Efficacy of Inhaled Ciclesonide for Outpatient Treatment of Adolescents and Adults With Symptomatic COVID-19
et al., JAMA Internal Medicine, doi:10.1001/jamainternmed.2021.6759, NCT04377711, Nov 2021
Budesonide for COVID-19
27th treatment shown to reduce risk in
September 2021, now with p = 0.0000042 from 14 studies, recognized in 10 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 400 outpatients in the USA, showing significantly lower ER visits/hospital admission with ciclesonide treatment. NCT04377711 (history).
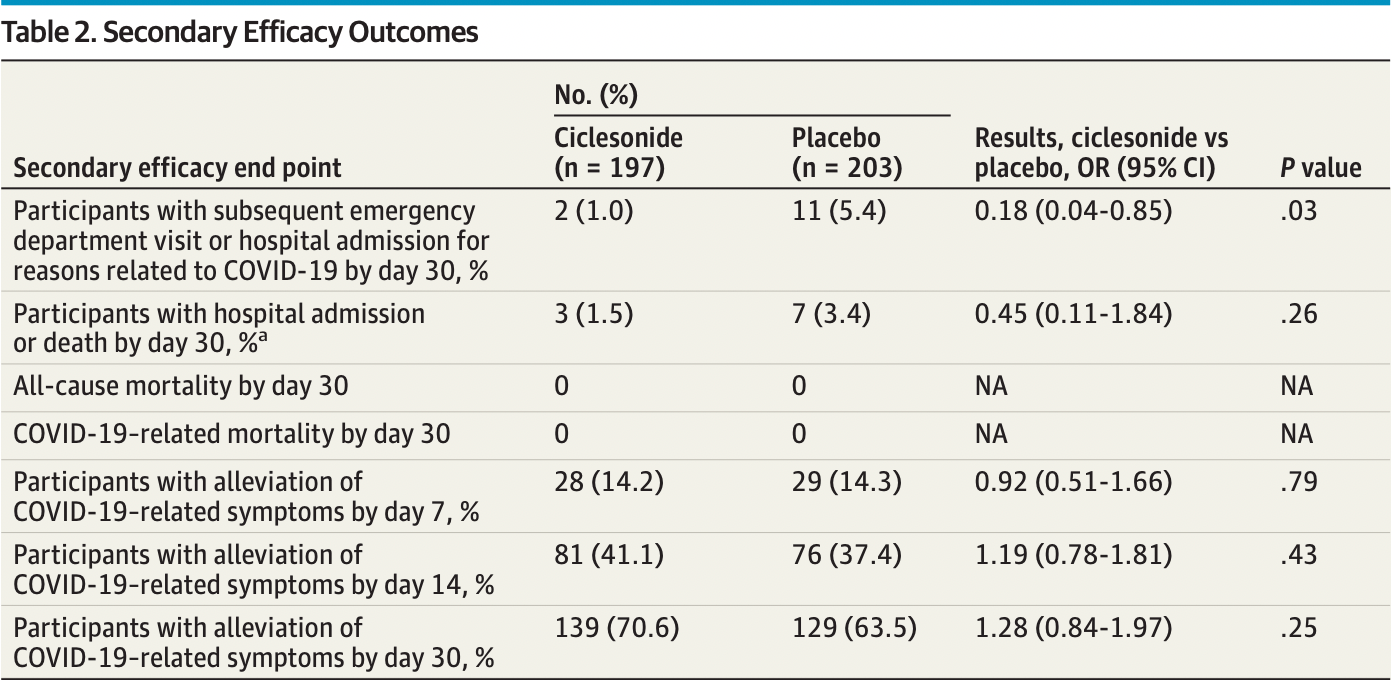
Publication was delayed about a year after completion and the primary endpoint was changed about 4 months after the end of the trial on April 9, 20211,2. The original primary outcome was the more important ER visits/hospital admission, which shows a statistically significant improvement OR 0.18 [0.04-0.85].
Targeted administration to the respiratory tract provides treatment directly
to the typical source of initial SARS-CoV-2 infection and replication, and
allows for rapid onset of action, higher local drug concentration, and reduced systemic side effects.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments3.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of hospitalization, 54.1% lower, RR 0.46, p = 0.26, treatment 3 of 197 (1.5%), control 7 of 203 (3.4%), NNT 52, odds ratio converted to relative risk.
|
|
risk of hospitalization/ER, 81.2% lower, RR 0.19, p = 0.03, treatment 2 of 197 (1.0%), control 11 of 203 (5.4%), NNT 23, odds ratio converted to relative risk, primary outcome.
|
|
risk of no recovery, 15.1% lower, RR 0.85, p = 0.25, treatment 58 of 197 (29.4%), control 74 of 203 (36.5%), NNT 14, inverted to make RR<1 favor treatment, odds ratio converted to relative risk, day 30.
|
|
recovery time, 7.4% lower, relative time 0.93, p = 0.55, treatment 197, control 203, inverted to make RR<1 favor treatment, Cox regression, post-hoc primary outcome.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Clemency et al., 22 Nov 2021, Double Blind Randomized Controlled Trial, USA, peer-reviewed, 7 authors, study period 11 June, 2020 - 3 November, 2020, trial NCT04377711 (history).
Efficacy of Inhaled Ciclesonide for Outpatient Treatment of Adolescents and Adults With Symptomatic COVID-19
JAMA Internal Medicine, doi:10.1001/jamainternmed.2021.6759
IMPORTANCE Systemic corticosteroids are commonly used in treating severe COVID-19. However, the role of inhaled corticosteroids in the treatment of patients with mild to moderate disease is less clear. OBJECTIVE To determine the efficacy of the inhaled steroid ciclesonide in reducing the time to alleviation of all COVID-19-related symptoms among nonhospitalized participants with symptomatic COVID-19 infection. DESIGN, SETTING, AND PARTICIPANTS This phase 3, multicenter, double-blind, randomized clinical trial was conducted at 10 centers throughout the US and assessed the safety and efficacy of a ciclesonide metered-dose inhaler (MDI) for treating nonhospitalized participants with symptomatic COVID-19 infection who were screened from June 11, 2020, to November 3, 2020. INTERVENTIONS Participants were randomly assigned to receive ciclesonide MDI, 160 μg per actuation, for a total of 2 actuations twice a day (total daily dose, 640 μg) or placebo for 30 days. MAIN OUTCOMES AND MEASURES The primary end point was time to alleviation of all COVID-19-related symptoms (cough, dyspnea, chills, feeling feverish, repeated shaking with chills, muscle pain, headache, sore throat, and new loss of taste or smell) by day 30. Secondary end points included subsequent emergency department visits or hospital admissions for reasons attributable to COVID-19. RESULTS A total of 413 participants were screened and 400 (96.9%) were enrolled and randomized (197 [49.3%] in the ciclesonide arm and 203 [50.7%] in the placebo arm; mean [SD] age, 43.3 [16.9] years; 221 [55.3%] female; 2 [0.5%] Asian, 47 [11.8%] Black or African American, 3 [0.8%] Native Hawaiian or other Pacific Islander, 345 [86.3%] White, and 1 multiracial individuals [0.3%]; 172 Hispanic or Latino individuals [43.0%] ). The median time to alleviation of all COVID-19-related symptoms was 19.0 days (95% CI, 14.0-21.0) in the ciclesonide arm and 19.0 days (95% CI, 16.0-23.0) in the placebo arm. There was no difference in resolution of all symptoms by day 30 (odds ratio, 1.28; 95% CI, 0.84-1.97). Participants who were treated with ciclesonide had fewer subsequent emergency department visits or hospital admissions for reasons related to COVID-19 (odds ratio, 0.18; 95% CI, 0.04-0.85). No participants died during the study.
CONCLUSIONS AND RELEVANCE The results of this randomized clinical trial demonstrated that ciclesonide did not achieve the primary efficacy end point of reduced time to alleviation of all COVID-19-related symptoms. TRIAL REGISTRATION ClinicalTrials.gov Identifier: NCT04377711
ARTICLE INFORMATION Accepted for Publication: October 3, 2021. Published Online: November 22, 2021. doi:10.1001/jamainternmed.2021.6759 Author Contributions: Dr Clemency and Mr Koster had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Funding/Support: The study was sponsored and funded by Covis Pharma GmbH. Research at the primary site was also supported by the National Center for Advancing Translational Sciences of the (NIH) (grant UL1TR001412; Drs Clemency and Varughese) and the National Heart, Lung, and Blood Institute of the NIH (grant K12HL138052; Dr Clemency). Role of the Funder/Sponsor: Covis Pharma GmbH had a role in the design and conduct of the study. Covis Pharma GmbH approved the plan for, but did not participate in, the collection, management and analysis of the data. Covis Pharma GmbH approved the decision to submit the manuscript for publication. Changes to the primary end point were made by the study sponsor in consultation with the study steering committee and the US Food and Drug Administration. The NIH had no role in the study design, data collection, data analysis, or decision to publish.
Disclaimer: The views expressed are those of the authors and not necessarily those of Covis Pharma GmbH or the NIH. Data Sharing Statement: See Supplement 3.
Additional Contributions: We thank Skyler Hime-Rupard, MS, and Yolaine Jeune-Smith, PhD, Cardinal Health Specialty..
References
Eedara, Alabsi, Encinas-Basurto, Polt, Ledford et al., Inhalation delivery for the treatment and prevention of COVID-19 infection, Pharmaceutics, doi:10.3390/pharmaceutics13071077
Fu, Cheng, Wu, Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools, Virol Sin, doi:10.1007/s12250-020-00207-4
Gross, Essien, Pasha, Gross, Wang et al., Racial and ethnic disparities in population-level COVID-19 mortality, J Gen Intern Med, doi:10.1007/s11606-020-06081-w
Horby, Lim, Emberson, Dexamethasone in hospitalized patients with COVID-19, N Engl J Med, doi:10.1056/NEJMoa2021436
Iwabuchi, Kurakami, Takahashi, Kato, Morishima, Therapeutic potential of ciclesonide inhalation for COVID-19 pneumonia: report of three cases, J Infect Chemother, doi:10.1016/j.jiac.2020.04.007
Jeon, Ko, Lee, Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs, Antimicrob Agents Chemother, doi:10.1128/AAC.00819-20
Jun, Nirenberg, Weinberger, Analysis of sex-specific risk factors and clinical outcomes in COVID-19, Commun Med, doi:10.1038/s43856-021-00006-2
Kimura, Kurusu, Sada, Molecular pharmacology of ciclesonide against SARS-CoV-2, J Allergy Clin Immunol, doi:10.1016/j.jaci.2020.05.029
Maruta, He, Matsuyama, Kawase, Nao, The inhaled steroid ciclesonide blocks SARS-CoV-2 RNA replication by targeting the viral replicationtranscription complex in cultured cells, Med Drug Discov, doi:10.1128/JVI.01648-20
Mesas, Cavero-Redondo, Álvarez-Bueno, Predictors of in-hospital COVID-19 mortality: a comprehensive systematic review and meta-analysis exploring differences by age, sex and health conditions, PLoS One, doi:10.1371/journal.pone.0241742
Milne, Li, Yang, Inhaled corticosteroids downregulate SARS-CoV-2-related genes in COPD: results from a randomised controlled trial, Eur Respir J, doi:10.1183/13993003.00130-2021
Nalbandian, Sehgal, Gupta, Post-acute COVID-19 syndrome, Nat Med, doi:10.1038/s41591-021-01283-z
Peters, Sajuthi, Deford, COVID-19-related genes in sputum cells in asthma: relationship to demographic features and corticosteroids, Am J Respir Crit Care Med, doi:10.1164/rccm.202003-0821OC
Petrocelli, Cutrupi, Salzano, Six-month smell and taste recovery rates in coronavirus disease 2019 patients: a prospective psychophysical study, J Laryngol Otol, doi:10.1017/S002221512100116X
Ramakrishnan, Nicolau, Jr, Langford, Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial, Lancet Respir Med
Shuto, Komiya, Yamasue, A systematic review of corticosteroid treatment for noncritically ill patients with COVID-19, Sci Rep, doi:10.1038/s41598-020-78054-2
Tartof, Qian, Hong, Obesity and mortality among patients diagnosed with COVID-19: results from an integrated health care organization, Ann Intern Med, doi:10.7326/M20-3742
Yu, Bafadhel, Dorward, Inhaled budesonide for COVID-19 in people at higher risk of adverse outcomes in the community: interim analyses from the PRINCIPLE trial, medRxiv, doi:10.1101/2021.04.10.21254672
Zhai, Ding, Wu, Long, Zhong et al., The epidemiology, diagnosis and treatment of COVID-19, Int J Antimicrob Agents, doi:10.1016/j.ijantimicag.2020.105955
DOI record:
{
"DOI": "10.1001/jamainternmed.2021.6759",
"ISSN": [
"2168-6106"
],
"URL": "http://dx.doi.org/10.1001/jamainternmed.2021.6759",
"author": [
{
"affiliation": [
{
"name": "Jacobs School of Medicine and Biomedical Sciences, University at Buffalo, Buffalo, New York"
}
],
"family": "Clemency",
"given": "Brian M.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Jacobs School of Medicine and Biomedical Sciences, University at Buffalo, Buffalo, New York"
}
],
"family": "Varughese",
"given": "Renoj",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Verus Clinical Research Corporation, Coral Gables, Florida"
}
],
"family": "Gonzalez-Rojas",
"given": "Yaneicy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Wake Forest School of Medicine, Winston-Salem, North Carolina"
}
],
"family": "Morse",
"given": "Caryn G.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Boston Children's Hospital, Harvard Medical School, Boston, Massachusetts"
}
],
"family": "Phipatanakul",
"given": "Wanda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Instat Clinical Research, Chatham, New Jersey"
}
],
"family": "Koster",
"given": "David J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Medical College of Georgia at Augusta University, Augusta"
}
],
"family": "Blaiss",
"given": "Michael S.",
"sequence": "additional"
}
],
"container-title": [
"JAMA Internal Medicine"
],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
11,
22
]
],
"date-time": "2021-11-22T16:04:03Z",
"timestamp": 1637597043000
},
"deposited": {
"date-parts": [
[
2021,
11,
22
]
],
"date-time": "2021-11-22T16:04:11Z",
"timestamp": 1637597051000
},
"indexed": {
"date-parts": [
[
2021,
12,
23
]
],
"date-time": "2021-12-23T21:07:08Z",
"timestamp": 1640293628510
},
"is-referenced-by-count": 2,
"issn-type": [
{
"type": "print",
"value": "2168-6106"
}
],
"issued": {
"date-parts": [
[
2021,
11,
22
]
]
},
"language": "en",
"link": [
{
"URL": "https://jamanetwork.com/journals/jamainternalmedicine/articlepdf/2786012/jamainternal_clemency_2021_oi_210069_1635873580.05405.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "10",
"original-title": [],
"prefix": "10.1001",
"published": {
"date-parts": [
[
2021,
11,
22
]
]
},
"published-online": {
"date-parts": [
[
2021,
11,
22
]
]
},
"publisher": "American Medical Association (AMA)",
"reference": [
{
"DOI": "10.1056/NEJMoa2021436",
"article-title": "Dexamethasone in hospitalized patients with COVID-19.",
"author": "Horby",
"doi-asserted-by": "crossref",
"first-page": "693",
"issue": "8",
"journal-title": "N Engl J Med",
"key": "ioi210069r1",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1038/s41598-020-78054-2",
"article-title": "A systematic review of corticosteroid treatment for noncritically ill patients with COVID-19.",
"author": "Shuto",
"doi-asserted-by": "publisher",
"first-page": "20935",
"issue": "1",
"journal-title": "Sci Rep",
"key": "ioi210069r2",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1007/s12250-020-00207-4",
"article-title": "Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools.",
"author": "Fu",
"doi-asserted-by": "publisher",
"first-page": "266",
"issue": "3",
"journal-title": "Virol Sin",
"key": "ioi210069r3",
"volume": "35",
"year": "2020"
},
{
"DOI": "10.1016/j.ijantimicag.2020.105955",
"article-title": "The epidemiology, diagnosis and treatment of COVID-19.",
"author": "Zhai",
"doi-asserted-by": "crossref",
"issue": "5",
"journal-title": "Int J Antimicrob Agents",
"key": "ioi210069r4",
"volume": "55",
"year": "2020"
},
{
"DOI": "10.1164/rccm.202003-0821OC",
"article-title": "COVID-19-related genes in sputum cells in asthma: relationship to demographic features and corticosteroids.",
"author": "Peters",
"doi-asserted-by": "publisher",
"first-page": "83",
"issue": "1",
"journal-title": "Am J Respir Crit Care Med",
"key": "ioi210069r5",
"volume": "202",
"year": "2020"
},
{
"DOI": "10.1183/13993003.00130-2021",
"article-title": "Inhaled corticosteroids downregulate SARS-CoV-2–related genes in COPD: results from a randomised controlled trial.",
"author": "Milne",
"doi-asserted-by": "crossref",
"issue": "1",
"journal-title": "Eur Respir J",
"key": "ioi210069r6",
"volume": "58",
"year": "2021"
},
{
"DOI": "10.1128/AAC.00819-20",
"article-title": "Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs.",
"author": "Jeon",
"doi-asserted-by": "publisher",
"first-page": "e00819",
"issue": "7",
"journal-title": "Antimicrob Agents Chemother",
"key": "ioi210069r7",
"volume": "64",
"year": "2020"
},
{
"DOI": "10.1016/j.jaci.2020.05.029",
"article-title": "Molecular pharmacology of ciclesonide against SARS-CoV-2.",
"author": "Kimura",
"doi-asserted-by": "publisher",
"first-page": "330",
"issue": "2",
"journal-title": "J Allergy Clin Immunol",
"key": "ioi210069r8",
"volume": "146",
"year": "2020"
},
{
"DOI": "10.1016/j.jiac.2020.04.007",
"article-title": "Therapeutic potential of ciclesonide inhalation for COVID-19 pneumonia: report of three cases.",
"author": "Iwabuchi",
"doi-asserted-by": "publisher",
"first-page": "625",
"issue": "6",
"journal-title": "J Infect Chemother",
"key": "ioi210069r9",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1007/s11606-020-06081-w",
"article-title": "Racial and ethnic disparities in population-level COVID-19 mortality.",
"author": "Gross",
"doi-asserted-by": "publisher",
"first-page": "3097",
"issue": "10",
"journal-title": "J Gen Intern Med",
"key": "ioi210069r10",
"volume": "35",
"year": "2020"
},
{
"DOI": "10.1038/s43856-021-00006-2",
"article-title": "Analysis of sex-specific risk factors and clinical outcomes in COVID-19.",
"author": "Jun",
"doi-asserted-by": "publisher",
"first-page": "3",
"journal-title": "Commun Med",
"key": "ioi210069r11",
"volume": "1",
"year": "2021"
},
{
"DOI": "10.1371/journal.pone.0241742",
"article-title": "Predictors of in-hospital COVID-19 mortality: a comprehensive systematic review and meta-analysis exploring differences by age, sex and health conditions.",
"author": "Mesas",
"doi-asserted-by": "crossref",
"issue": "11",
"journal-title": "PLoS One",
"key": "ioi210069r12",
"volume": "15",
"year": "2020"
},
{
"DOI": "10.7326/M20-3742",
"article-title": "Obesity and mortality among patients diagnosed with COVID-19: results from an integrated health care organization.",
"author": "Tartof",
"doi-asserted-by": "publisher",
"first-page": "773",
"issue": "10",
"journal-title": "Ann Intern Med",
"key": "ioi210069r13",
"volume": "173",
"year": "2020"
},
{
"DOI": "10.1017/S002221512100116X",
"article-title": "Six-month smell and taste recovery rates in coronavirus disease 2019 patients: a prospective psychophysical study.",
"author": "Petrocelli",
"doi-asserted-by": "publisher",
"first-page": "436",
"issue": "5",
"journal-title": "J Laryngol Otol",
"key": "ioi210069r14",
"volume": "135",
"year": "2021"
},
{
"DOI": "10.3390/pharmaceutics13071077",
"article-title": "Inhalation delivery for the treatment and prevention of COVID-19 infection.",
"author": "Eedara",
"doi-asserted-by": "publisher",
"first-page": "1077",
"issue": "7",
"journal-title": "Pharmaceutics",
"key": "ioi210069r15",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1016/j.medidd.2020.100039",
"article-title": "PAK1-blockers: potential therapeutics against COVID-19.",
"author": "Maruta",
"doi-asserted-by": "crossref",
"journal-title": "Med Drug Discov",
"key": "ioi210069r16",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1128/JVI.01648-20",
"article-title": "The inhaled steroid ciclesonide blocks SARS-CoV-2 RNA replication by targeting the viral replication-transcription complex in cultured cells.",
"author": "Matsuyama",
"doi-asserted-by": "publisher",
"first-page": "e01648",
"issue": "1",
"journal-title": "J Virol",
"key": "ioi210069r17",
"volume": "95",
"year": "2020"
},
{
"article-title": "Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial.",
"author": "Ramakrishnan",
"first-page": "1",
"issue": "21",
"journal-title": "Lancet Respir Med",
"key": "ioi210069r18",
"volume": "19",
"year": "2021"
},
{
"DOI": "10.1038/s41591-021-01283-z",
"article-title": "Post-acute COVID-19 syndrome.",
"author": "Nalbandian",
"doi-asserted-by": "publisher",
"first-page": "601",
"issue": "4",
"journal-title": "Nat Med",
"key": "ioi210069r20",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1101/2021.04.10.21254672",
"doi-asserted-by": "crossref",
"key": "ioi210069r19",
"unstructured": "Yu? LM, Bafadhel? M, Dorward? J, . Inhaled budesonide for COVID-19 in people at higher risk of adverse outcomes in the community: interim analyses from the PRINCIPLE trial.? medRxiv. Posted April 12, 2021. doi:10.1101/2021.04.10.21254672"
}
],
"reference-count": 20,
"references-count": 20,
"relation": {},
"score": 1,
"short-container-title": [
"JAMA Intern Med"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Internal Medicine"
],
"subtitle": [
"A Randomized Clinical Trial"
],
"title": [
"Efficacy of Inhaled Ciclesonide for Outpatient Treatment of Adolescents and Adults With Symptomatic COVID-19"
],
"type": "journal-article"
}
