in transport across the placenta, AIDS Lond. Engl
Neumanova, Cerveny, Greenwood, Ceckova, Staud, Effect of drug efflux transporters on placental transport of antiretroviral agent abacavir, Reprod Toxicol, doi:10.1016/j.reprotox.2015.07.070
Oseshnyuk, Nikiforova, Boroduleva, Yu, Bioequivalence study of generic nirmatrelvir in healthy volunteers, Farmatsiya Farmakol, doi:10.19163/2307-9266-2023-11-1-62-71
Owen, Allerton, Anderson, An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19, Science, doi:10.1126/science.abl4784
Painter, Human Safety, Tolerability, and Pharmacokinetics of Molnupiravir, a Novel Broad-Spectrum Oral Antiviral Agent with Activity against SARS-CoV-2, Antimicrob. Agents Chemother
Panigel, Pascaud, Brun, Radioangiographic study of circulation in the villi and intervillous space of isolated human placental cotyledon kept viable by perfusion, J. Physiol
Pasanen, The expression and regulation of drug metabolism in human placenta, Adv. Drug Deliv. Rev
Paxlovid Monograph, nirmatrelvir tablets; ritonavir tablets) | Pfizer Canada
Paxton, Briant, Alpha 1-acid glycoprotein concentrations and propranolol binding in elderly patients with acute illness, Br. J. Clin. Pharmacol
Petrovic, Kojovic, Cressman, Piquette-Miller, Maternal bacterial infections impact expression of drug transporters in human placenta, Int. Immunopharmacol
Pfeifer, Regulation of human placental drug transporters in HCV infection and their influence on direct acting antiviral medications, Placenta
Pilarska, Bizon, Sawicki, Influence of COVID-19 infection on placental function, Ginekol. Pol
Pinheiro, Stika, Drugs in pregnancy: Pharmacologic and physiologic changes that affect clinical care, Semin. Perinatol
Poulsen, Rytting, Mose, Knudsen, Modeling placental transport: Correlation of in vitro BeWo cell permeability and ex vivo human placental perfusion, Toxicol. In Vitro
Qu, Su, Xiang, Population pharmacokinetic modeling and simulation for nirmatrelvir exposure assessment in Chinese older patients with COVID-19 infection, Eur J Pharm Sci Off J Eur Fed Pharm Sci, doi:10.1016/j.ejps.2023.106535
Ring, Ghabrial, Ching, Smallwood, Morgan, Fetal hepatic drug elimination, Pharmacol. Ther
Rough, Zidovudine use in pregnancy and congenital malformations, AIDS Lond. Engl
Rubinchik-Stern, Eyal, Drug Interactions at the Human Placenta: What is the Evidence?, Front Pharmacol, doi:10.3389/fphar.2012.00126
Rubinchik-Stern, Eyal, Drug Interactions at the Human Placenta: What is the Evidence?, Front. Pharmacol
Rumpler, Sams, Colahan, Pharmacokinetics of glycopyrrolate following intravenous administration in the horse: Glycopyrrolate pharmacokinetics in the horse, J. Vet. Pharmacol. Ther
Rytting, Audus, Novel Organic Cation Transporter 2-Mediated Carnitine Uptake in Placental Choriocarcinoma (BeWo) Cells, J. Pharmacol. Exp. Ther
Sagawa, Lin, Jaini, Di, Physiologically-Based Pharmacokinetic Modeling of PAXLOVID TM with First-Order Absorption Kinetics, Pharm Res, doi:10.1007/s11095-023-03538-5
Saravolatz, Depcinski, Sharma, Molnupiravir and Nirmatrelvir-Ritonavir: Oral COVID Antiviral Drugs, Clin Infect Dis Off Publ Infect Dis Soc Am, doi:10.1093/cid/ciac180
Saravolatz, Depcinski, Sharma, Molnupiravir and Nirmatrelvir-Ritonavir: Oral COVID Antiviral Drugs, Clin Infect Dis Off Publ Infect Dis Soc Am, doi:10.1093/cid/ciac180
Sata, Functional Analysis of Organic Cation Transporter 3 Expressed in Human Placenta, J. Pharmacol. Exp. Ther
Schalkwijk, Buaben, Freriksen, Prediction of Fetal Darunavir Exposure by Integrating Human Ex-Vivo Placental Transfer and Physiologically Based Pharmacokinetic Modeling, Clin Pharmacokinet, doi:10.1007/s40262-017-0583-8
Schalkwijk, The pharmacokinetics of abacavir 600 mg once daily in HIV-1positive pregnant women, AIDS
Schmolling, Jung, Reinsberg, Schlebusch, Digoxin transfer across the isolated placenta is influenced by maternal and fetal albumin concentrations, Reprod. Fertil. Dev
Schneider, Dancis, None, J
Schneider, Heilmann, Harenberg, Placental transfer of low-molecular weight heparin, Geburtshilfe Frauenheilkd
Schneider, Panigel, Dancis, Transfer across the perfused human placenta of antipyrine, sodium, and leucine, Am. J. Obstet. Gynecol
Sentilhes, Coronavirus disease 2019 in pregnancy was associated with maternal morbidity and preterm birth, Am. J. Obstet. Gynecol
Sentilhes, Marcillac, Jouffrieau, Coronavirus disease 2019 in pregnancy was associated with maternal morbidity and preterm birth, Am J Obstet Gynecol, doi:10.1016/j.ajog.2020.06.022
Sessa, Filardo, Masciullo, SARS-CoV-2 Infection in Pregnancy: Clues and Proof of Adverse Outcomes, Int J Environ Res Public Health, doi:10.3390/ijerph20032616
Shanes, Placental Pathology in COVID-19, Am. J. Clin. Pathol
Sicard, Squires, Mullah, Daley, Characteristics and clinical outcomes of nirmatrelvir/ritonavir (PaxlovidTM) recipients in Canada, 2022: a descriptive cohort study, Can Commun Dis Rep, doi:10.14745/ccdr.v49i10a05
Singh, Singh, Singh, Misra, An updated practical guideline on use of molnupiravir and comparison with agents having emergency use authorization for treatment of COVID-19, Diabetes Metab. Syndr
Singh, Toussi, Hackman, Innovative Randomized Phase I Study and Dosing Regimen Selection to Accelerate and Inform Pivotal COVID-19 Trial of Nirmatrelvir, Clin Pharmacol Ther, doi:10.1002/cpt.2603
Singh, Walker, Kadar, Metabolism and Excretion of Nirmatrelvir in Humans Using Quantitative Fluorine Nuclear Magnetic Resonance Spectroscopy: A Novel Approach for Accelerating Drug Development, Clin Pharmacol Ther, doi:10.1002/cpt.2683
Smith, Maternal and neonatal outcomes associated with COVID-19 infection: A systematic review, PLOS ONE
Smith, Seo, Warty, Maternal and neonatal outcomes associated with COVID-19 infection: A systematic review, PLOS ONE, doi:10.1371/journal.pone.0234187
Soares, Chakraborty, Rumi, Konno, Renaud, Rat placentation: an experimental model for investigating the hemochorial maternal-fetal interface, Placenta, doi:10.1016/j.placenta.2011.11.026
Song, Toward Greater Insights on Applications of Modeling and Simulation in Pregnancy, Curr. Drug Metab
Sousa Mendes, A Physiologically-Based Pharmacokinetic Model to Predict Human Fetal Exposure for a Drug Metabolized by Several CYP450 Pathways, Clin. Pharmacokinet
Sousa Mendes, Hirt, Vinot, Prediction of human fetal pharmacokinetics using ex vivo human placenta perfusion studies and physiologically based models: Human fetal concentration prediction, Br J Clin Pharmacol, doi:10.1111/bcp.12815
Spiess, Transplacental passage of hyperforin, hypericin, and valerenic acid, Front. Pharmacol
Staud, Cerveny, Ceckova, Pharmacotherapy in pregnancy; effect of ABC and SLC transporters on drug transport across the placenta and fetal drug exposure, J. Drug Target
Stock, Pregnancy outcomes after SARS-CoV-2 infection in periods dominated by delta and omicron variants in Scotland: a population-based cohort study, Lancet Respir. Med
Straka, Mercury toxicokinetics of the healthy human term placenta involve amino acid transporters and ABC transporters, Toxicology
Sun, Expression of the Multidrug Resistance P-Glycoprotein, (ABCB1 glycoprotein) in the Human Placenta Decreases with Advancing Gestation, Placenta
Syme, Paxton, Keelan, Drug Transfer and Metabolism by the Human Placenta, Clin Pharmacokinet, doi:10.2165/00003088-200443080-00001
Syme, Paxton, Keelan, Drug Transfer and Metabolism by the Human Placenta, Clin. Pharmacokinet
Szeto, Maternal-Fetal Pharmacokinetics and Fetal Dose-Response Relationships, Ann. N. Y. Acad. Sci
Timircan, Bratosin, Vidican, Exploring Pregnancy Outcomes Associated with SARS-CoV-2 Infection, Medicina (Mex), doi:10.3390/medicina57080796
Tomi, Nishimura, Nakashima, Mother-to-fetus transfer of antiviral drugs and the involvement of transporters at the placental barrier, J Pharm Sci, doi:10.1002/jps.22642
Tomson, Ohman, Vitols, Lamotrigine in pregnancy and lactation: a case report, Epilepsia
Toure, Oral Nirmatrelvir-Ritonavir Use and Clinical Outcomes in Pregnant Patients With Coronavirus Disease 2019 (COVID-19), Obstet. Gynecol
Toure, Panakam, Johns, Butler, Tuomala et al., Oral Nirmatrelvir-Ritonavir Use and Clinical Outcomes in Pregnant Patients With Coronavirus Disease 2019 (COVID-19), Obstet Gynecol, doi:10.1097/AOG.0000000000005471
Toure, Panakam, Johns, Butler, Tuomala et al., Oral Nirmatrelvir-Ritonavir Use and Clinical Outcomes in Pregnant Patients With Coronavirus Disease 2019 (COVID-19), Obstet Gynecol, doi:10.1097/AOG.0000000000005471
Toussi, Neutel, Navarro, Pharmacokinetics of Oral Nirmatrelvir/Ritonavir, a Protease Inhibitor for Treatment of COVID-19, in Subjects With Renal Impairment, Clin Pharmacol Ther, doi:10.1002/cpt.2688
Toussi, Pharmacokinetics of Oral Nirmatrelvir/Ritonavir, a Protease Inhibitor for Treatment of COVID-19, in Subjects With Renal Impairment, Clin. Pharmacol. Ther
Tracy, Venkataramanan, Glover, Caritis, Temporal changes in drug metabolism (CYP1A2, CYP2D6 and CYP3A Activity) during pregnancy, Am. J. Obstet. Gynecol
Ugele, Bahn, Rex-Haffner, Functional differences in steroid sulfate uptake of organic anion transporter 4 (OAT4) and organic anion transporting polypeptide 2B1 (OATP2B1) in human placenta, J. Steroid Biochem. Mol. Biol
Vincenzetti, Cambi, Balducci, Natalini, Volpini et al., Human placenta cytidine deaminase: a zinc metalloprotein, IUBMB Life, doi:10.1080/15216549700202871
Vricella, Emerging understanding and measurement of plasma volume expansion in pregnancy, Am J Clin Nutr, doi:10.3945/ajcn.117.155903
Vricella, Emerging understanding and measurement of plasma volume expansion in pregnancy, Am. J. Clin. Nutr
Vähäkangas, Myllynen, Experimental methods to study human transplacental exposure to genotoxic agents, Mutat. Res. Toxicol. Environ. Mutagen
Wang, Ding, Wang, The Development of an Oral Solution Containing Nirmatrelvir and Ritonavir and Assessment of Its Pharmacokinetics and Stability, Pharmaceutics, doi:10.3390/pharmaceutics16010109
Ward, Drug therapy of the fetus, J. Clin. Pharmacol
Westberg, Su, Zou, Design of SARS-CoV-2 protease inhibitors with improved affinity and reduced sensitivity to mutations, bioRxiv, doi:10.1101/2023.07.19.549739
Wildman, Review: Toward an integrated evolutionary understanding of the mammalian placenta, Placenta, doi:10.1016/j.placenta.2011.01.005
Williams, Davison, Chronic kidney disease in pregnancy, BMJ
Wong, Lau, Chung, Nirmatrelvir/ritonavir use in pregnant women with SARS-CoV-2 Omicron infection: a target trial emulation, Nat Med, doi:10.1038/s41591-023-02674-0
Wong, Lau, Chung, Nirmatrelvir/ritonavir use in pregnant women with SARS-CoV-2 Omicron infection: a target trial emulation, Nat Med, doi:10.1038/s41591-023-02674-0
Wong, Nirmatrelvir/ritonavir use in pregnant women with SARS-CoV-2 Omicron infection: a target trial emulation, Nat. Med
Wood, Wood, Changes in plasma drug binding and α1-acid glycoprotein in mother and newborn infant, Clin. Pharmacol. Ther
Wu, Fang, Wang, Deng, Liao, Management of Pregnancy during the COVID-19 Pandemic, Glob. Chall
Xu Z Liang, Li C Jian, Qian, A validated LC-MS/MS method for determination of six Anti-SARS-CoV-2 drugs in plasma and its application for a pharmacokinetic study in rats, J Chromatogr B, doi:10.1016/j.jchromb.2024.124038
Yamashita, Markert, Overview of Drug Transporters in Human Placenta, Int J Mol Sci, doi:10.3390/ijms222313149
Yan, Coronavirus disease 2019 in pregnant women: a report based on 116 cases, Am. J. Obstet. Gynecol
Yang, You, Shu, Design, synthesis and biological evaluation of peptidomimetic benzothiazolyl ketones as 3CLpro inhibitors against SARS-CoV-2, Eur J Med Chem, doi:10.1016/j.ejmech.2023.115512
Yeboah, Expression of breast cancer resistance protein (BCRP/ABCG2) in human placenta throughout gestation and at term before and after labor, Can. J. Physiol. Pharmacol
Young, Allen, Audus, Efflux transporters of the human placenta, Adv. Drug Deliv. Rev
Zaigham, Andersson, Maternal and perinatal outcomes with COVID-19: A systematic review of 108 pregnancies, Acta Obstet. Gynecol. Scand
Zeng, Chen, Jiang, Population pharmacokinetics and pharmacodynamics of nirmatrelvir in Chinese patients with COVID-19, Fundam Clin Pharmacol, doi:10.1111/fcp.12989
Zeng, Multiple Drug Transporters Contribute to the Placental Transfer of Emtricitabine, Antimicrob. Agents Chemother, doi:10.1128/aac.00199-19
Zeng, Zhou, Peng, Dong, Qin, The prevention and treatment of COVID-19 in patients treated with hemodialysis, Eur J Med Res, doi:10.1186/s40001-023-01389-9
Zhang, Bastian, Zhao, Pregnancy Alters CYP-and UGT-Mediated Metabolism of Buprenorphine, Ther Drug Monit, doi:10.1097/FTD.0000000000000724
Zhang, Kalluri, Bastian, Gestational changes in buprenorphine exposure: A physiologically-based pharmacokinetic analysis, Br J Clin Pharmacol, doi:10.1111/bcp.13642
Zhang, Pregnancy Alters CYP-and UGT-Mediated Metabolism of Buprenorphine, Ther. Drug Monit
Zhao, Gockenbach, Grimstein, Characterization of Plasma Protein Alterations in Pregnant and Postpartum Individuals Living With HIV to Support Physiologically-Based Pharmacokinetic Model Development, Front Pediatr, doi:10.3389/fped.2021.721059
Zhao, Xiang, Han, Simultaneous quantification of nirmatrelvir/ritonavir in human serum by LC-HRMS, J Pharm Biomed Anal, doi:10.1016/j.jpba.2023.115796
Zheng, Safety, efficacy, and pharmacokinetics of nirmatrelvir and ritonavir in patients with severe COVID-19 and renal impairment: A case report, Int. J. Obstet. Anesth
Zhou, Hill, Sarkar, β-d-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells, J Infect Dis, doi:10.1093/infdis/jiab247
Zhou, β-d-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells, J. Infect. Dis
Zhu, Fu, You, Design, synthesis and biological evaluation of covalent peptidomimetic 3CL protease inhibitors containing nitrile moiety, Bioorg Med Chem, doi:10.1016/j.bmc.2023.117316