Inhaled ciclesonide for outpatient treatment of COVID-19 in adults at risk of adverse outcomes: a randomised controlled trial (COVERAGE)
et al., Clinical Microbiology and Infection, doi:10.1016/j.cmi.2022.02.031, NCT04356495, Mar 2022
Budesonide for COVID-19
27th treatment shown to reduce risk in
September 2021, now with p = 0.0000042 from 14 studies, recognized in 10 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
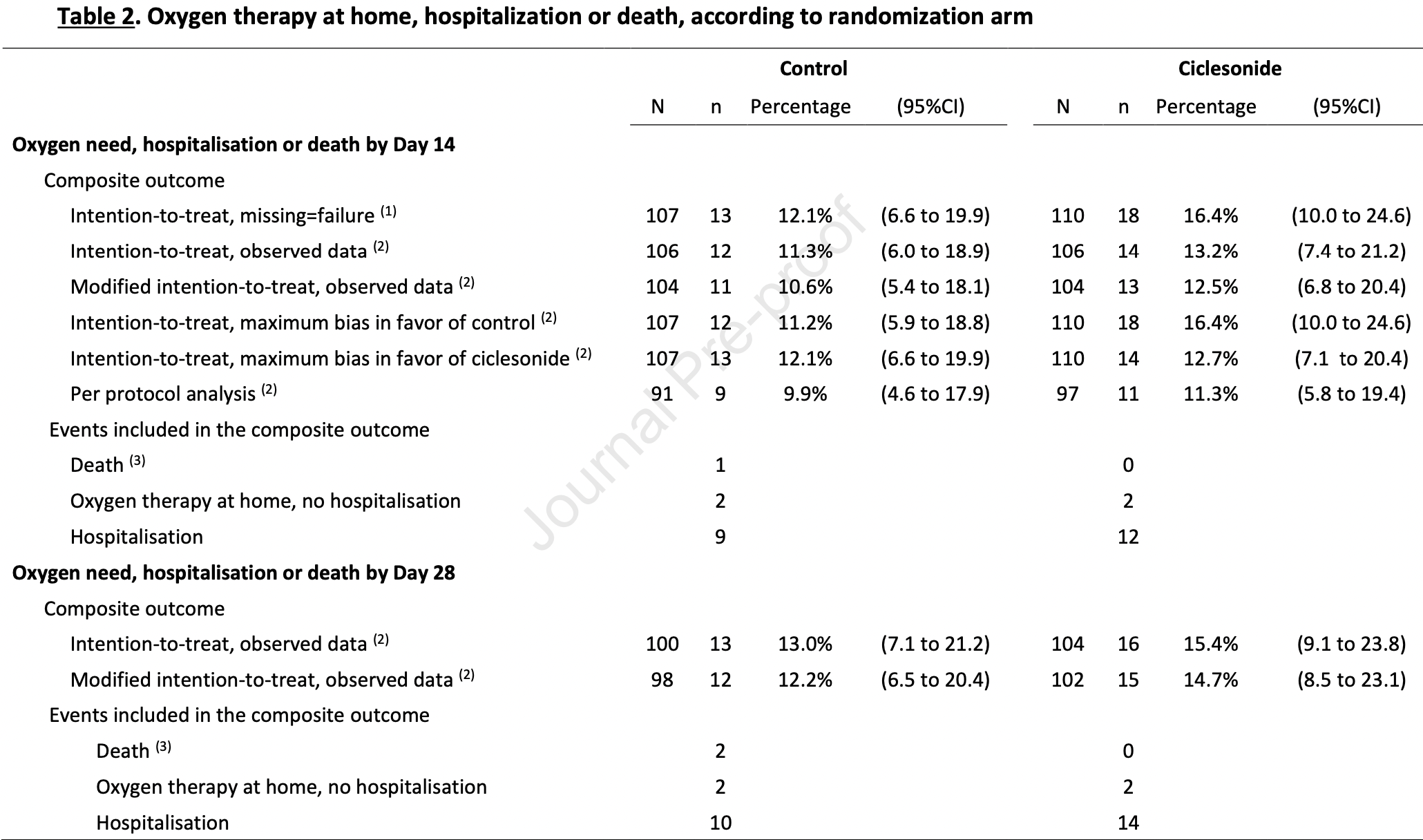
Early terminated RCT with 110 ciclesonide patients and 107 patients treated with vitamins A, B1, B2, B3, B5, B6, B8, B9, B12, C, D3, and E, calcium, chromium, copper, iron, manganese, molybdenum, selenium, and zinc (Azinc Vitality), showing no significant differences between the two treatments. There was no control group. NCT04356495 (history). EudraCT 2020-001435-27.
Targeted administration to the respiratory tract provides treatment directly
to the typical source of initial SARS-CoV-2 infection and replication, and
allows for rapid onset of action, higher local drug concentration, and reduced systemic side effects.
This study is excluded in meta-analysis:
study compares against another treatment showing significant efficacy.
|
risk of death, 80.2% lower, RR 0.20, p = 0.24, treatment 0 of 110 (0.0%), control 2 of 107 (1.9%), NNT 54, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), day 28.
|
|
risk of death, 67.0% lower, RR 0.33, p = 0.49, treatment 0 of 110 (0.0%), control 1 of 107 (0.9%), NNT 107, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), day 14.
|
|
risk of hospitalization, 36.2% higher, RR 1.36, p = 0.52, treatment 14 of 110 (12.7%), control 10 of 107 (9.3%), day 28.
|
|
risk of hospitalization, 29.7% higher, RR 1.30, p = 0.65, treatment 12 of 110 (10.9%), control 9 of 107 (8.4%), day 14.
|
|
risk of progression, 19.7% higher, RR 1.20, p = 0.69, treatment 16 of 110 (14.5%), control 13 of 107 (12.1%), oxygen, hospitalization, or death, day 28.
|
|
risk of progression, 13.5% higher, RR 1.13, p = 0.84, treatment 14 of 110 (12.7%), control 12 of 107 (11.2%), oxygen, hospitalization, or death, day 14, primary outcome.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Duvignaud et al., 16 Mar 2022, Randomized Controlled Trial, France, peer-reviewed, median age 63.0, 314 authors, study period 29 July, 2020 - 22 October, 2021, average treatment delay 4.0 days, this trial compares with another treatment - results may be better when compared to placebo, trial NCT04356495 (history).
Inhaled ciclesonide for outpatient treatment of COVID-19 in adults at risk of adverse outcomes: a randomised controlled trial (COVERAGE)
Clinical Microbiology and Infection, doi:10.1016/j.cmi.2022.02.031
This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contribution statement Alexandre Duvignaud (AD), Edouard Lhomme (EL), Racha Onaisi, Rémi Sitta (RS), Duc Nguyen (DN), Thierry Pistone (TP), Rodolphe Thiébaut, Jean-Philippe Joseph, Laura Richert (LR), Linda
Confit of interest All authors declare no conflict of interest.
Trial registration The trial protocol was registered in the European database EudraCT (2020-001435-27) and on clinicaltrials.gov (NCT04356495).
J o u r n a l P r e -p r o o f
Role of funding source The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
J o u r n a l P r e -p r o o f
LIST OF FIGURES Figure 1. CONSORT Flow Diagram
Footnotes to Figure 1 This figure shows the flow of participants through Day 14. The number of participants included in the analyses covering the 28-day follow-up is shown in Tables 2 and 3 . ITT=intention-to-treat; mITT=modified intention-to-treat; PP=per protocol; (1) Participant on chronic inhaled corticosteroid therapy (exclusion criteria) (2) Participants who have not been lost to follow-up before Day-9 and have not completed the 10-day treatment for reasons other than the occurrence of an adverse event. (3) Patients lost to follow-up were excluded from the ITT analysis (4) Reasons: lost to follow-up, n=2 ; major of eligibility criteria, n=2 (untreated diabetes, n=1; patient requiring oxygen therapy before randomisation, n=1) (5) Reasons: lost to follow-up, n=4 ; major violation..
References
Andrade, Sequí-Dominguez, Predictors of in-hospital COVID-19 mortality: A comprehensive systematic review and meta-analysis exploring differences by age, sex and health conditions, PloS One, doi:10.1371/journal.pone.0241742
Bateman, Karpel, Casale, Wenzel, Banerji, Ciclesonide reduces the need for oral steroid use in adult patients with severe, persistent asthma, Chest, doi:10.1378/chest.129.5.1176.JournalPre-proof
Clemency, Varughese, Gonzalez-Rojas, Morse, Phipatanakul et al., Efficacy of Inhaled Ciclesonide for Outpatient Treatment of Adolescents and Adults With Symptomatic COVID-19: A Randomized Clinical Trial, JAMA Intern Med, doi:10.1001/jamainternmed.2021.6759
Creatininemia, None, µmol/L
Dillman, Zoratti, Park, Hsu, Dron et al., The Landscape of Emerging Randomized Clinical Trial Evidence for COVID-19 Disease Stages: A Systematic Review of Global Trial Registries, Infect Drug Resist, doi:10.2147/IDR.S288399
Duvignaud, Anglaret, Research on COVID-19 therapy: Putting the cart alongside the horse, EBioMedicine, doi:10.1016/j.ebiom.2021.103342
Ezer, Belga, Daneman, Smith, Daniels, Inhaled and intranasal ciclesonide for the treatment of covid-19 in adult outpatients: CONTAIN phase II randomised controlled trial, BMJ, doi:10.1136/bmj-2021-068060
Ferritinemia, None, µg/L
Gude-Sampedro, Fernández-Merino, Ferreiro, Lado-Baleato, Espasandín-Domínguez et al., Development and validation of a prognostic model based on comorbidities to predict COVID-19 severity: a population-based study, Int J Epidemiol, doi:10.1093/ije/dyaa209
Indari, Jakhmola, Manivannan, Jha, An Update on Antiviral Therapy Against SARS-CoV-2: How Far Have We Come?, Front Pharmacol, doi:10.3389/fphar.2021.632677
J O U R N A L P R E, -p r o o f
James, Sydes, Clarke, Mason, Dearnaley et al., Systemic therapy for advancing or metastatic prostate cancer (STAMPEDE): a multi-arm, multistage randomized controlled trial, BJU Int, doi:10.1111/j.1464-410X.2008.08034.x
Jeon, Ko, Lee, Choi, Byun et al., Identification of Antiviral Drug Candidates against SARS-CoV-2 from FDA-Approved Drugs, Antimicrob Agents Chemother, doi:10.1128/AAC.00819-20
Ko, Chang, Byun, Ianevski, Choi et al., Screening of FDA-Approved Drugs Using a MERS-CoV Clinical Isolate from South Korea Identifies Potential Therapeutic Options for COVID-19, Viruses, doi:10.3390/v13040651
Liesenborghs, Spriet, Jochmans, Belmans, Gyselinck et al., Itraconazole for COVID-19: preclinical studies and a proof-of-concept randomized clinical trial, EBioMedicine, doi:10.1016/j.ebiom.2021.103288
Matsuyama, Kawase, Nao, Shirato, Ujike et al., The Inhaled Steroid Ciclesonide Blocks SARS-CoV-2 RNA Replication by Targeting the Viral Replication-Transcription Complex in Cultured Cells, J Virol, doi:10.1128/JVI.01648-20
Mesas, Cavero-Redondo, Álvarez-Bueno, Cabrera, De, None
Noor, Pett, Esmail, Crook, Vale et al., Adaptive platform trials using multi-arm, multi-stage protocols: getting fast answers in pandemic settings, doi:10.12688/f1000research.26253.2
Ramakrishnan, Nicolau, Langford, Mahdi, Jeffers et al., Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial, Lancet Respir Med, doi:10.1016/S2213-2600(21)00160-0
Reilev, Kristensen, Pottegård, Lund, Hallas et al., Characteristics and predictors of hospitalization and death in the first 11 122 cases with a positive RT-PCR test for SARS-CoV-2 in Denmark: a nationwide cohort, Int J Epidemiol, doi:10.1093/ije/dyaa140
Song, Yoon, Seo, Lee, Eom et al., Ciclesonide Inhaler Treatment for Mild-to-Moderate COVID-19: A Randomized, Open-Label, Phase 2 Trial, J Clin Med, doi:10.3390/jcm10163545
Tzou, Tao, Nouhin, Rhee, Hu et al., Coronavirus Antiviral Research Database (CoV-RDB): An Online Database Designed to Facilitate Comparisons between Candidate Anti-Coronavirus Compounds, Viruses, doi:10.3390/v12091006
Ukena, Biberger, Steinijans, Von Behren, Malek et al., Ciclesonide is more effective than budesonide in the treatment of persistent asthma, Pulm Pharmacol Ther, doi:10.1016/j.pupt.2006.05.007
Yu, Bafadhel, Dorward, Hayward, Saville et al., Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial, The Lancet, doi:10.1016/S0140-6736(21)01744-X
DOI record:
{
"DOI": "10.1016/j.cmi.2022.02.031",
"ISSN": [
"1198-743X"
],
"URL": "http://dx.doi.org/10.1016/j.cmi.2022.02.031",
"alternative-id": [
"S1198743X22001082"
],
"author": [
{
"affiliation": [],
"family": "Duvignaud",
"given": "Alexandre",
"sequence": "first"
},
{
"affiliation": [],
"family": "Lhomme",
"given": "Edouard",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Onaisi",
"given": "Racha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sitta",
"given": "Rémi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gelley",
"given": "Ambre",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chastang",
"given": "Julie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Piroth",
"given": "Lionel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Binquet",
"given": "Christine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dupouy",
"given": "Julie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Makinson",
"given": "Alain",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lefèvre",
"given": "Benjamin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Naccache",
"given": "Jean-Marc",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Roussillon",
"given": "Caroline",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Landman",
"given": "Roland",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wallet",
"given": "Cédrick",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Karcher",
"given": "Sophie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Journot",
"given": "Valérie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nguyen",
"given": "Duc",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pistone",
"given": "Thierry",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bouchet",
"given": "Stéphane",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lafon",
"given": "Marie-Edith",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Molimard",
"given": "Mathieu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thiébaut",
"given": "Rodolphe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "de Lamballerie",
"given": "Xavier",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Joseph",
"given": "Jean-Philippe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Richert",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saint-Lary",
"given": "Olivier",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Djabarouti",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wittkop",
"given": "Linda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Anglaret",
"given": "Xavier",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Malvy",
"given": "Denis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Duluc",
"given": "G.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Arma",
"given": "D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Beaufrère",
"given": "P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Belcastro",
"given": "J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Brice",
"given": "T.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cassai",
"given": "P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Desjardins",
"given": "S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Durrieu",
"given": "J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Georgevail",
"given": "S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gimbert",
"given": "A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Marchi",
"given": "S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Marty",
"given": "V.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nacka",
"given": "F.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pinilla",
"given": "R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Poulizac",
"given": "P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Regueme",
"given": "S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rousset",
"given": "M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Roussillon",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Salvo",
"given": "F.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Soria",
"given": "J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vautrat",
"given": "S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Richert",
"given": "L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Allais",
"given": "F.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Canete",
"given": "S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chaghil",
"given": "N.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Daoui",
"given": "S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dupouy",
"given": "G.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Esterle",
"given": "L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Favreau",
"given": "V.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gelley",
"given": "A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gillet",
"given": "S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hardel",
"given": "L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kanté",
"given": "M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lhomme",
"given": "E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Martiren",
"given": "S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moinot",
"given": "L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pinoges",
"given": "L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rouch",
"given": "E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schwimmer",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sitta",
"given": "R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Termote",
"given": "M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wallet",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wittkop",
"given": "L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Anglaret",
"given": "X.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Balestre",
"given": "E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Beuscart",
"given": "A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bonnier",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cazes",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chazallon",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Clouet",
"given": "G.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Daures",
"given": "M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gabillard",
"given": "D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Habiyambere",
"given": "G.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Journot",
"given": "V.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Loniewski",
"given": "M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Karcher",
"given": "S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Le Carrou",
"given": "J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Marcy",
"given": "O.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Murat",
"given": "V.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Orne Gliemann",
"given": "J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Plazy",
"given": "M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Djabarouti",
"given": "S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ghezzoul",
"given": "B.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Delignac",
"given": "L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Etienne",
"given": "P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fulda",
"given": "V.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gigan",
"given": "M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Langlade",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Le Chanjour",
"given": "J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Marque",
"given": "P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mora",
"given": "P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Plessis",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sourisseau",
"given": "B.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pellegrin",
"given": "I.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bouchet",
"given": "S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cognet",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Garrigue",
"given": "I.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jeanpetit",
"given": "J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lafon",
"given": "M.-E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pouzet",
"given": "A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tarricone",
"given": "A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Trimoulet",
"given": "P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Voldoire",
"given": "A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lacabaratz",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hocini",
"given": "H.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Anglaret",
"given": "X.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Duvignaud",
"given": "A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bez",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bironneau",
"given": "E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Collomb",
"given": "E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Contamin",
"given": "A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dubourdieu",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Faure",
"given": "N.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Galinski",
"given": "M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gibaud",
"given": "P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gil-Jardine",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Guillot-Warin",
"given": "J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lebouc",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Leger",
"given": "A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lengline",
"given": "V.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Loizeau",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mayenc",
"given": "M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Merle",
"given": "N.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nadiri-Kahraman",
"given": "H.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nguyen",
"given": "D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Odorico",
"given": "M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Onaisi",
"given": "R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pistone",
"given": "T.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sacher",
"given": "F.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Scandella",
"given": "J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Velardo",
"given": "F.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wiet",
"given": "A.S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Anglaret",
"given": "X.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Boudon",
"given": "T.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bouissière",
"given": "O.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Brégéras",
"given": "R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Broennec",
"given": "N.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Condé",
"given": "S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gazille",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Grenier",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kouame",
"given": "G.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Martins-Calado",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "N’Takpe",
"given": "J.-B.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pascual",
"given": "Z.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Piroth",
"given": "L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Court Devilliers",
"given": "S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Darley",
"given": "J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ducherpozat",
"given": "F.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Eberard",
"given": "N.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Faure",
"given": "J.L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Portier",
"given": "H.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rochelet",
"given": "R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ruffino",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thevenoud",
"given": "R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Binquet",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Creusvaux-Nguyen",
"given": "T.T.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Desbiolles",
"given": "N.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Grattard",
"given": "A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lamotte Felin",
"given": "A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rossye",
"given": "L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schaeffer",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Silvestre",
"given": "F.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Simonel",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lefèvre",
"given": "B.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Baronnet",
"given": "G.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Baux",
"given": "E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Di Santolo",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ferry",
"given": "M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Goehringer",
"given": "F.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Daguin",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dauchy",
"given": "E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gilg",
"given": "M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rossignol",
"given": "P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dupouy",
"given": "J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Boucault",
"given": "A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gimenez",
"given": "L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Burguier",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Couderc",
"given": "M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fradet",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gervais",
"given": "A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lavergne",
"given": "J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Landon",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mathe",
"given": "S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ortala",
"given": "B.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ainaoui",
"given": "N.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Auriac",
"given": "S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "S.Bras",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Flasquin",
"given": "S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fraysse",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gabriel",
"given": "M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gauteul",
"given": "P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Germain",
"given": "J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gross",
"given": "F.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lebely",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Riviere",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Robert",
"given": "D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thalamas",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Darnaud",
"given": "T.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Poitrenaud",
"given": "D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Giusti",
"given": "I.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Provent",
"given": "S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Makinson",
"given": "A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Montoya-Ferrer",
"given": "A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Battery",
"given": "J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Coux",
"given": "N.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Crantelle",
"given": "L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Galtier",
"given": "F.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Martin",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Picot",
"given": "M.C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Le Bel",
"given": "J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chastang",
"given": "J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nguyen",
"given": "A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saint-Lary",
"given": "O.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Giraud",
"given": "I.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Naccache",
"given": "J.M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jouveshomme",
"given": "J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Devaud",
"given": "E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ben Nasr",
"given": "N.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Monkam",
"given": "R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sacco",
"given": "E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rulle",
"given": "S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Landman",
"given": "R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Amat",
"given": "K.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Benalicherif",
"given": "A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sylla",
"given": "B.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Begue",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rat",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bouve",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Huard",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Morgand",
"given": "S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Guegan",
"given": "E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fairier",
"given": "D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Loiez",
"given": "A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alcouffe",
"given": "T.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Briand",
"given": "V.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Coudray",
"given": "S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Charles",
"given": "D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jacquet",
"given": "H.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Levy-Marchal",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Raude",
"given": "J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vellozzo",
"given": "P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Whithenay",
"given": "P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "de Lamballerie (Chair)",
"given": "X.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Anglaret",
"given": "X.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Atlani-Duault",
"given": "L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Begue",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chauvin",
"given": "F.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chastang",
"given": "J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Darmon",
"given": "D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Darnaud",
"given": "T.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Druais",
"given": "P.-L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dubee",
"given": "V.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dupouy",
"given": "J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Duvignaud",
"given": "A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gimbert",
"given": "A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Journot",
"given": "V.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Landman",
"given": "R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lebeaux",
"given": "D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lefèvre",
"given": "B.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lhomme",
"given": "E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Makinson",
"given": "A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Malvy",
"given": "D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mentré",
"given": "F.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Michel",
"given": "J.-F.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Molimard",
"given": "M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Montastruc",
"given": "J.L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Naccache",
"given": "J.M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Orne Gliemann",
"given": "J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Piroth",
"given": "L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rat",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Richert",
"given": "L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Roussillon",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saint Lary",
"given": "O.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thiebaut",
"given": "R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Weiss",
"given": "L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wittkop",
"given": "L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bouchet",
"given": "S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cazenave",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Conde",
"given": "S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cremer",
"given": "A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Djabarouti",
"given": "S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gil Jardine",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hardel",
"given": "L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Joseph",
"given": "J.-P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lafon",
"given": "M.-E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Le Bel",
"given": "J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Letinier",
"given": "L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Marchi",
"given": "S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moinot",
"given": "L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Montoya-Ferrer",
"given": "A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nguyen",
"given": "J.D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Onaisi",
"given": "R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pellegrin",
"given": "I.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pistone",
"given": "T.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Poitrenaud",
"given": "D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schaeverbeke",
"given": "T.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Costagliola",
"given": "D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bellissant",
"given": "E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gavazzi",
"given": "G.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Locher",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Taburet",
"given": "A.-M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tattevin",
"given": "P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Walker",
"given": "S.",
"sequence": "additional"
}
],
"container-title": [
"Clinical Microbiology and Infection"
],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
3,
15
]
],
"date-time": "2022-03-15T07:41:41Z",
"timestamp": 1647330101000
},
"deposited": {
"date-parts": [
[
2022,
3,
20
]
],
"date-time": "2022-03-20T03:00:38Z",
"timestamp": 1647745238000
},
"indexed": {
"date-parts": [
[
2022,
4,
2
]
],
"date-time": "2022-04-02T15:37:45Z",
"timestamp": 1648913865815
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "print",
"value": "1198-743X"
}
],
"issued": {
"date-parts": [
[
2022,
3
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
3,
1
]
],
"date-time": "2022-03-01T00:00:00Z",
"timestamp": 1646092800000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 13,
"start": {
"date-parts": [
[
2022,
3,
14
]
],
"date-time": "2022-03-14T00:00:00Z",
"timestamp": 1647216000000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1198743X22001082?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1198743X22001082?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022,
3
]
]
},
"published-print": {
"date-parts": [
[
2022,
3
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1371/journal.pone.0241742",
"article-title": "Predictors of in-hospital COVID-19 mortality: A comprehensive systematic review and meta-analysis exploring differences by age, sex and health conditions",
"author": "Mesas",
"doi-asserted-by": "crossref",
"journal-title": "PloS One",
"key": "10.1016/j.cmi.2022.02.031_bib1",
"volume": "15",
"year": "2020"
},
{
"DOI": "10.1093/ije/dyaa140",
"article-title": "Characteristics and predictors of hospitalization and death in the first 11 122 cases with a positive RT-PCR test for SARS-CoV-2 in Denmark: a nationwide cohort",
"author": "Reilev",
"doi-asserted-by": "crossref",
"first-page": "1468",
"journal-title": "Int J Epidemiol",
"key": "10.1016/j.cmi.2022.02.031_bib2",
"volume": "49",
"year": "2020"
},
{
"DOI": "10.1093/ije/dyaa209",
"article-title": "Development and validation of a prognostic model based on comorbidities to predict COVID-19 severity: a population-based study",
"author": "Gude-Sampedro",
"doi-asserted-by": "crossref",
"first-page": "64",
"journal-title": "Int J Epidemiol",
"key": "10.1016/j.cmi.2022.02.031_bib3",
"volume": "50",
"year": "2021"
},
{
"DOI": "10.3389/fphar.2021.632677",
"article-title": "An Update on Antiviral Therapy Against SARS-CoV-2: How Far Have We Come?",
"author": "Indari",
"doi-asserted-by": "crossref",
"first-page": "632677",
"journal-title": "Front Pharmacol",
"key": "10.1016/j.cmi.2022.02.031_bib4",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1111/j.1464-410X.2008.08034.x",
"article-title": "Systemic therapy for advancing or metastatic prostate cancer (STAMPEDE): a multi-arm, multistage randomized controlled trial",
"author": "James",
"doi-asserted-by": "crossref",
"first-page": "464",
"journal-title": "BJU Int",
"key": "10.1016/j.cmi.2022.02.031_bib5",
"volume": "103",
"year": "2009"
},
{
"DOI": "10.12688/f1000research.26253.1",
"article-title": "Adaptive platform trials using multi-arm, multi-stage protocols: getting fast answers in pandemic settings",
"author": "Noor",
"doi-asserted-by": "crossref",
"first-page": "1109",
"journal-title": "F1000Research",
"key": "10.1016/j.cmi.2022.02.031_bib6",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.2147/IDR.S288399",
"article-title": "The Landscape of Emerging Randomized Clinical Trial Evidence for COVID-19 Disease Stages: A Systematic Review of Global Trial Registries",
"author": "Dillman",
"doi-asserted-by": "crossref",
"first-page": "4577",
"journal-title": "Infect Drug Resist",
"key": "10.1016/j.cmi.2022.02.031_bib7",
"volume": "13",
"year": "2020"
},
{
"DOI": "10.3390/v12091006",
"article-title": "Coronavirus Antiviral Research Database (CoV-RDB): An Online Database Designed to Facilitate Comparisons between Candidate Anti-Coronavirus Compounds",
"author": "Tzou",
"doi-asserted-by": "crossref",
"first-page": "1006",
"journal-title": "Viruses",
"key": "10.1016/j.cmi.2022.02.031_bib8",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1016/j.ebiom.2021.103342",
"article-title": "Research on COVID-19 therapy: Putting the cart alongside the horse",
"author": "Duvignaud",
"doi-asserted-by": "crossref",
"first-page": "103342",
"journal-title": "EBioMedicine",
"key": "10.1016/j.cmi.2022.02.031_bib9",
"volume": "67",
"year": "2021"
},
{
"DOI": "10.1378/chest.129.5.1176",
"article-title": "Ciclesonide reduces the need for oral steroid use in adult patients with severe, persistent asthma",
"author": "Bateman",
"doi-asserted-by": "crossref",
"first-page": "1176",
"journal-title": "Chest",
"key": "10.1016/j.cmi.2022.02.031_bib10",
"volume": "129",
"year": "2006"
},
{
"DOI": "10.1016/j.pupt.2006.05.007",
"article-title": "Ciclesonide is more effective than budesonide in the treatment of persistent asthma",
"author": "Ukena",
"doi-asserted-by": "crossref",
"first-page": "562",
"journal-title": "Pulm Pharmacol Ther",
"key": "10.1016/j.cmi.2022.02.031_bib11",
"volume": "20",
"year": "2007"
},
{
"DOI": "10.1128/JVI.01648-20",
"article-title": "The Inhaled Steroid Ciclesonide Blocks SARS-CoV-2 RNA Replication by Targeting the Viral Replication-Transcription Complex in Cultured Cells",
"author": "Matsuyama",
"doi-asserted-by": "crossref",
"journal-title": "J Virol",
"key": "10.1016/j.cmi.2022.02.031_bib12",
"volume": "95",
"year": "2020"
},
{
"DOI": "10.3390/v13040651",
"article-title": "Screening of FDA-Approved Drugs Using a MERS-CoV Clinical Isolate from South Korea Identifies Potential Therapeutic Options for COVID-19",
"author": "Ko",
"doi-asserted-by": "crossref",
"first-page": "651",
"journal-title": "Viruses",
"key": "10.1016/j.cmi.2022.02.031_bib13",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1128/AAC.00819-20",
"article-title": "Identification of Antiviral Drug Candidates against SARS-CoV-2 from FDA-Approved Drugs",
"author": "Jeon",
"doi-asserted-by": "crossref",
"first-page": "e00819",
"journal-title": "Antimicrob Agents Chemother",
"key": "10.1016/j.cmi.2022.02.031_bib14",
"volume": "64",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(21)01744-X",
"article-title": "Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial",
"author": "Yu",
"doi-asserted-by": "crossref",
"first-page": "843",
"journal-title": "The Lancet",
"key": "10.1016/j.cmi.2022.02.031_bib15",
"volume": "398",
"year": "2021"
},
{
"DOI": "10.3390/jcm10163545",
"article-title": "Ciclesonide Inhaler Treatment for Mild-to-Moderate COVID-19: A Randomized, Open-Label, Phase 2 Trial",
"author": "Song",
"doi-asserted-by": "crossref",
"first-page": "3545",
"journal-title": "J Clin Med",
"key": "10.1016/j.cmi.2022.02.031_bib16",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(21)00160-0",
"article-title": "Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial",
"author": "Ramakrishnan",
"doi-asserted-by": "crossref",
"first-page": "763",
"journal-title": "Lancet Respir Med",
"key": "10.1016/j.cmi.2022.02.031_bib17",
"volume": "9",
"year": "2021"
},
{
"article-title": "Efficacy of Inhaled Ciclesonide for Outpatient Treatment of Adolescents and Adults With Symptomatic COVID-19: A Randomized Clinical Trial",
"author": "Clemency",
"journal-title": "JAMA Intern Med",
"key": "10.1016/j.cmi.2022.02.031_bib18",
"year": "2021"
},
{
"article-title": "Inhaled and intranasal ciclesonide for the treatment of covid-19 in adult outpatients: CONTAIN phase II randomised controlled trial",
"author": "Ezer",
"journal-title": "BMJ",
"key": "10.1016/j.cmi.2022.02.031_bib19",
"volume": "375",
"year": "2021"
},
{
"DOI": "10.1016/j.ebiom.2021.103288",
"article-title": "Itraconazole for COVID-19: preclinical studies and a proof-of-concept randomized clinical trial",
"author": "Liesenborghs",
"doi-asserted-by": "crossref",
"first-page": "103288",
"journal-title": "EBioMedicine",
"key": "10.1016/j.cmi.2022.02.031_bib20",
"volume": "66",
"year": "2021"
},
{
"article-title": "Insufficient data on use of inhaled corticosteroids to treat COVID-19",
"journal-title": "Eur Med Agency",
"key": "10.1016/j.cmi.2022.02.031_bib21",
"year": "2021"
}
],
"reference-count": 21,
"references-count": 21,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S1198743X22001082"
}
},
"score": 1,
"short-container-title": [
"Clinical Microbiology and Infection"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Microbiology (medical)",
"General Medicine"
],
"subtitle": [],
"title": [
"Inhaled ciclesonide for outpatient treatment of COVID-19 in adults at risk of adverse outcomes: a randomised controlled trial (COVERAGE)"
],
"type": "journal-article"
}
