Clinical efficacy of inhaled corticosteroids in patients with coronavirus disease-2019: an updated systematic review and meta-analysis
, S., Open Forum Infectious Diseases, doi:10.1093/ofid/ofad500.600, Nov 2023
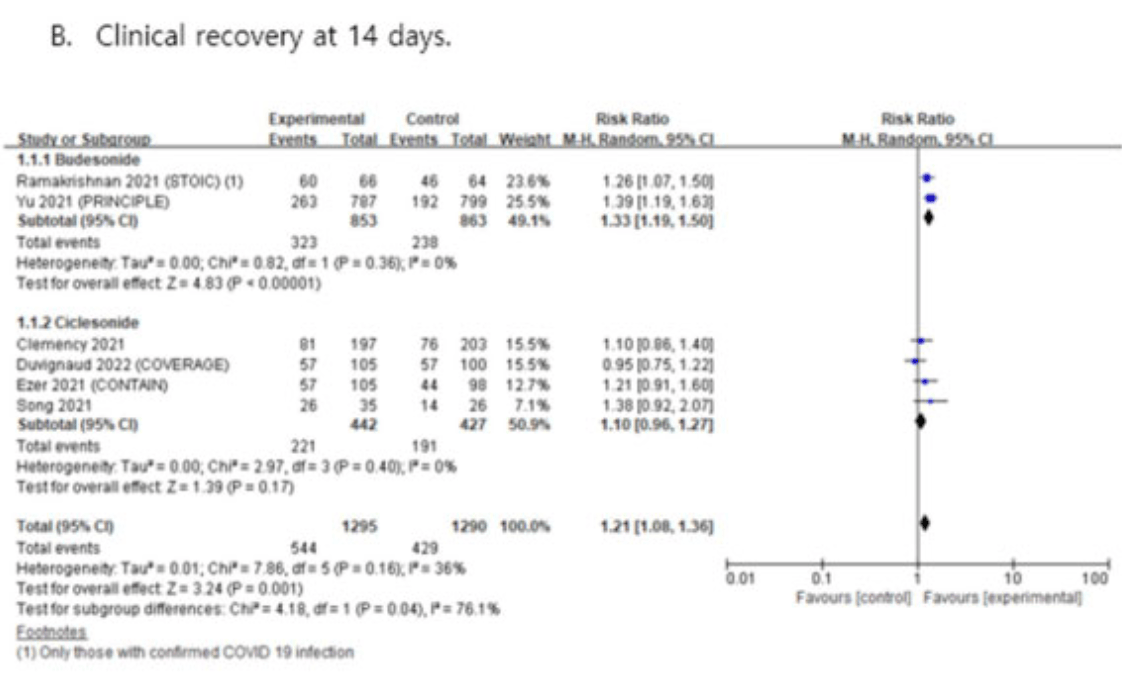
Budesonide for COVID-19
27th treatment shown to reduce risk in
September 2021, now with p = 0.0000042 from 14 studies, recognized in 10 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Systematic review and meta analysis showing improved recovery with budesonide.
There are significant differences between budesonide and ciclesonide that
may contribute to the lower efficacy of ciclesonide.
Pharmacokinetics and pharmacodynamics: budesonide has a more
rapid onset of action and a shorter half-life, which can lead to a more
immediate and potent anti-inflammatory effect. Ciclesonide is a prodrug
that requires conversion to its active form, which may delay therapeutic
effects.
Receptor affinity and potency: budesonide has a higher affinity for
glucocorticoid receptors and a higher potency compared to ciclesonide.
This can result in more effective suppression of inflammation and immune
modulation in the respiratory tract, potentially leading to better
clinical outcomes.
Bioavailability: budesonide typically has higher lung bioavailability
compared to ciclesonide.
Currently there are 14 budesonide for COVID-19 studies, showing 25% lower mortality [14‑35%], 15% lower ventilation [-73‑58%], 67% lower ICU admission [28‑85%], 29% lower hospitalization [10‑43%], and 25% fewer cases [14‑35%].
|
risk of no recovery, 24.8% lower, RR 0.75, p < 0.001, inverted to make RR<1 favor treatment.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Jeong et al., 27 Nov 2023, peer-reviewed, 1 author.
Abstract: Methods. Randomized controlled trials comparing the efficacy of ICS treatment
in patients with COVID-19 were identified through literature electronic databases
searches up to March 10, 2023. Meta-analyses were conducted for predefined outcomes, and the certainty of evidence was graded using the grading of recommendations, assessment, development, and evaluation approach.
Results. Overall seven trials (eight articles) were included in this systematic review. Compared with usual care, ICS was associated with significantly improved clinical recovery at 7 and 14 days in patients with COVID-19. In subgroup analysis, only
budesonide showed significant efficacy in clinical recovery, whereas no significant
benefit was observed for ciclesonide. Moreover, ICS use was not significantly associated with all-cause hospitalization, all-cause mortality, admission to intensive care
unit, or the use of mechanical ventilation. Our systematic review used evidence
with very low to moderate certainty.
Conclusion. The results of this meta-analysis revealed the clinical efficacy of ICS
treatment compared with usual care. Based on limited evidence, our results suggest
that ICS treatment, especially budesonide, improves the clinical recovery of patients
with COVID-19. The safety of ICS treatment in patients with COVID-19 has not
yet been established; however, ICS are relatively safe and widely available. In addition,
short-term ICS use in the early stages of COVID-19 did not increase the risk of pulmonary infections or side effects. Subsequent randomized clinical trials with a larger
number of patients, as well as meta-analyses, are needed to determine the usefulness of
ICS treatment in improving the clinical outcomes of patients with COVID-19.
Disclosures. All Authors: No reported disclosures
Abstract citation ID: ofad500.600
531. Clinical efficacy of inhaled corticosteroids in patients with coronavirus
disease-2019: an updated systematic review and meta-analysis
Su Jin Jeong, MD, PhD1; 1Yonsei University College of Medicine, Seoul,
Seoul-t’ukpyolsi, Republic of Korea
Session: 47. COVID-19: Treatment
Thursday, October 12, 2023: 12:15 PM
Background. Inhaled corticosteroids (ICS) are known to be relatively safe for
long-term use in inflammatory respiratory diseases, and it has been repurposed as
one of the potential therapies for outpatients with coronavirus diseases 2019
(COVID-19). However, ICS have not been accepted for COVID-19 as a standard therapy because of its lack of proven benefits. Therefore, this study aimed to evaluate the
effectiveness of ICS in patients with COVID-19.
S320 • OFID 2023:10 (Suppl 2) • Poster Abstracts
1
Pfizer Inc, Collegeville,
Pennsylvania 2Pfizer, Sandwich, England, United Kingdom
1
Shionogi & Co., Ltd., Osaka, Osaka, Japan 2SHIONOGI & CO., LTD., Osaka, Osaka,
Japan 3Clinical Pharmacology & Pharmacokinetics, Project Management
Department, Shionogi & Co., Ltd. Osaka, Japan, Osaka, Osaka, Japan
DOI record:
{
"DOI": "10.1093/ofid/ofad500.600",
"ISSN": [
"2328-8957"
],
"URL": "http://dx.doi.org/10.1093/ofid/ofad500.600",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Inhaled corticosteroids (ICS) are known to be relatively safe for long-term use in inflammatory respiratory diseases, and it has been repurposed as one of the potential therapies for outpatients with coronavirus diseases 2019 (COVID-19). However, ICS have not been accepted for COVID-19 as a standard therapy because of its lack of proven benefits. Therefore, this study aimed to evaluate the effectiveness of ICS in patients with COVID-19.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>Randomized controlled trials comparing the efficacy of ICS treatment in patients with COVID-19 were identified through literature electronic databases searches up to March 10, 2023. Meta-analyses were conducted for predefined outcomes, and the certainty of evidence was graded using the grading of recommendations, assessment, development, and evaluation approach.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>Overall seven trials (eight articles) were included in this systematic review. Compared with usual care, ICS was associated with significantly improved clinical recovery at 7 and 14 days in patients with COVID-19. In subgroup analysis, only budesonide showed significant efficacy in clinical recovery, whereas no significant benefit was observed for ciclesonide. Moreover, ICS use was not significantly associated with all-cause hospitalization, all-cause mortality, admission to intensive care unit, or the use of mechanical ventilation. Our systematic review used evidence with very low to moderate certainty.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusion</jats:title>\n <jats:p>The results of this meta-analysis revealed the clinical efficacy of ICS treatment compared with usual care. Based on limited evidence, our results suggest that ICS treatment, especially budesonide, improves the clinical recovery of patients with COVID-19. The safety of ICS treatment in patients with COVID-19 has not yet been established; however, ICS are relatively safe and widely available. In addition, short-term ICS use in the early stages of COVID-19 did not increase the risk of pulmonary infections or side effects. Subsequent randomized clinical trials with a larger number of patients, as well as meta-analyses, are needed to determine the usefulness of ICS treatment in improving the clinical outcomes of patients with COVID-19.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Disclosures</jats:title>\n <jats:p>All Authors: No reported disclosures</jats:p>\n </jats:sec>",
"author": [
{
"affiliation": [
{
"name": "Yonsei University College of Medicine , Seoul, Seoul-t'ukpyolsi , Republic of Korea"
}
],
"family": "Jeong",
"given": "Su Jin",
"sequence": "first"
}
],
"container-title": "Open Forum Infectious Diseases",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
11,
27
]
],
"date-time": "2023-11-27T01:49:57Z",
"timestamp": 1701049797000
},
"deposited": {
"date-parts": [
[
2023,
11,
27
]
],
"date-time": "2023-11-27T01:49:57Z",
"timestamp": 1701049797000
},
"indexed": {
"date-parts": [
[
2023,
11,
27
]
],
"date-time": "2023-11-27T05:18:08Z",
"timestamp": 1701062288670
},
"is-referenced-by-count": 0,
"issue": "Supplement_2",
"issued": {
"date-parts": [
[
2023,
11,
27
]
]
},
"journal-issue": {
"issue": "Supplement_2",
"published-print": {
"date-parts": [
[
2023,
11,
27
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
11,
27
]
],
"date-time": "2023-11-27T00:00:00Z",
"timestamp": 1701043200000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/ofid/article-pdf/10/Supplement_2/ofad500.600/53761256/ofad500.600.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/ofid/article-pdf/10/Supplement_2/ofad500.600/53761256/ofad500.600.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2023,
11,
27
]
]
},
"published-online": {
"date-parts": [
[
2023,
11,
27
]
]
},
"published-other": {
"date-parts": [
[
2023,
12
]
]
},
"published-print": {
"date-parts": [
[
2023,
11,
27
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/ofid/article/doi/10.1093/ofid/ofad500.600/7447200"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Oncology"
],
"subtitle": [],
"title": "531. Clinical efficacy of inhaled corticosteroids in patients with coronavirus disease-2019: an updated systematic review and meta-analysis",
"type": "journal-article",
"volume": "10"
}
