TRPC6 inhibitor (BI 764198) to reduce risk and severity of ARDS due to COVID-19: a phase II randomised controlled trial
et al., Thorax, doi:10.1136/thorax-2022-219668, NCT04604184, Apr 2023
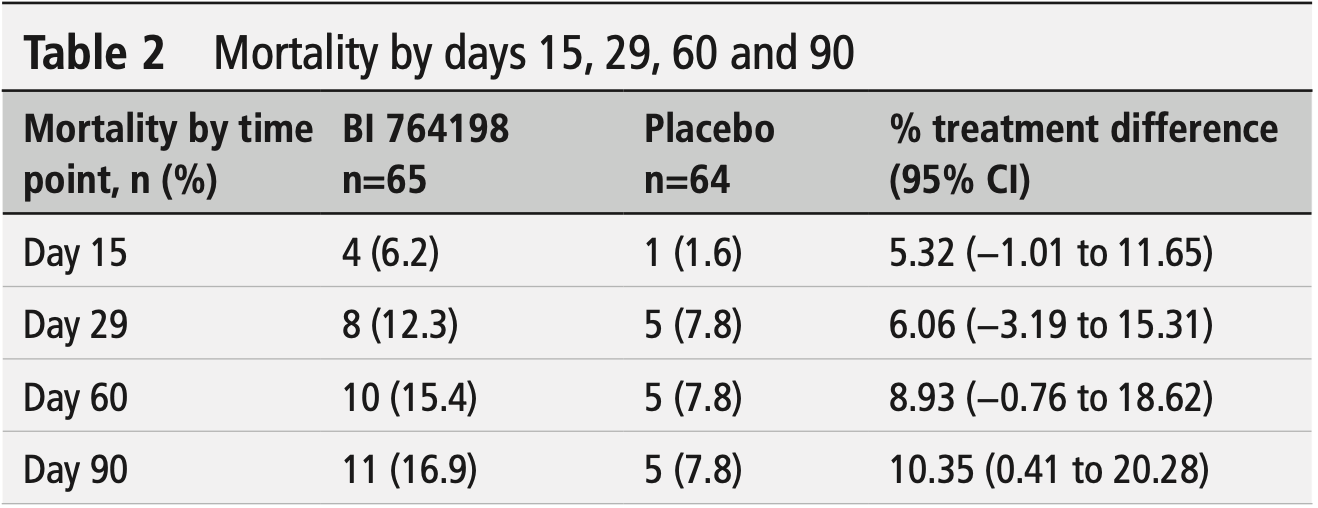
RCT 129 hospitalized COVID-19 patients requiring non-invasive oxygen support showing no benefit and potential harm with BI 764198 (TRPC6 inhibitor). The study was terminated early due to lack of efficacy and an imbalance of fatal events (11 deaths vs 5 deaths in placebo group). Patients receiving BI 764198 had longer hospitalizations (+3.4 days), longer time to recovery, and longer oxygen use duration compared to placebo.
|
risk of death, 116.6% higher, RR 2.17, p = 0.18, treatment 11 of 65 (16.9%), control 5 of 64 (7.8%), day 90.
|
|
risk of death, 96.9% higher, RR 1.97, p = 0.27, treatment 10 of 65 (15.4%), control 5 of 64 (7.8%), day 60.
|
|
risk of death, 57.5% higher, RR 1.58, p = 0.56, treatment 8 of 65 (12.3%), control 5 of 64 (7.8%), day 29.
|
|
risk of death, 293.8% higher, RR 3.94, p = 0.37, treatment 4 of 65 (6.2%), control 1 of 64 (1.6%), day 15.
|
|
risk of progression, 11.6% higher, RR 1.12, p = 0.84, treatment 17 of 65 (26.2%), control 15 of 64 (23.4%), death, ICU admission, or mechanical ventilation, day 29.
|
|
risk of no recovery, 42.2% higher, RR 1.42, p = 0.48, treatment 13 of 65 (20.0%), control 9 of 64 (14.1%), day 29.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Ware et al., 6 Apr 2023, Double Blind Randomized Controlled Trial, placebo-controlled, multiple countries, peer-reviewed, mean age 63.7, 10 authors, study period 12 November, 2020 - 24 February, 2021, average treatment delay 8.3 days, trial NCT04604184 (history).
TRPC6 inhibitor (BI 764198) to reduce risk and severity of ARDS due to COVID-19: a phase II randomised controlled trial
Thorax, doi:10.1136/thorax-2022-219668
Background Despite the availability of COVID-19 vaccinations, there remains a need to investigate treatments to reduce the risk or severity of potentially fatal complications of COVID-19, such as acute respiratory distress syndrome (ARDS). This study evaluated the efficacy and safety of the transient receptor potential channel C6 (TRPC6) inhibitor, BI 764198, in reducing the risk and/or severity of ARDS in patients hospitalised for COVID-19 and requiring noninvasive, supplemental oxygen support (oxygen by mask or nasal prongs, oxygen by non-invasive ventilation or high-flow nasal oxygen). Methods Multicentre, double-blind, randomised phase II trial comparing once-daily oral BI 764198 (n=65) with placebo (n=64) for 28 days (+2-month follow-up). Primary endpoint: proportion of patients alive and free of mechanical ventilation at day 29. Secondary endpoints: proportion of patients alive and discharged without oxygen (day 29); occurrence of either in-hospital mortality, intensive care unit admission or mechanical ventilation (day 29); time to first response (clinical improvement/recovery); ventilator-free days (day 29); and mortality (days 15, 29, 60 and 90). Results No difference was observed for the primary endpoint: BI 764198 (83.1%) versus placebo (87.5%) (estimated risk difference -5.39%; 95% CI -16.08 to 5.30; p=0.323). For secondary endpoints, a longer time to first response (rate ratio 0.67; 95% CI 0.46 to 0.99; p=0.045) and longer hospitalisation (+3.41 days; 95% CI 0.49 to 6.34; p=0.023) for BI 764198 versus placebo was observed; no other significant differences were observed. On-treatment adverse events were similar between trial arms and more fatal events were reported for BI 764198 (n=7) versus placebo (n=2). Treatment was stopped early based on an interim observation of a lack of efficacy and an imbalance of fatal events (Data Monitoring Committee recommendation). Conclusions TRPC6 inhibition was not effective in reducing the risk and/or severity of ARDS in patients with COVID-19 requiring non-invasive, supplemental oxygen support.
Respiratory infection investigators (online supplemental table 1) for their dedicated work in this trial. A special recognition goes to all patients and their families for their invaluable time and participation which made the conduct of this trial possible. The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). The authors did not receive payment for the development of the manuscript. Shivani Singh, PhD, of Meditech Media, provided writing assistance, which was contracted and funded by Boehringer Ingelheim. Boehringer Ingelheim were given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations. The study was supported and funded by Boehringer Ingelheim. Contributors Conception and design: LW, NS, DFM, RK, WC, AG and TW. Acquisition of data/design of data acquisition platform: LW, NS, VE, GAD, PL, RK, WC and AG. Principal investigators at study sites: VE, GAD and PL. Statistical analysis: NS, RK, WC and AG. Data interpretation, edited and reviewed the manuscript, and approved the final version of the manuscript: LW, NS, DFM, VE, GAD, PL, RK, WC, AG and TW. LW accepts full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Supplementary Methods
Exclusion criteria The exclusion criteria included: pulmonary oedema/respiratory failure due to cardiogenic insult;..
References
Ai, Zhang, Zhang, Omicron variant showed lower neutralizing sensitivity than other SARS-CoV-2 variants to immune sera elicited by vaccines after boost, Emerg Microbes Infect, doi:10.1080/22221751.2021.2022440
Baraniuk, Where are we with drug treatments for COVID-19, BMJ, doi:10.1136/bmj.n1109
Brown, Alazawi, Kanoni, Interleukin-6 receptor antagonists in critically ill patients with COVID-19, N Engl J Med, doi:10.1056/NEJMc2108482
Clinicaltrials, Gov, A study to test how well healthy men tolerate different doses of BI 764198
Dietrich, Modulators of transient receptor potential (Trp) channels as therapeutic options in lung disease, Pharmaceuticals, doi:10.3390/ph12010023
Group, Tocilizumab in patients admitted to hospital with COVID-19 (recovery): a randomised, controlled, open-label, platform trial, Lancet, doi:10.1016/S0140-6736(21)00676-0
Guan, Ni, Hu, Clinical characteristics of coronavirus disease 2019 in China, N Engl J Med, doi:10.1056/NEJMoa2002032
Guimarães, Quirk, Furtado, Tofacitinib in patients hospitalized with COVID-19 pneumonia, N Engl J Med, doi:10.1056/NEJMoa2101643
Gurusamy, Nasseri, Lee, Kinin B1 receptor antagonist BI113823 reduces allergen-induced airway inflammation and mucus secretion in mice, Pharmacol Res, doi:10.1016/j.phrs.2015.12.017
Hajjar, Costa, Da, Rizk, Intensive care management of patients with COVID-19: a practical approach, Ann Intensive Care, doi:10.1186/s13613-021-00820-w
Hofmann, Fiedler, Vierkotten, Classical transient receptor potential 6 (TRPC6) channels support myofibroblast differentiation and development of experimental pulmonary fibrosis, Biochim Biophys Acta Mol Basis Dis, doi:10.1016/j.bbadis.2016.12.002
Hohlfeld, Badorrek, Faulenbach, BI 1026706, a bradykinin 1 antagonist, did not reduce pulmonary inflammation upon segmental endotoxin challenge in healthy current smoker subjects compared with placebo
Horby, Lim, Emberson, Dexamethasone in hospitalized patients with COVID-19, N Engl J Med, doi:10.1056/NEJMoa2021436
Huang, Wang, Li, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet, doi:10.1016/S0140-6736(20)30183-5
Jaffal, Abbas, TRP channels in COVID-19 disease: potential targets for prevention and treatment, Chem Biol Interact, doi:10.1016/j.cbi.2021.109567
Kalil, Patterson, Mehta, Baricitinib plus remdesivir for hospitalized adults with COVID-19, N Engl J Med, doi:10.1056/NEJMoa2031994
Kirsten, Singh, Pedersen, Safety and tolerability of four weeks' treatment with a bradykinin 1 receptor antagonist (BI 1026706) in patients with COPD (GOLD I-III)
Malczyk, Erb, Veith, The role of transient receptor potential channel 6 channels in the pulmonary vasculature, Front Immunol, doi:10.3389/fimmu.2017.00707
Marconi, Ramanan, De Bono, Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial, Lancet Respir Med, doi:10.1016/S2213-2600(21)00331-3
Marshall, Murthy, Diaz, A minimal common outcome measure set for COVID-19 clinical research, Lancet Infect Dis, doi:10.1016/S1473-3099(20)30483-7
Matera, Rogliani, Calzetta, Pharmacological management of COVID-19 patients with ARDS (CARDS): a narrative review, Respir Med, doi:10.1016/j.rmed.2020.106114
Montenegro, Unigarro, Paredes, Acute respiratory distress syndrome (ARDS) caused by the novel coronavirus disease (COVID-19): a practical comprehensive literature review, Expert Rev Respir Med, doi:10.1080/17476348.2020.1820329
Murugesan, Jung, Lee, Kinin B1 receptor inhibition with BI113823 reduces inflammatory response, mitigates organ injury, and improves survival among rats with severe sepsis, J Infect Dis, doi:10.1093/infdis/jiv426
Nasseri, Gurusamy, Jung, Kinin B1 receptor antagonist BI113823 reduces acute lung injury, Crit Care Med, doi:10.1097/CCM.0000000000001268
Ndwandwe, Wiysonge, COVID-19 vaccines, Curr Opin Immunol, doi:10.1016/j.coi.2021.07.003
Reilly, Bellamy, Shashaty, Heterogeneous phenotypes of acute respiratory distress syndrome after major trauma, Ann Am Thorac Soc, doi:10.1513/AnnalsATS.201308-280OC
Singh, Knezevic, Ahmmed, Galphaq-TRPC6-mediated Ca2+ entry induces RhoA activation and resultant endothelial cell shape change in response to thrombin, J Biol Chem, doi:10.1074/jbc.M608288200
Viana, Moyo, Amoako, Rapid epidemic expansion of the SARS-CoV-2 omicron variant in southern africa, Nature, doi:10.1038/s41586-022-04411-y
Wang, Hu, Hu, Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China, JAMA, doi:10.1001/jama.2020.1585
Ware, None, Thorax, doi:10.1136/thorax-2022-219668Protectedbycopyright
Ware, Pathophysiology of acute respiratory distress syndrome
Weissmann, Sydykov, Kalwa, Activation of TRPC6 channels is essential for lung ischaemia-reperfusion induced oedema in mice, Nat Commun, doi:10.1038/ncomms1660
Welte, Ambrose, Sibbring, Current evidence for COVID-19 therapies: a systematic literature review, Eur Respir Rev, doi:10.1183/16000617.0384-2020
Yang, Yu, Xu, Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study, Lancet Respir Med, doi:10.1016/S2213-2600(20)30079-5
DOI record:
{
"DOI": "10.1136/thorax-2022-219668",
"ISSN": [
"0040-6376",
"1468-3296"
],
"URL": "http://dx.doi.org/10.1136/thorax-2022-219668",
"abstract": "<jats:sec><jats:title>Background</jats:title><jats:p>Despite the availability of COVID-19 vaccinations, there remains a need to investigate treatments to reduce the risk or severity of potentially fatal complications of COVID-19, such as acute respiratory distress syndrome (ARDS). This study evaluated the efficacy and safety of the transient receptor potential channel C6 (TRPC6) inhibitor, BI 764198, in reducing the risk and/or severity of ARDS in patients hospitalised for COVID-19 and requiring non-invasive, supplemental oxygen support (oxygen by mask or nasal prongs, oxygen by non-invasive ventilation or high-flow nasal oxygen).</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>Multicentre, double-blind, randomised phase II trial comparing once-daily oral BI 764198 (n=65) with placebo (n=64) for 28 days (+2-month follow-up). Primary endpoint: proportion of patients alive and free of mechanical ventilation at day 29. Secondary endpoints: proportion of patients alive and discharged without oxygen (day 29); occurrence of either in-hospital mortality, intensive care unit admission or mechanical ventilation (day 29); time to first response (clinical improvement/recovery); ventilator-free days (day 29); and mortality (days 15, 29, 60 and 90).</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>No difference was observed for the primary endpoint: BI 764198 (83.1%) versus placebo (87.5%) (estimated risk difference –5.39%; 95% CI –16.08 to 5.30; p=0.323). For secondary endpoints, a longer time to first response (rate ratio 0.67; 95% CI 0.46 to 0.99; p=0.045) and longer hospitalisation (+3.41 days; 95% CI 0.49 to 6.34; p=0.023) for BI 764198 versus placebo was observed; no other significant differences were observed. On-treatment adverse events were similar between trial arms and more fatal events were reported for BI 764198 (n=7) versus placebo (n=2). Treatment was stopped early based on an interim observation of a lack of efficacy and an imbalance of fatal events (Data Monitoring Committee recommendation).</jats:p></jats:sec><jats:sec><jats:title>Conclusions</jats:title><jats:p>TRPC6 inhibition was not effective in reducing the risk and/or severity of ARDS in patients with COVID-19 requiring non-invasive, supplemental oxygen support.</jats:p></jats:sec><jats:sec><jats:title>Trial registration number</jats:title><jats:p><jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"clintrialgov\" xlink:href=\"NCT04604184\">NCT04604184</jats:ext-link>.</jats:p></jats:sec>",
"accepted": {
"date-parts": [
[
2023,
1,
31
]
]
},
"alternative-id": [
"10.1136/thorax-2022-219668"
],
"author": [
{
"affiliation": [],
"family": "Ware",
"given": "Lorraine B",
"sequence": "first"
},
{
"affiliation": [],
"family": "Soleymanlou",
"given": "Nima",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McAuley",
"given": "Danny Francis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Estrada",
"given": "Vicente",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Diaz",
"given": "George A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lacamera",
"given": "Peter",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kaste",
"given": "Renee",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Choi",
"given": "Wansuk",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gupta",
"given": "Abhya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Welte",
"given": "Tobias",
"sequence": "additional"
}
],
"clinical-trial-number": [
{
"clinical-trial-number": "nct04604184",
"registry": "10.18810/clinical-trials-gov"
},
{
"clinical-trial-number": "nct03854552",
"registry": "10.18810/clinical-trials-gov"
},
{
"clinical-trial-number": "nct04102462",
"registry": "10.18810/clinical-trials-gov"
}
],
"container-title": "Thorax",
"container-title-short": "Thorax",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"bmj.com"
]
},
"created": {
"date-parts": [
[
2023,
4,
6
]
],
"date-time": "2023-04-06T15:05:13Z",
"timestamp": 1680793513000
},
"deposited": {
"date-parts": [
[
2024,
5,
14
]
],
"date-time": "2024-05-14T03:53:08Z",
"timestamp": 1715658788000
},
"funder": [
{
"DOI": "10.13039/100001003",
"award": [
"N/A"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/100001003",
"id-type": "DOI"
}
],
"name": "Boehringer Ingelheim"
}
],
"indexed": {
"date-parts": [
[
2025,
10,
21
]
],
"date-time": "2025-10-21T15:50:13Z",
"timestamp": 1761061813516,
"version": "3.37.3"
},
"is-referenced-by-count": 6,
"issue": "8",
"issued": {
"date-parts": [
[
2023,
4,
6
]
]
},
"journal-issue": {
"issue": "8",
"published-online": {
"date-parts": [
[
2023,
7,
13
]
]
},
"published-print": {
"date-parts": [
[
2023,
8
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
4,
6
]
],
"date-time": "2023-04-06T00:00:00Z",
"timestamp": 1680739200000
}
}
],
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1136/thorax-2022-219668",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "239",
"original-title": [],
"page": "816-824",
"prefix": "10.1136",
"published": {
"date-parts": [
[
2023,
4,
6
]
]
},
"published-online": {
"date-parts": [
[
2023,
4,
6
]
]
},
"published-print": {
"date-parts": [
[
2023,
8
]
]
},
"publisher": "BMJ",
"reference": [
{
"DOI": "10.1056/NEJMoa2002032",
"doi-asserted-by": "publisher",
"key": "2024051320382765000_78.8.816.1"
},
{
"DOI": "10.1183/16000617.0384-2020",
"article-title": "Current evidence for COVID-19 therapies: a systematic literature review",
"author": "Welte",
"doi-asserted-by": "crossref",
"journal-title": "Eur Respir Rev",
"key": "2024051320382765000_78.8.816.2",
"volume": "30",
"year": "2021"
},
{
"key": "2024051320382765000_78.8.816.3",
"unstructured": "Centers for Disease Control and Prevention (CDC) . Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19). 2021. Available: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html [Accessed 4 Feb 2022]."
},
{
"DOI": "10.1001/jama.2020.1585",
"doi-asserted-by": "publisher",
"key": "2024051320382765000_78.8.816.4"
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"doi-asserted-by": "publisher",
"key": "2024051320382765000_78.8.816.5"
},
{
"DOI": "10.1016/S2213-2600(20)30079-5",
"article-title": "Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study",
"author": "Yang",
"doi-asserted-by": "crossref",
"first-page": "475",
"journal-title": "Lancet Respir Med",
"key": "2024051320382765000_78.8.816.6",
"volume": "8",
"year": "2020"
},
{
"key": "2024051320382765000_78.8.816.7",
"unstructured": "National Institutes of Health . Therapeutic management of hospitalized adults with COVID-19. 2021. Available: https://www.covid19treatmentguidelines.nih.gov/management/clinical-management/hospitalized-adults--therapeutic-management [Accessed 1 Nov 2022]."
},
{
"DOI": "10.1056/NEJMoa2021436",
"doi-asserted-by": "publisher",
"key": "2024051320382765000_78.8.816.8"
},
{
"DOI": "10.1016/S0140-6736(21)00676-0",
"doi-asserted-by": "publisher",
"key": "2024051320382765000_78.8.816.9"
},
{
"DOI": "10.1056/NEJMc2108482",
"article-title": "Interleukin-6 receptor antagonists in critically ill patients with COVID-19",
"author": "Brown",
"doi-asserted-by": "crossref",
"first-page": "1147",
"journal-title": "N Engl J Med",
"key": "2024051320382765000_78.8.816.10",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(21)00331-3",
"article-title": "Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial",
"author": "Marconi",
"doi-asserted-by": "crossref",
"first-page": "1407",
"journal-title": "Lancet Respir Med",
"key": "2024051320382765000_78.8.816.11",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1056/nejmoa2101643",
"doi-asserted-by": "publisher",
"key": "2024051320382765000_78.8.816.12"
},
{
"DOI": "10.1056/nejmoa2031994",
"doi-asserted-by": "publisher",
"key": "2024051320382765000_78.8.816.13"
},
{
"key": "2024051320382765000_78.8.816.14",
"unstructured": "U.S. Food and Drug Administration . Sotrovimab emergency use authorization. 2021. Available: https://www.sotrovimab.com/content/dam/cf-pharma/hcp-sotrovimab-phase2/en_US/sotrovimab-fda-letter-of-authorization.pdf [Accessed 16 Dec 2021]."
},
{
"key": "2024051320382765000_78.8.816.15",
"unstructured": "U.S. Food and Drug Administration . Bamlanivimab and etesevimab emergency use authorization. 2022. Available: https://www.fda.gov/media/145801/download [Accessed 16 Dec 2021]."
},
{
"key": "2024051320382765000_78.8.816.16",
"unstructured": "U.S. Food and Drug Administration . REGEN-COV emergency use authorization. 2022. Available: https://www.fda.gov/media/145610/download [Accessed 24 Jan 2022]."
},
{
"key": "2024051320382765000_78.8.816.17",
"unstructured": "U.S. Food and Drug Administration . Molnupiravir emergency use authorization. 2021. Available: https://www.fda.gov/media/155053/download [Accessed 7 Feb 2022]."
},
{
"key": "2024051320382765000_78.8.816.18",
"unstructured": "U.S. Food and Drug Administration . Paxlovid emergency use authorization. 2021. Available: https://www.fda.gov/media/155049/download [Accessed 7 Feb 2022]."
},
{
"DOI": "10.1016/j.coi.2021.07.003",
"doi-asserted-by": "publisher",
"key": "2024051320382765000_78.8.816.19"
},
{
"DOI": "10.1080/22221751.2021.2022440",
"article-title": "Omicron variant showed lower neutralizing sensitivity than other SARS-CoV-2 variants to immune sera elicited by vaccines after boost",
"author": "Ai",
"doi-asserted-by": "crossref",
"first-page": "337",
"journal-title": "Emerg Microbes Infect",
"key": "2024051320382765000_78.8.816.20",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1038/s41586-022-04411-y",
"article-title": "Rapid epidemic expansion of the SARS-CoV-2 omicron variant in southern africa",
"author": "Viana",
"doi-asserted-by": "crossref",
"first-page": "679",
"journal-title": "Nature",
"key": "2024051320382765000_78.8.816.21",
"volume": "603",
"year": "2022"
},
{
"DOI": "10.1093/med/9780199600830.003.0108",
"doi-asserted-by": "crossref",
"key": "2024051320382765000_78.8.816.22",
"unstructured": "Ware LB . Pathophysiology of acute respiratory distress syndrome. In: Webb A , Angus D , Finfer S , et al , eds. Oxford Textbook of Critical Care. Oxford University Press, 2016: 497–500."
},
{
"DOI": "10.1513/AnnalsATS.201308-280OC",
"doi-asserted-by": "publisher",
"key": "2024051320382765000_78.8.816.23"
},
{
"key": "2024051320382765000_78.8.816.24",
"unstructured": "GTEx Portal . Gene page. 2021. Available: https://gtexportal.org/home/gene/TRPC6 [Accessed 1 Nov 2021]."
},
{
"DOI": "10.3390/ph12010023",
"article-title": "Modulators of transient receptor potential (Trp) channels as therapeutic options in lung disease",
"author": "Dietrich",
"doi-asserted-by": "crossref",
"journal-title": "Pharmaceuticals (Basel)",
"key": "2024051320382765000_78.8.816.25",
"volume": "12",
"year": "2019"
},
{
"DOI": "10.1074/jbc.M608288200",
"doi-asserted-by": "publisher",
"key": "2024051320382765000_78.8.816.26"
},
{
"key": "2024051320382765000_78.8.816.27",
"unstructured": "ClinicalTrials.gov . NCT03854552 safety, tolerability and pharmacokinetics of single rising oral doses of BI 764198 in healthy male subjects (single-blind, partially randomised, placebo-controlled, parallel group design). 2021. Available: https://clinicaltrials.gov/ct2/show/NCT03854552 [Accessed 15 Dec 2020]."
},
{
"key": "2024051320382765000_78.8.816.28",
"unstructured": "ClinicalTrials.gov . A study to test how well healthy men tolerate different doses of BI 764198. 2020. Available: https://clinicaltrials.gov/ct2/show/NCT04102462 [Accessed 28 Sep 2022]."
},
{
"DOI": "10.1016/S1473-3099(20)30483-7",
"doi-asserted-by": "publisher",
"key": "2024051320382765000_78.8.816.29"
},
{
"key": "2024051320382765000_78.8.816.30",
"unstructured": "Infectious Diseases Society of America . IDSA guidelines on the treatment and management of patients with COVID-19. 2021. Available: https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management [Accessed 1 Nov 2021]."
},
{
"key": "2024051320382765000_78.8.816.31",
"unstructured": "National Institutes of Health . Coronavirus disease 2019 (COVID-19) treatment guidelines. 2022. Available: https://www.covid19treatmentguidelines.nih.gov [Accessed 1 Nov 2021]."
},
{
"key": "2024051320382765000_78.8.816.32",
"unstructured": "EBSCO Medical . Government of Spain Ministry of Health (Gobierno de Espana Ministerio de Sanidad) guidelines on management of hospitalized patients with COVID-19. 2022. Available: https://covid-19.ebscomedical.com/government-spain-ministry-health-gobierno-de-espana-ministerio-de-sanidad-guidelines-management [Accessed 1 Nov 2021]."
},
{
"DOI": "10.1038/ncomms1660",
"article-title": "Activation of TRPC6 channels is essential for lung ischaemia-reperfusion induced oedema in mice",
"author": "Weissmann",
"doi-asserted-by": "crossref",
"journal-title": "Nat Commun",
"key": "2024051320382765000_78.8.816.33",
"volume": "3",
"year": "2012"
},
{
"DOI": "10.1016/j.cbi.2021.109567",
"article-title": "TRP channels in COVID-19 disease: potential targets for prevention and treatment",
"author": "Jaffal",
"doi-asserted-by": "crossref",
"first-page": "109567",
"journal-title": "Chem Biol Interact",
"key": "2024051320382765000_78.8.816.34",
"volume": "345",
"year": "2021"
},
{
"DOI": "10.1016/j.bbadis.2016.12.002",
"doi-asserted-by": "publisher",
"key": "2024051320382765000_78.8.816.35"
},
{
"DOI": "10.3389/fimmu.2017.00707",
"article-title": "The role of transient receptor potential channel 6 channels in the pulmonary vasculature",
"author": "Malczyk",
"doi-asserted-by": "crossref",
"journal-title": "Front Immunol",
"key": "2024051320382765000_78.8.816.36",
"volume": "8",
"year": "2017"
},
{
"DOI": "10.1097/CCM.0000000000001268",
"article-title": "Kinin B1 receptor antagonist BI113823 reduces acute lung injury",
"author": "Nasseri",
"doi-asserted-by": "crossref",
"first-page": "e499",
"journal-title": "Crit Care Med",
"key": "2024051320382765000_78.8.816.37",
"volume": "43",
"year": "2015"
},
{
"DOI": "10.1016/j.phrs.2015.12.017",
"article-title": "Kinin B1 receptor antagonist BI113823 reduces allergen-induced airway inflammation and mucus secretion in mice",
"author": "Gurusamy",
"doi-asserted-by": "crossref",
"first-page": "132",
"journal-title": "Pharmacol Res",
"key": "2024051320382765000_78.8.816.38",
"volume": "104",
"year": "2016"
},
{
"DOI": "10.1093/infdis/jiv426",
"doi-asserted-by": "publisher",
"key": "2024051320382765000_78.8.816.39"
},
{
"DOI": "10.1164/ajrccm-conference.2019.199.1_MeetingAbstracts.A2450",
"doi-asserted-by": "crossref",
"key": "#cr-split#-2024051320382765000_78.8.816.40.1",
"unstructured": "Kirsten A-M , Singh D , Pedersen F , et al . Safety and tolerability of four weeks' treatment with a bradykinin 1 receptor antagonist (BI 1026706) in patients with COPD (GOLD I-III). American Thoracic Society 2019 International Conference, May 17-22, 2019 - Dallas, TX"
},
{
"DOI": "10.1164/ajrccm-conference.2019.199.1_MeetingAbstracts.A2450",
"doi-asserted-by": "crossref",
"key": "#cr-split#-2024051320382765000_78.8.816.40.2",
"unstructured": "May 2019 doi:10.1164/ajrccm-conference.2019.199.1_MeetingAbstracts.A2450"
},
{
"DOI": "10.1164/ajrccm-conference.2019.199.1_MeetingAbstracts.A4874",
"doi-asserted-by": "crossref",
"key": "#cr-split#-2024051320382765000_78.8.816.41.1",
"unstructured": "Hohlfeld JM , Badorrek P , Faulenbach C , et al . BI 1026706, a bradykinin 1 antagonist, did not reduce pulmonary inflammation upon segmental endotoxin challenge in healthy current smoker subjects compared with placebo. American Thoracic Society 2019 International Conference, May 17-22, 2019 - Dallas, TX"
},
{
"DOI": "10.1164/ajrccm-conference.2019.199.1_MeetingAbstracts.A4874",
"doi-asserted-by": "crossref",
"key": "#cr-split#-2024051320382765000_78.8.816.41.2",
"unstructured": "May 2019 doi:10.1164/ajrccm-conference.2019.199.1_MeetingAbstracts.A4874"
},
{
"DOI": "10.1186/s13613-021-00820-w",
"article-title": "Intensive care management of patients with COVID-19: a practical approach",
"author": "Hajjar",
"doi-asserted-by": "crossref",
"journal-title": "Ann Intensive Care",
"key": "2024051320382765000_78.8.816.42",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1080/17476348.2020.1820329",
"doi-asserted-by": "publisher",
"key": "2024051320382765000_78.8.816.43"
},
{
"DOI": "10.1016/j.rmed.2020.106114",
"article-title": "Pharmacological management of COVID-19 patients with ARDS (CARDS): a narrative review",
"author": "Matera",
"doi-asserted-by": "crossref",
"first-page": "106114",
"journal-title": "Respir Med",
"key": "2024051320382765000_78.8.816.44",
"volume": "171",
"year": "2020"
},
{
"DOI": "10.1136/bmj.n1109",
"article-title": "Where are we with drug treatments for COVID-19?",
"author": "Baraniuk",
"doi-asserted-by": "crossref",
"journal-title": "BMJ",
"key": "2024051320382765000_78.8.816.45",
"volume": "373",
"year": "2021"
},
{
"key": "2024051320382765000_78.8.816.46",
"unstructured": "National Institutes of Health . COVID-19 treatment guidelines. Table 2c. Therapeutic management of adults hospitalized for COVID-19 based on disease severity. Available: https://www.covid19treatmentguidelines.nih.gov/tables/management-of-hospitalized-adults-summary [Accessed 4 Nov 2022]."
},
{
"key": "2024051320382765000_78.8.816.47",
"unstructured": "U.S. Food & Drug Administration . Emergency use authorization 099 [press release]. 2021."
}
],
"reference-count": 49,
"references-count": 49,
"relation": {},
"resource": {
"primary": {
"URL": "https://thorax.bmj.com/lookup/doi/10.1136/thorax-2022-219668"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "TRPC6 inhibitor (BI 764198) to reduce risk and severity of ARDS due to COVID-19: a phase II randomised controlled trial",
"type": "journal-article",
"update-policy": "https://doi.org/10.1136/crossmarkpolicy",
"volume": "78"
}