Clinical efficacy of inhaled corticosteroids in patients with coronavirus disease 2019: A living review and meta-analysis
et al., PLOS ONE, doi:10.1371/journal.pone.0294872, Nov 2023
Budesonide for COVID-19
27th treatment shown to reduce risk in
September 2021, now with p = 0.0000042 from 14 studies, recognized in 10 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
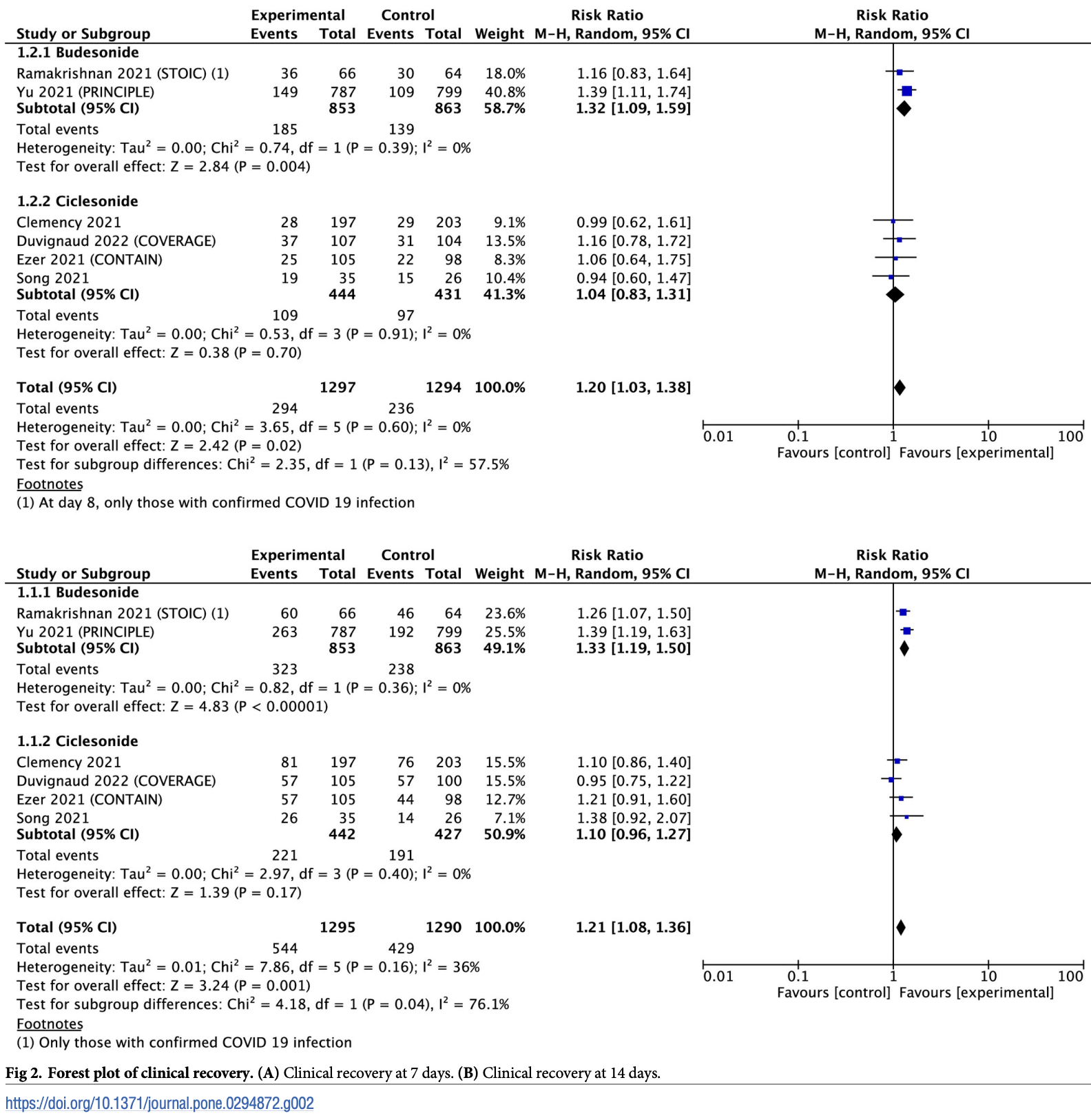
Systematic review and meta-analysis of 8 randomized controlled trials (2,788 patients) comparing inhaled corticosteroids (ICS) to placebo or usual care for the treatment of COVID-19. Overall, ICS treatment was associated with improved clinical recovery at 7 days (risk ratio 1.20) and 14 days (risk ratio 1.21), but improvements for hospitalization, mortality, ICU admission, and ventilation were not statistically significant. In subgroup analyses, the ICS budesonide improved clinical recovery but ciclesonide did not.
There are significant differences between budesonide and ciclesonide that
may contribute to the lower efficacy of ciclesonide.
Pharmacokinetics and pharmacodynamics: budesonide has a more
rapid onset of action and a shorter half-life, which can lead to a more
immediate and potent anti-inflammatory effect. Ciclesonide is a prodrug
that requires conversion to its active form, which may delay therapeutic
effects.
Receptor affinity and potency: budesonide has a higher affinity for
glucocorticoid receptors and a higher potency compared to ciclesonide.
This can result in more effective suppression of inflammation and immune
modulation in the respiratory tract, potentially leading to better
clinical outcomes.
Bioavailability: budesonide typically has higher lung bioavailability
compared to ciclesonide.
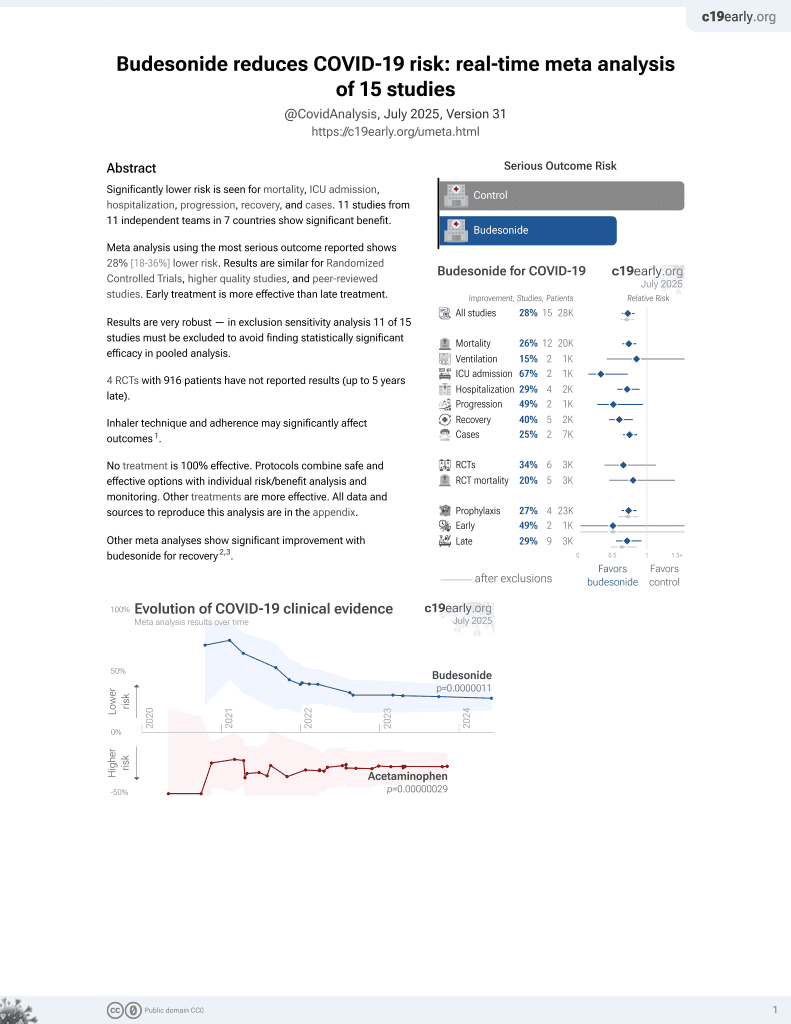
Currently there are 14 budesonide for COVID-19 studies, showing 25% lower mortality [14‑35%], 15% lower ventilation [-73‑58%], 67% lower ICU admission [28‑85%], 29% lower hospitalization [10‑43%], and 25% fewer cases [14‑35%].
|
risk of hospitalization, 55.0% lower, RR 0.45, p = 0.26.
|
|
risk of no recovery, 24.8% lower, RR 0.75, p < 0.001, inverted to make RR<1 favor treatment.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Yu et al., 28 Nov 2023, peer-reviewed, 7 authors.
Clinical efficacy of inhaled corticosteroids in patients with coronavirus disease 2019: A living review and meta-analysis
PLOS ONE, doi:10.1371/journal.pone.0294872
Inhaled corticosteroids are known to be relatively safe for long-term use in inflammatory respiratory diseases and it has been repurposed as one of the potential therapies for outpatients with coronavirus disease 2019 (COVID-19). However, inhaled corticosteroids have not been accepted for COVID-19 as a standard therapy because of its lack of proven benefits. Therefore, this study aimed to evaluate the effectiveness of inhaled corticosteroids in patients with COVID-19. Randomized controlled trials comparing the efficacy of inhaled corticosteroid treatment in patients with COVID-19 were identified through literature electronic database searches up to March 10, 2023. Meta-analyses were conducted for predefined outcomes, and the certainty of evidence was graded using the grading of recommendations, assessment, development, and evaluation approach. Overall, seven trials (eight articles) were included in this systematic review. Compared with usual care, inhaled corticosteroids was associated with significantly improved clinical recovery at 7 and 14 days in patients with COVID-19. In subgroup analysis, only budesonide showed significant efficacy in clinical recovery, whereas no significant benefit was observed for ciclesonide. Moreover, inhaled corticosteroids use was not significantly associated with all-cause hospitalization, all-cause mortality, admission to intensive care unit, or the use of mechanical ventilation. Our systematic review used evidence with very low to moderate certainty. Although based on limited evidence, our results suggest that inhaled corticosteroids treatment, especially budesonide, improves the clinical recovery of patients with COVID-19. More trials and meta-analyses are needed to assess the efficacy of inhaled corticosteroids for COVID-19 treatment.
References
Brodin, Tornhammar, Ueda, Krifors, Westerlund et al., Inhaled ciclesonide in adults hospitalised with COVID-19: a randomised controlled open-label trial (HALT COVID-19), BMJ open, doi:10.1136/bmjopen-2022-064374
Clemency, Varughese, Gonzalez-Rojas, Morse, Phipatanakul et al., Efficacy of inhaled ciclesonide for outpatient treatment of adolescents and adults with symptomatic COVID-19: a randomized clinical trial, JAMA internal medicine, doi:10.1001/jamainternmed.2021.6759
Cumpston, Li, Page, Chandler, Welch et al., Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions, Cochrane Database Syst Rev, doi:10.1002/14651858.ED000142
Doi, Tomita, Okura, Matsuyama, Frequent occurrence of mutations in nsp3 and nsp4 of SARS-CoV-2, presumably caused by the inhaled asthma drug ciclesonide, PNAS Nexus, doi:10.1093/pnasnexus/pgac197
Donahue, Weiss, Livingston, Goetsch, Greineder et al., Inhaled steroids and the risk of hospitalization for asthma, JAMA-JOURNAL OF THE AMERICAN MEDICAL ASSOCIATION
Duvignaud, Lhomme, Onaisi, Sitta, Gelley et al., Inhaled ciclesonide for outpatient treatment of COVID-19 in adults at risk of adverse outcomes: a randomised controlled trial (COV-ERAGE), Clinical Microbiology and Infection, doi:10.1016/j.cmi.2022.02.031
Ezer, Belga, Daneman, Smith, Daniels, Inhaled and intranasal ciclesonide for the treatment of covid-19 in adult outpatients: CONTAIN phase II randomised controlled trial, BMJ (Clinical Research, doi:10.1136/bmj-2021-068060
Griesel, Wagner, Mikolajewska, Stegemann, Fichtner et al., Inhaled corticosteroids for the treatment of COVID-19, Cochrane Database of Systematic Reviews (Online
Guyatt, Thorlund, Oxman, Walter, Patrick et al., GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles-continuous outcomes, J Clin Epidemiol, doi:10.1016/j.jclinepi.2012.08.001
Horby, Lim, Emberson, Mafham, Bell et al., Dexamethasone in Hospitalized Patients with Covid-19, New England Journal of Medicine, doi:10.1056/NEJMoa2021436
Irwin, Baumann, Bolser, Boulet, Braman et al., Diagnosis and management of cough executive summary: ACCP evidence-based clinical practice guidelines, Chest, doi:10.1378/chest.129.1_suppl.1S
Jeon, Ko, Lee, Choi, Byun et al., Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs, Antimicrobial Agents and Chemotherapy, doi:10.1128/AAC.00819-20
Kelly, Comparison of inhaled corticosteroids: an update, ANNALS OF PHARMACOTHERAPY, doi:10.1345/aph.1L546
Kew, Seniukovich, Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease, Cochrane Database of Systematic Reviews (Online, doi:10.1002/14651858.CD010115.pub2
Latorre, Novelli, Vagaggini, Braido, Papi et al., Differences in the efficacy and safety among inhaled corticosteroids (ICS)/long-acting beta2-agonists (LABA) combinations in the treatment of chronic obstructive pulmonary disease (COPD): role of ICS, Pulmonary Pharmacology & Therapeutics, doi:10.1016/j.pupt.2014.10.006
Lee, Pickard, Au, Bartle, Weiss, Risk for death associated with medications for recently diagnosed chronic obstructive pulmonary disease, Annals of Internal Medicine, doi:10.7326/0003-4819-149-6-200809160-00004
Liberati, Altman, Tetzlaff, Mulrow, Gøtzsche et al., The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration, Journal of clinical epidemiology, doi:10.1016/j.jclinepi.2009.06.006
Masjedi, Esmaeil, Saffaei, Naeini, Pourazizi et al., Cytokine Indexes in Pemphigus Vulgaris: Perception of Its Immunpathogenesis and Hopes for Non-Steroidal Treatment, Iranian journal of pharmaceutical research
Matsuyama, Kawase, Nao, Shirato, Ujike et al., The inhaled steroid ciclesonide blocks SARS-CoV-2 RNA replication by targeting the viral replication-transcription complex in cultured cells, Journal of Virology, doi:10.1128/JVI.01648-20
Mattos-Silva, Felix, Silva, Robba, Battaglini et al., Pros and cons of corticosteroid therapy for COVID-19 patients, Respiratory Physiology & Neurobiology, doi:10.1016/j.resp.2020.103492
Meduri, Muthiah, Carratu, Eltorky, Chrousos, Nuclear factor-kappaB-and glucocorticoid receptor alpha-mediated mechanisms in the regulation of systemic and pulmonary inflammation during sepsis and acute respiratory distress syndrome. Evidence for inflammation-induced target tissue resistance to glucocorticoids, Neuroimmunomodulation, doi:10.1159/000091126
Milne, Li, Yang, Filho, ´ndez Cordero et al., Inhaled corticosteroids downregulate SARS-CoV-2-related genes in COPD: results from a randomised controlled trial, Eur Respir J, doi:10.1183/13993003.00130-2021
Moghadasi, Shabany, Heidari, Eskandarieh, Can pulse steroid therapy increase the risk of infection by COVID-19 in patients with multiple sclerosis?, Clinical Neurology and Neurosurgery, doi:10.1016/j.clineuro.2021.106563
Mutch, Nave, Mccracken, Zech, Williams, The role of esterases in the metabolism of ciclesonide to desisobutyryl-ciclesonide in human tissue, Biochemical Pharmacology, doi:10.1016/j.bcp.2007.01.031
Nave, Clinical pharmacokinetic and pharmacodynamic profile of inhaled ciclesonide, Clinical Pharmacokinetics, doi:10.2165/00003088-200948040-00002
Nieto, Mazon, Pamies, Linana, Lanuza et al., Adverse effects of inhaled corticosteroids in funded and nonfunded studies, Archives of Internal Medicine, doi:10.1001/archinte.167.19.2047
Peters, Sajuthi, Deford, Christenson, Rios et al., COVID-19-related Genes in Sputum Cells in Asthma. Relationship to Demographic Features and Corticosteroids, Am J Respir Crit Care Med, doi:10.1164/rccm.202003-0821OC
Ramakrishnan, Nicolau, Jr, Langford, Mahdi et al., Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial, The Lancet Respiratory Medicine, doi:10.1016/S2213-2600(21)00160-0
Singanayagam, Glanville, Girkin, Ching, Marcellini et al., Corticosteroid suppression of antiviral immunity increases bacterial loads and mucus production in COPD exacerbations, Nature Communications
Song, Yoon, Seo, Lee, Eom et al., Ciclesonide inhaler treatment for mild-tomoderate COVID-19: a randomized, open-label, phase 2 trial, Journal of Clinical Medicine, doi:10.3390/jcm10163545
Southworth, Pattwell, Khan, Mowbray, Strieter et al., Increased type 2 inflammation post rhinovirus infection in patients with moderate asthma, Cytokine, doi:10.1016/j.cyto.2019.154857
Sterne, Murthy, Diaz, Slutsky, Villar et al., Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis, JAMA-JOURNAL OF THE AMERICAN MEDICAL ASSOCIATION, doi:10.1001/jama.2020.17023
Terada-Hirashima, Suzuki, Tsujimoto, Hamamoto, Uemura et al., Impact of inhaled ciclesonide on asymptomatic or mild COVID-19: A randomized trial, Drug Discov Ther, doi:10.5582/ddt.2022.01068
Van Rensen, Straathof, Veselic-Charvat, Zwinderman, Bel et al., Effect of inhaled steroids on airway hyperresponsiveness, sputum eosinophils, and exhaled nitric oxide levels in patients with asthma, Thorax, doi:10.1136/thx.54.5.403
Yang, Du, Chen, Jiang, Xu, Inhaled corticosteroids and risk of pneumonia in patients with chronic obstructive pulmonary disease: a meta-analysis of randomized controlled trials, International Immunopharmacology, doi:10.1016/j.intimp.2019.105950
Yang, Zhang, Chen, Lin, Zeng et al., Inhaled corticosteroids and risk of upper respiratory tract infection in patients with asthma: a meta-analysis, Infection, doi:10.1007/s15010-018-1229-y
Yu, Bafadhel, Dorward, Hayward, Saville et al., Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial, Lancet, doi:10.1016/S0140-6736(21)01744-X
Zhu, Zhang, Li, Yang, Song, A Novel Coronavirus from Patients with Pneumonia in China, 2019, New England Journal of Medicine, doi:10.1056/NEJMoa2001017
DOI record:
{
"DOI": "10.1371/journal.pone.0294872",
"ISSN": [
"1932-6203"
],
"URL": "http://dx.doi.org/10.1371/journal.pone.0294872",
"abstract": "<jats:p>Inhaled corticosteroids are known to be relatively safe for long-term use in inflammatory respiratory diseases and it has been repurposed as one of the potential therapies for outpatients with coronavirus disease 2019 (COVID-19). However, inhaled corticosteroids have not been accepted for COVID-19 as a standard therapy because of its lack of proven benefits. Therefore, this study aimed to evaluate the effectiveness of inhaled corticosteroids in patients with COVID-19. Randomized controlled trials comparing the efficacy of inhaled corticosteroid treatment in patients with COVID-19 were identified through literature electronic database searches up to March 10, 2023. Meta-analyses were conducted for predefined outcomes, and the certainty of evidence was graded using the grading of recommendations, assessment, development, and evaluation approach. Overall, seven trials (eight articles) were included in this systematic review. Compared with usual care, inhaled corticosteroids was associated with significantly improved clinical recovery at 7 and 14 days in patients with COVID-19. In subgroup analysis, only budesonide showed significant efficacy in clinical recovery, whereas no significant benefit was observed for ciclesonide. Moreover, inhaled corticosteroids use was not significantly associated with all-cause hospitalization, all-cause mortality, admission to intensive care unit, or the use of mechanical ventilation. Our systematic review used evidence with very low to moderate certainty. Although based on limited evidence, our results suggest that inhaled corticosteroids treatment, especially budesonide, improves the clinical recovery of patients with COVID-19. More trials and meta-analyses are needed to assess the efficacy of inhaled corticosteroids for COVID-19 treatment.</jats:p>",
"author": [
{
"affiliation": [],
"family": "Yu",
"given": "Su-Yeon",
"sequence": "first"
},
{
"affiliation": [],
"family": "Choi",
"given": "Miyoung",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ryoo",
"given": "Seungeun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cheong",
"given": "Chelim",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Huh",
"given": "Kyungmin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yoon",
"given": "Young Kyung",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4025-4542",
"affiliation": [],
"authenticated-orcid": true,
"family": "Jeong",
"given": "Su Jin",
"sequence": "additional"
}
],
"container-title": "PLOS ONE",
"container-title-short": "PLoS ONE",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"www.plosone.org"
]
},
"created": {
"date-parts": [
[
2023,
11,
28
]
],
"date-time": "2023-11-28T18:35:04Z",
"timestamp": 1701196504000
},
"deposited": {
"date-parts": [
[
2023,
11,
28
]
],
"date-time": "2023-11-28T18:35:29Z",
"timestamp": 1701196529000
},
"editor": [
{
"affiliation": [],
"family": "Gilchrist",
"given": "Francis John",
"sequence": "first"
}
],
"funder": [
{
"DOI": "10.13039/501100003670",
"award": [
"NECA-P-21-004"
],
"doi-asserted-by": "publisher",
"name": "National Evidence-based Healthcare Collaborating Agency"
},
{
"DOI": "10.13039/501100003670",
"award": [
"NECA-A-22-008"
],
"doi-asserted-by": "publisher",
"name": "National Evidence-based Healthcare Collaborating Agency"
},
{
"DOI": "10.13039/501100003670",
"award": [
"NECA-A-23-010"
],
"doi-asserted-by": "publisher",
"name": "National Evidence-based Healthcare Collaborating Agency"
},
{
"DOI": "10.13039/501100002510",
"award": [
"2023"
],
"doi-asserted-by": "publisher",
"name": "Kongju National University"
}
],
"indexed": {
"date-parts": [
[
2023,
11,
29
]
],
"date-time": "2023-11-29T01:04:12Z",
"timestamp": 1701219852810
},
"is-referenced-by-count": 0,
"issue": "11",
"issued": {
"date-parts": [
[
2023,
11,
28
]
]
},
"journal-issue": {
"issue": "11",
"published-online": {
"date-parts": [
[
2023,
11,
28
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
11,
28
]
],
"date-time": "2023-11-28T00:00:00Z",
"timestamp": 1701129600000
}
}
],
"link": [
{
"URL": "https://dx.plos.org/10.1371/journal.pone.0294872",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "340",
"original-title": [],
"page": "e0294872",
"prefix": "10.1371",
"published": {
"date-parts": [
[
2023,
11,
28
]
]
},
"published-online": {
"date-parts": [
[
2023,
11,
28
]
]
},
"publisher": "Public Library of Science (PLoS)",
"reference": [
{
"DOI": "10.1056/NEJMoa2001017",
"article-title": "A Novel Coronavirus from Patients with Pneumonia in China, 2019",
"author": "N Zhu",
"doi-asserted-by": "crossref",
"first-page": "727",
"issue": "8",
"journal-title": "New England Journal of Medicine",
"key": "pone.0294872.ref001",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2021436",
"article-title": "Dexamethasone in Hospitalized Patients with Covid-19",
"author": "P Horby",
"doi-asserted-by": "crossref",
"first-page": "693",
"issue": "8",
"journal-title": "New England Journal of Medicine",
"key": "pone.0294872.ref002",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.17023",
"article-title": "Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis",
"author": "JAC Sterne",
"doi-asserted-by": "crossref",
"first-page": "1330",
"issue": "13",
"journal-title": "JAMA-JOURNAL OF THE AMERICAN MEDICAL ASSOCIATION",
"key": "pone.0294872.ref003",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1159/000091126",
"article-title": "Nuclear factor-kappaB- and glucocorticoid receptor alpha- mediated mechanisms in the regulation of systemic and pulmonary inflammation during sepsis and acute respiratory distress syndrome. Evidence for inflammation-induced target tissue resistance to glucocorticoids",
"author": "GU Meduri",
"doi-asserted-by": "crossref",
"first-page": "321",
"issue": "6",
"journal-title": "Neuroimmunomodulation",
"key": "pone.0294872.ref004",
"volume": "12",
"year": "2005"
},
{
"article-title": "Cytokine Indexes in Pemphigus Vulgaris: Perception of Its Immunpathogenesis and Hopes for Non-Steroidal Treatment",
"author": "M Masjedi",
"first-page": "1223",
"issue": "3",
"journal-title": "Iranian journal of pharmaceutical research",
"key": "pone.0294872.ref005",
"volume": "16",
"year": "2017"
},
{
"DOI": "10.1016/j.resp.2020.103492",
"article-title": "Pros and cons of corticosteroid therapy for COVID-19 patients",
"author": "P Mattos-Silva",
"doi-asserted-by": "crossref",
"first-page": "103492",
"journal-title": "Respiratory Physiology & Neurobiology",
"key": "pone.0294872.ref006",
"volume": "280",
"year": "2020"
},
{
"DOI": "10.1016/j.clineuro.2021.106563",
"article-title": "Can pulse steroid therapy increase the risk of infection by COVID-19 in patients with multiple sclerosis?",
"author": "AN Moghadasi",
"doi-asserted-by": "crossref",
"first-page": "106563",
"journal-title": "Clinical Neurology and Neurosurgery",
"key": "pone.0294872.ref007",
"volume": "203",
"year": "2021"
},
{
"DOI": "10.1001/jama.1997.03540350037030",
"article-title": "Inhaled steroids and the risk of hospitalization for asthma",
"author": "JG Donahue",
"doi-asserted-by": "crossref",
"first-page": "887",
"issue": "11",
"journal-title": "JAMA-JOURNAL OF THE AMERICAN MEDICAL ASSOCIATION",
"key": "pone.0294872.ref008",
"volume": "277",
"year": "1997"
},
{
"DOI": "10.1183/13993003.00130-2021",
"article-title": "Inhaled corticosteroids downregulate SARS-CoV-2-related genes in COPD: results from a randomised controlled trial",
"author": "S Milne",
"doi-asserted-by": "crossref",
"issue": "1",
"journal-title": "Eur Respir J",
"key": "pone.0294872.ref009",
"volume": "58",
"year": "2021"
},
{
"DOI": "10.1164/rccm.202003-0821OC",
"article-title": "COVID-19-related Genes in Sputum Cells in Asthma. Relationship to Demographic Features and Corticosteroids",
"author": "MC Peters",
"doi-asserted-by": "crossref",
"first-page": "83",
"issue": "1",
"journal-title": "Am J Respir Crit Care Med",
"key": "pone.0294872.ref010",
"volume": "202",
"year": "2020"
},
{
"DOI": "10.1001/archinte.167.19.2047",
"article-title": "Adverse effects of inhaled corticosteroids in funded and nonfunded studies",
"author": "A Nieto",
"doi-asserted-by": "crossref",
"first-page": "2047",
"issue": "19",
"journal-title": "Archives of Internal Medicine (1960)",
"key": "pone.0294872.ref011",
"volume": "167",
"year": "2007"
},
{
"DOI": "10.7326/0003-4819-149-6-200809160-00004",
"article-title": "Risk for death associated with medications for recently diagnosed chronic obstructive pulmonary disease",
"author": "TA Lee",
"doi-asserted-by": "crossref",
"first-page": "380",
"issue": "6",
"journal-title": "Annals of Internal Medicine",
"key": "pone.0294872.ref012",
"volume": "149",
"year": "2008"
},
{
"DOI": "10.1016/j.jclinepi.2009.06.006",
"article-title": "The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration",
"author": "A Liberati",
"doi-asserted-by": "crossref",
"first-page": "e1",
"issue": "10",
"journal-title": "Journal of clinical epidemiology",
"key": "pone.0294872.ref013",
"volume": "62",
"year": "2009"
},
{
"article-title": "Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions",
"author": "M Cumpston",
"first-page": "Ed000142",
"journal-title": "Cochrane Database Syst Rev",
"key": "pone.0294872.ref014",
"volume": "10",
"year": "2019"
},
{
"DOI": "10.1016/j.jclinepi.2012.08.001",
"article-title": "GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles-continuous outcomes",
"author": "GH Guyatt",
"doi-asserted-by": "crossref",
"first-page": "173",
"issue": "2",
"journal-title": "J Clin Epidemiol",
"key": "pone.0294872.ref015",
"volume": "66",
"year": "2013"
},
{
"DOI": "10.1016/S2213-2600(21)00160-0",
"article-title": "Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial",
"author": "S Ramakrishnan",
"doi-asserted-by": "crossref",
"first-page": "763",
"issue": "7",
"journal-title": "The Lancet Respiratory Medicine",
"key": "pone.0294872.ref016",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(21)01744-X",
"article-title": "Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial",
"author": "L-M Yu",
"doi-asserted-by": "crossref",
"first-page": "843",
"issue": "10303",
"journal-title": "Lancet",
"key": "pone.0294872.ref017",
"volume": "398",
"year": "2021"
},
{
"DOI": "10.5582/ddt.2022.01068",
"article-title": "Impact of inhaled ciclesonide on asymptomatic or mild COVID-19: A randomized trial",
"author": "J Terada-Hirashima",
"doi-asserted-by": "crossref",
"first-page": "225",
"issue": "5",
"journal-title": "Drug Discov Ther",
"key": "pone.0294872.ref018",
"volume": "16",
"year": "2022"
},
{
"DOI": "10.1001/jamainternmed.2021.6759",
"article-title": "Efficacy of inhaled ciclesonide for outpatient treatment of adolescents and adults with symptomatic COVID-19: a randomized clinical trial",
"author": "BM Clemency",
"doi-asserted-by": "crossref",
"first-page": "42",
"issue": "1",
"journal-title": "JAMA internal medicine",
"key": "pone.0294872.ref019",
"volume": "182",
"year": "2022"
},
{
"article-title": "Inhaled ciclesonide for outpatient treatment of COVID-19 in adults at risk of adverse outcomes: a randomised controlled trial (COVERAGE)",
"author": "A Duvignaud",
"journal-title": "Clinical Microbiology and Infection",
"key": "pone.0294872.ref020",
"year": "2022"
},
{
"article-title": "Inhaled and intranasal ciclesonide for the treatment of covid-19 in adult outpatients: CONTAIN phase II randomised controlled trial",
"author": "N Ezer",
"first-page": "375",
"journal-title": "BMJ (Clinical Research ed.)",
"key": "pone.0294872.ref021",
"year": "2021"
},
{
"DOI": "10.3390/jcm10163545",
"article-title": "Ciclesonide inhaler treatment for mild-to-moderate COVID-19: a randomized, open-label, phase 2 trial",
"author": "J-Y Song",
"doi-asserted-by": "crossref",
"first-page": "3545",
"issue": "16",
"journal-title": "Journal of Clinical Medicine",
"key": "pone.0294872.ref022",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1136/bmjopen-2022-064374",
"article-title": "Inhaled ciclesonide in adults hospitalised with COVID-19: a randomised controlled open-label trial (HALT COVID-19)",
"author": "D Brodin",
"doi-asserted-by": "crossref",
"first-page": "e064374",
"issue": "2",
"journal-title": "BMJ open",
"key": "pone.0294872.ref023",
"volume": "13",
"year": "2023"
},
{
"article-title": "Inhaled corticosteroids for the treatment of COVID‐19",
"author": "M Griesel",
"issue": "3",
"journal-title": "Cochrane Database of Systematic Reviews (Online)",
"key": "pone.0294872.ref024",
"year": "2022"
},
{
"DOI": "10.1378/chest.129.1_suppl.1S",
"article-title": "Diagnosis and management of cough executive summary: ACCP evidence-based clinical practice guidelines",
"author": "RS Irwin",
"doi-asserted-by": "crossref",
"first-page": "1S",
"issue": "1",
"journal-title": "Chest",
"key": "pone.0294872.ref025",
"volume": "129",
"year": "2006"
},
{
"DOI": "10.1136/thx.54.5.403",
"article-title": "Effect of inhaled steroids on airway hyperresponsiveness, sputum eosinophils, and exhaled nitric oxide levels in patients with asthma",
"author": "EL van Rensen",
"doi-asserted-by": "crossref",
"first-page": "403",
"issue": "5",
"journal-title": "Thorax",
"key": "pone.0294872.ref026",
"volume": "54",
"year": "1999"
},
{
"article-title": "Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease",
"author": "KM Kew",
"issue": "3",
"journal-title": "Cochrane Database of Systematic Reviews (Online)",
"key": "pone.0294872.ref027",
"year": "2014"
},
{
"DOI": "10.1038/s41467-018-04574-1",
"article-title": "Corticosteroid suppression of antiviral immunity increases bacterial loads and mucus production in COPD exacerbations",
"author": "A Singanayagam",
"doi-asserted-by": "crossref",
"first-page": "1",
"issue": "1",
"journal-title": "Nature Communications",
"key": "pone.0294872.ref028",
"volume": "9",
"year": "2018"
},
{
"DOI": "10.1016/j.cyto.2019.154857",
"article-title": "Increased type 2 inflammation post rhinovirus infection in patients with moderate asthma",
"author": "T Southworth",
"doi-asserted-by": "crossref",
"first-page": "154857",
"journal-title": "Cytokine",
"key": "pone.0294872.ref029",
"volume": "125",
"year": "2020"
},
{
"DOI": "10.1016/j.pupt.2014.10.006",
"article-title": "Differences in the efficacy and safety among inhaled corticosteroids (ICS)/long-acting beta2-agonists (LABA) combinations in the treatment of chronic obstructive pulmonary disease (COPD): role of ICS",
"author": "M Latorre",
"doi-asserted-by": "crossref",
"first-page": "44",
"journal-title": "Pulmonary Pharmacology & Therapeutics",
"key": "pone.0294872.ref030",
"volume": "30",
"year": "2015"
},
{
"DOI": "10.1345/aph.1L546",
"article-title": "Comparison of inhaled corticosteroids: an update",
"author": "HW Kelly",
"doi-asserted-by": "crossref",
"first-page": "519",
"issue": "3",
"journal-title": "ANNALS OF PHARMACOTHERAPY",
"key": "pone.0294872.ref031",
"volume": "43",
"year": "2009"
},
{
"DOI": "10.1007/s15010-018-1229-y",
"article-title": "Inhaled corticosteroids and risk of upper respiratory tract infection in patients with asthma: a meta-analysis",
"author": "M Yang",
"doi-asserted-by": "crossref",
"first-page": "377",
"issue": "3",
"journal-title": "Infection",
"key": "pone.0294872.ref032",
"volume": "47",
"year": "2019"
},
{
"DOI": "10.1016/j.intimp.2019.105950",
"article-title": "Inhaled corticosteroids and risk of pneumonia in patients with chronic obstructive pulmonary disease: a meta-analysis of randomized controlled trials",
"author": "M Yang",
"doi-asserted-by": "crossref",
"first-page": "105950",
"journal-title": "International Immunopharmacology",
"key": "pone.0294872.ref033",
"volume": "77",
"year": "2019"
},
{
"DOI": "10.1016/j.bcp.2007.01.031",
"article-title": "The role of esterases in the metabolism of ciclesonide to desisobutyryl-ciclesonide in human tissue",
"author": "E Mutch",
"doi-asserted-by": "crossref",
"first-page": "1657",
"issue": "10",
"journal-title": "Biochemical Pharmacology",
"key": "pone.0294872.ref034",
"volume": "73",
"year": "2007"
},
{
"DOI": "10.2165/00003088-200948040-00002",
"article-title": "Clinical pharmacokinetic and pharmacodynamic profile of inhaled ciclesonide",
"author": "R. Nave",
"doi-asserted-by": "crossref",
"first-page": "243",
"issue": "4",
"journal-title": "Clinical Pharmacokinetics",
"key": "pone.0294872.ref035",
"volume": "48",
"year": "2009"
},
{
"DOI": "10.1128/AAC.00819-20",
"article-title": "Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs",
"author": "S Jeon",
"doi-asserted-by": "crossref",
"first-page": "e00819",
"issue": "7",
"journal-title": "Antimicrobial Agents and Chemotherapy",
"key": "pone.0294872.ref036",
"volume": "64",
"year": "2020"
},
{
"DOI": "10.1128/JVI.01648-20",
"article-title": "The inhaled steroid ciclesonide blocks SARS-CoV-2 RNA replication by targeting the viral replication-transcription complex in cultured cells",
"author": "S Matsuyama",
"doi-asserted-by": "crossref",
"first-page": "e01648",
"issue": "1",
"journal-title": "Journal of Virology",
"key": "pone.0294872.ref037",
"volume": "95",
"year": "2020"
},
{
"article-title": "Frequent occurrence of mutations in nsp3 and nsp4 of SARS-CoV-2, presumably caused by the inhaled asthma drug ciclesonide",
"author": "A Doi",
"issue": "4",
"journal-title": "PNAS Nexus",
"key": "pone.0294872.ref038",
"volume": "1",
"year": "2022"
}
],
"reference-count": 38,
"references-count": 38,
"relation": {},
"resource": {
"primary": {
"URL": "https://dx.plos.org/10.1371/journal.pone.0294872"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Multidisciplinary"
],
"subtitle": [],
"title": "Clinical efficacy of inhaled corticosteroids in patients with coronavirus disease 2019: A living review and meta-analysis",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1371/journal.pone.corrections_policy",
"volume": "18"
}