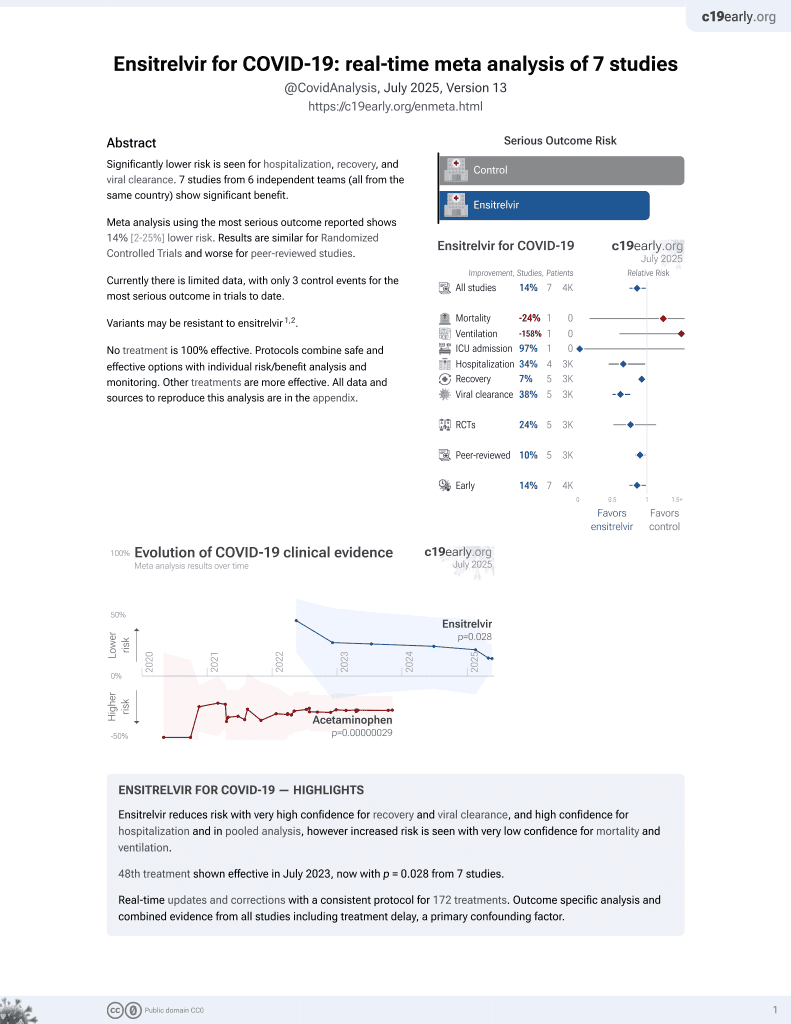
Real-World Efficacy of Ensitrelvir in Hospitalized Patients With COVID-19 in Japan: A Retrospective Observational Study
et al., Cureus, doi:10.7759/cureus.61048, Feb 2024 (preprint)
50th treatment shown to reduce risk in
July 2023, now with p = 0.015 from 8 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
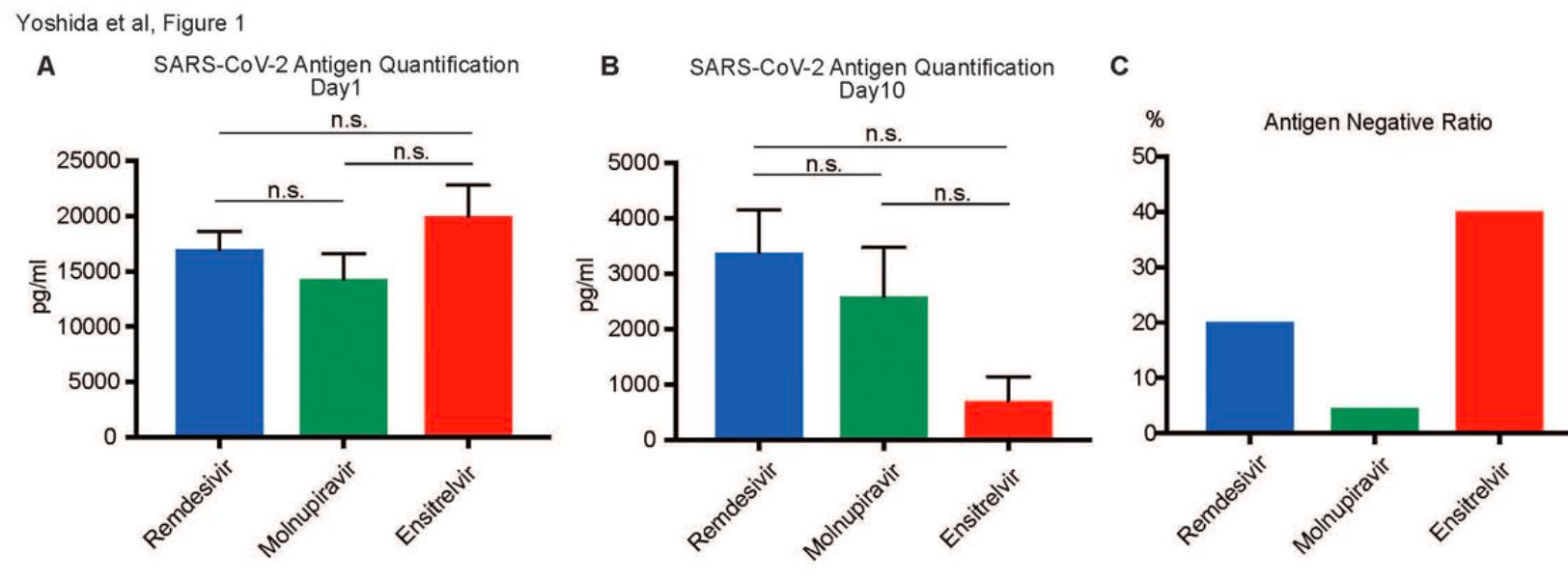
Retrospective 154 hospitalized COVID-19 patients in Japan showing faster viral clearance and shorter hospitalization with ensitrelvir treatment compared to remdesivir or molnupiravir. There was no significant difference for fever resolution time.
Study covers ensitrelvir, remdesivir, and molnupiravir.
Yoshida et al., 6 Feb 2024, retrospective, Japan, peer-reviewed, 3 authors, study period 1 July, 2022 - 15 September, 2023.
Contact: yryohei@asahikawa-med.ac.jp.
Real-World Efficacy of Ensitrelvir in Hospitalized Patients With COVID-19 in Japan: A Retrospective Observational Study
Cureus, doi:10.7759/cureus.61048
Background and aim: The coronavirus disease 2019 (COVID-19) pandemic necessitates continuously evaluating antiviral treatments, especially for high-risk groups, including older individuals. This study aimed to compare the efficacy of three antiviral drugs, including remdesivir, molnupiravir, and ensitrelvir, in hospitalized patients as measured by our own institution's antigen test, focusing on outcomes, such as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antigen levels, hospitalization duration, and fever resolution. Methods: This retrospective observational study was conducted at Yoshida Hospital, Asahikawa City, Japan, enrolling 154 patients who received antiviral treatment upon COVID-19 diagnosis from July 1, 2022, to September 15, 2023. The diagnosis was confirmed by proprietary antigen tests or loop-mediated isothermal amplification assays. Patients who received treatment outside the hospital or with consistently negative antigen results were excluded. Drug administration was determined by attending physicians, considering oral administration challenges and renal dysfunction. The data were statistically analyzed using an unpaired two-tailed Student's t-test and one-way analysis of variance complemented by the Tukey post-hoc test for detailed group comparisons. Results: No significant differences were observed in the initial antigen levels among the treatment groups. By day 10, the ensitrelvir group showed lower antigen levels than the other groups, but not significantly. The ensitrelvir group had a higher antigen-negative conversion rate and a significantly shorter hospital stay than the molnupiravir group. However, no significant differences were noted in the fever resolution time among the groups. Conclusion: This study suggests the potential benefits of ensitrelvir in reducing antigen levels and hospitalization duration. However, the overall efficacy of the antiviral agents for symptomatic relief appears similar. These findings underscore the need for further research to optimize COVID-19 management by considering personalized treatment approaches and long-term outcomes.
Additional Information Author Contributions All authors have reviewed the final version to be published and agreed to be accountable for all aspects of the work.
Concept and design: Ryohei Yoshida, Yoshinobu Ohsaki Drafting of the manuscript: Ryohei Yoshida Acquisition, analysis, or interpretation of data: Takaaki Sasaki Critical review of the manuscript for important intellectual content: Takaaki Sasaki, Yoshinobu Ohsaki Supervision: Takaaki Sasaki, Yoshinobu Ohsaki
Disclosures Human subjects: Consent was obtained or waived by all participants in this study. The Institutional Review Board of Yoshida Hospital issued approval 20230809003. The study protocol was approved by the Institutional Review Board of Yoshida Hospital (approval no. 20230809003) and conducted in accordance with the principles of the Declaration of Helsinki. Informed consent was obtained from all study patients using the opt-out method. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other..
References
Baden, Sahly, Essink, Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine, N Engl J Med, doi:10.1056/NEJMoa2035389
Beigel, Tomashek, Dodd, Remdesivir for the Treatment of Covid-19 -Final Report, N Engl J Med, doi:10.1056/NEJMoa2007764
Bernal, Da Silva, Musungaie, Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients, N Engl J Med, doi:10.1056/NEJMoa2116044
Chen, Klein, Garibaldi, Aging in COVID-19: Vulnerability, immunity and intervention, Ageing Res Rev, doi:10.1016/j.arr.2020.101205
Dessie, Zewotir, Mortality-related risk factors of COVID-19: a systematic review and meta-analysis of 42 studies and 423,117 patients, BMC Infect Dis, doi:10.1186/s12879-021-06536-3
Ejaz, Alsrhani, Zafar, COVID-19 and comorbidities: Deleterious impact on infected patients, J Infect Public Health, doi:10.1016/j.jiph.2020.07.014
Fan, Lou, Li, Tong, The emergence of powerful oral anti-COVID-19 drugs in the post-vaccine era, Lancet Microbe, doi:10.1016/S2666-5247(21)00278-0
Hashimoto, Yahiro, Khan, Seroprevalence kinetics of SARS-CoV-2 antibodies in pediatric patients visiting a hospital during COVID-19 pandemic in Japan, J Infect Chemother, doi:10.1016/j.jiac.2023.09.020
Huang, Wang, Liu, COVID-19 patients with hypertension have more severe disease: a multicenter retrospective observational study, Hypertens Res, doi:10.1038/s41440-020-0485-2
Li, Hilgenfeld, Whitley, Clercq, Therapeutic strategies for COVID-19: progress and lessons learned, Nat Rev Drug Discov, doi:10.1038/s41573-023-00672-y
Mukae, Yotsuyanagi, Ohmagari, A Randomized Phase 2/3 Study of Ensitrelvir, a Novel Oral SARS-CoV-2 3C-Like Protease Inhibitor, in Japanese Patients with Mild-to-Moderate COVID-19 or Asymptomatic SARS-CoV-2 Infection: Results of the Phase 2a Part, Antimicrob Agents Chemother, doi:10.1128/aac.00697-22
Mukae, Yotsuyanagi, Ohmagari, Efficacy and Safety of Ensitrelvir in Patients With Mild-to-Moderate Coronavirus Disease 2019: The Phase 2b Part of a Randomized, Placebo-Controlled, Phase 2/3 Study, Clin Infect Dis, doi:10.1093/cid/ciac933
Nobori, Fukao, Kuroda, Efficacy of ensitrelvir against SARS-CoV-2 in a delayed-treatment mouse model, J Antimicrob Chemother, doi:10.1093/jac/dkac257
Pan, Peto, Henao-Restrepo, Repurposed Antiviral Drugs for Covid-19 -Interim WHO Solidarity Trial Results, N Engl J Med, doi:10.1056/NEJMoa2023184
Polack, Thomas, Kitchin, Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine, N Engl J Med, doi:10.1056/NEJMoa2034577
Richardson, Hirsch, Narasimhan, Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area, JAMA, doi:10.1001/jama.2020.6775
Wong, Au, Lau, Lau, Cowling et al., Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among communitydwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: an observational study, Lancet, doi:10.1016/S0140-6736(22)01586-0
Yotsuyanagi, Ohmagari, Doi, Efficacy and Safety of 5-Day Oral Ensitrelvir for Patients With Mild to Moderate COVID-19: The SCORPIO-SR Randomized Clinical Trial, JAMA Netw Open, doi:10.1001/jamanetworkopen.2023.54991
DOI record:
{
"DOI": "10.7759/cureus.61048",
"ISSN": [
"2168-8184"
],
"URL": "http://dx.doi.org/10.7759/cureus.61048",
"author": [
{
"affiliation": [],
"family": "Yoshida",
"given": "Ryohei",
"sequence": "first"
},
{
"affiliation": [],
"family": "Sasaki",
"given": "Takaaki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ohsaki",
"given": "Yoshinobu",
"sequence": "additional"
}
],
"container-title": "Cureus",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
5,
25
]
],
"date-time": "2024-05-25T11:56:11Z",
"timestamp": 1716638171000
},
"deposited": {
"date-parts": [
[
2024,
5,
25
]
],
"date-time": "2024-05-25T11:56:14Z",
"timestamp": 1716638174000
},
"indexed": {
"date-parts": [
[
2024,
5,
26
]
],
"date-time": "2024-05-26T00:16:02Z",
"timestamp": 1716682562854
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
5,
25
]
]
},
"language": "en",
"link": [
{
"URL": "https://www.cureus.com/articles/242222-real-world-efficacy-of-ensitrelvir-in-hospitalized-patients-with-covid-19-in-japan-a-retrospective-observational-study",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.7759",
"published": {
"date-parts": [
[
2024,
5,
25
]
]
},
"published-print": {
"date-parts": [
[
2024,
5,
25
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1056/NEJMoa2035389",
"article-title": "Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine",
"author": "Baden LR",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "ref1",
"unstructured": "Baden LR, El Sahly HM, Essink B, et al.. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021, 384:403-16. 10.1056/NEJMoa2035389",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2034577",
"article-title": "Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine",
"author": "Polack FP",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "ref2",
"unstructured": "Polack FP, Thomas SJ, Kitchin N, et al.. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020, 383:2603-15. 10.1056/NEJMoa2034577",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1186/s12879-021-06536-3",
"article-title": "Mortality-related risk factors of COVID-19: a systematic review and meta-analysis of 42 studies and 423,117 patients",
"author": "Dessie ZG",
"doi-asserted-by": "publisher",
"journal-title": "BMC Infect Dis",
"key": "ref3",
"unstructured": "Dessie ZG, Zewotir T. Mortality-related risk factors of COVID-19: a systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect Dis. 2021, 21:855. 10.1186/s12879-021-06536-3",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.6775",
"article-title": "Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area",
"author": "Richardson S",
"doi-asserted-by": "publisher",
"journal-title": "JAMA",
"key": "ref4",
"unstructured": "Richardson S, Hirsch JS, Narasimhan M, et al.. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020, 323:2052-9. 10.1001/jama.2020.6775",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1038/s41440-020-0485-2",
"article-title": "COVID-19 patients with hypertension have more severe disease: a multicenter retrospective observational study",
"author": "Huang S",
"doi-asserted-by": "publisher",
"journal-title": "Hypertens Res",
"key": "ref5",
"unstructured": "Huang S, Wang J, Liu F, et al.. COVID-19 patients with hypertension have more severe disease: a multicenter retrospective observational study. Hypertens Res. 2020, 43:824-31. 10.1038/s41440-020-0485-2",
"volume": "43",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(22)01586-0",
"article-title": "Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: an observational study",
"author": "Wong CK",
"doi-asserted-by": "publisher",
"journal-title": "Lancet",
"key": "ref6",
"unstructured": "Wong CK, Au IC, Lau KT, Lau EH, Cowling BJ, Leung GM. Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: an observational study. Lancet. 2022, 400:1213-22. 10.1016/S0140-6736(22)01586-0",
"volume": "400",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2023184",
"article-title": "Repurposed Antiviral Drugs for Covid-19 - Interim WHO Solidarity Trial Results",
"author": "Pan H",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "ref7",
"unstructured": "Pan H, Peto R, Henao-Restrepo AM, et al.. Repurposed Antiviral Drugs for Covid-19 - Interim WHO Solidarity Trial Results. N Engl J Med. 2021, 384:497-511. 10.1056/NEJMoa2023184",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1038/s41573-023-00672-y",
"article-title": "Therapeutic strategies for COVID-19: progress and lessons learned",
"author": "Li G",
"doi-asserted-by": "publisher",
"journal-title": "Nat Rev Drug Discov",
"key": "ref8",
"unstructured": "Li G, Hilgenfeld R, Whitley R, De Clercq E. Therapeutic strategies for COVID-19: progress and lessons learned. Nat Rev Drug Discov. 2023, 22:449-75. 10.1038/s41573-023-00672-y",
"volume": "22",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the Treatment of Covid-19 - Final Report",
"author": "Beigel JH",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "ref9",
"unstructured": "Beigel JH, Tomashek KM, Dodd LE, et al.. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med. 2020, 383:1813-26. 10.1056/NEJMoa2007764",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2116044",
"article-title": "Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients",
"author": "Jayk Bernal A",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "ref10",
"unstructured": "Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al.. Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients. N Engl J Med. 2022, 386:509-20. 10.1056/NEJMoa2116044",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1093/cid/ciac933",
"article-title": "Efficacy and Safety of Ensitrelvir in Patients With Mild-to-Moderate Coronavirus Disease 2019: The Phase 2b Part of a Randomized, Placebo-Controlled, Phase 2/3 Study",
"author": "Mukae H",
"doi-asserted-by": "publisher",
"journal-title": "Clin Infect Dis",
"key": "ref11",
"unstructured": "Mukae H, Yotsuyanagi H, Ohmagari N, et al.. Efficacy and Safety of Ensitrelvir in Patients With Mild-to-Moderate Coronavirus Disease 2019: The Phase 2b Part of a Randomized, Placebo-Controlled, Phase 2/3 Study. Clin Infect Dis. 2023, 76:1403-11. 10.1093/cid/ciac933",
"volume": "76",
"year": "2023"
},
{
"DOI": "10.1016/S2666-5247(21)00278-0",
"article-title": "The emergence of powerful oral anti-COVID-19 drugs in the post-vaccine era",
"author": "Fan H",
"doi-asserted-by": "publisher",
"journal-title": "Lancet Microbe",
"key": "ref12",
"unstructured": "Fan H, Lou F, Fan J, Li M, Tong Y. The emergence of powerful oral anti-COVID-19 drugs in the post-vaccine era. Lancet Microbe. 2022, 3:e91. 10.1016/S2666-5247(21)00278-0",
"volume": "3",
"year": "2022"
},
{
"DOI": "10.1128/aac.00697-22",
"article-title": "A Randomized Phase 2/3 Study of Ensitrelvir, a Novel Oral SARS-CoV-2 3C-Like Protease Inhibitor, in Japanese Patients with Mild-to-Moderate COVID-19 or Asymptomatic SARS-CoV-2 Infection: Results of the Phase 2a Part",
"author": "Mukae H",
"doi-asserted-by": "publisher",
"journal-title": "Antimicrob Agents Chemother",
"key": "ref13",
"unstructured": "Mukae H, Yotsuyanagi H, Ohmagari N, et al.. A Randomized Phase 2/3 Study of Ensitrelvir, a Novel Oral SARS-CoV-2 3C-Like Protease Inhibitor, in Japanese Patients with Mild-to-Moderate COVID-19 or Asymptomatic SARS-CoV-2 Infection: Results of the Phase 2a Part. Antimicrob Agents Chemother. 2022, 66:e0069722. 10.1128/aac.00697-22",
"volume": "66",
"year": "2022"
},
{
"DOI": "10.1093/jac/dkac257",
"article-title": "Efficacy of ensitrelvir against SARS-CoV-2 in a delayed-treatment mouse model",
"author": "Nobori H",
"doi-asserted-by": "publisher",
"journal-title": "J Antimicrob Chemother",
"key": "ref14",
"unstructured": "Nobori H, Fukao K, Kuroda T, et al.. Efficacy of ensitrelvir against SARS-CoV-2 in a delayed-treatment mouse model. J Antimicrob Chemother. 2022, 77:2984-91. 10.1093/jac/dkac257",
"volume": "77",
"year": "2022"
},
{
"DOI": "10.1016/j.jiac.2023.09.020",
"article-title": "Seroprevalence kinetics of SARS-CoV-2 antibodies in pediatric patients visiting a hospital during COVID-19 pandemic in Japan",
"author": "Hashimoto T",
"doi-asserted-by": "publisher",
"journal-title": "J Infect Chemother",
"key": "ref15",
"unstructured": "Hashimoto T, Yahiro T, Khan S, et al.. Seroprevalence kinetics of SARS-CoV-2 antibodies in pediatric patients visiting a hospital during COVID-19 pandemic in Japan. J Infect Chemother. 2024, 30:169-71. 10.1016/j.jiac.2023.09.020",
"volume": "30",
"year": "2024"
},
{
"DOI": "10.1016/j.jiph.2020.07.014",
"article-title": "COVID-19 and comorbidities: Deleterious impact on infected patients",
"author": "Ejaz H",
"doi-asserted-by": "publisher",
"journal-title": "J Infect Public Health",
"key": "ref16",
"unstructured": "Ejaz H, Alsrhani A, Zafar A, et al.. COVID-19 and comorbidities: Deleterious impact on infected patients. J Infect Public Health. 2020, 13:1833-9. 10.1016/j.jiph.2020.07.014",
"volume": "13",
"year": "2020"
},
{
"DOI": "10.1016/j.arr.2020.101205",
"article-title": "Aging in COVID-19: Vulnerability, immunity and intervention",
"author": "Chen Y",
"doi-asserted-by": "publisher",
"journal-title": "Ageing Res Rev",
"key": "ref17",
"unstructured": "Chen Y, Klein SL, Garibaldi BT, et al.. Aging in COVID-19: Vulnerability, immunity and intervention. Ageing Res Rev. 2021, 65:101205. 10.1016/j.arr.2020.101205",
"volume": "65",
"year": "2021"
},
{
"DOI": "10.1001/jamanetworkopen.2023.54991",
"article-title": "Efficacy and Safety of 5-Day Oral Ensitrelvir for Patients With Mild to Moderate COVID-19: The SCORPIO-SR Randomized Clinical Trial",
"author": "Yotsuyanagi H",
"doi-asserted-by": "publisher",
"journal-title": "JAMA Netw Open",
"key": "ref18",
"unstructured": "Yotsuyanagi H, Ohmagari N, Doi Y, et al.. Efficacy and Safety of 5-Day Oral Ensitrelvir for Patients With Mild to Moderate COVID-19: The SCORPIO-SR Randomized Clinical Trial. JAMA Netw Open. 2024, 7:e2354991. 10.1001/jamanetworkopen.2023.54991",
"volume": "7",
"year": "2024"
}
],
"reference-count": 18,
"references-count": 18,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.cureus.com/articles/242222-real-world-efficacy-of-ensitrelvir-in-hospitalized-patients-with-covid-19-in-japan-a-retrospective-observational-study"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Real-World Efficacy of Ensitrelvir in Hospitalized Patients With COVID-19 in Japan: A Retrospective Observational Study",
"type": "journal-article"
}