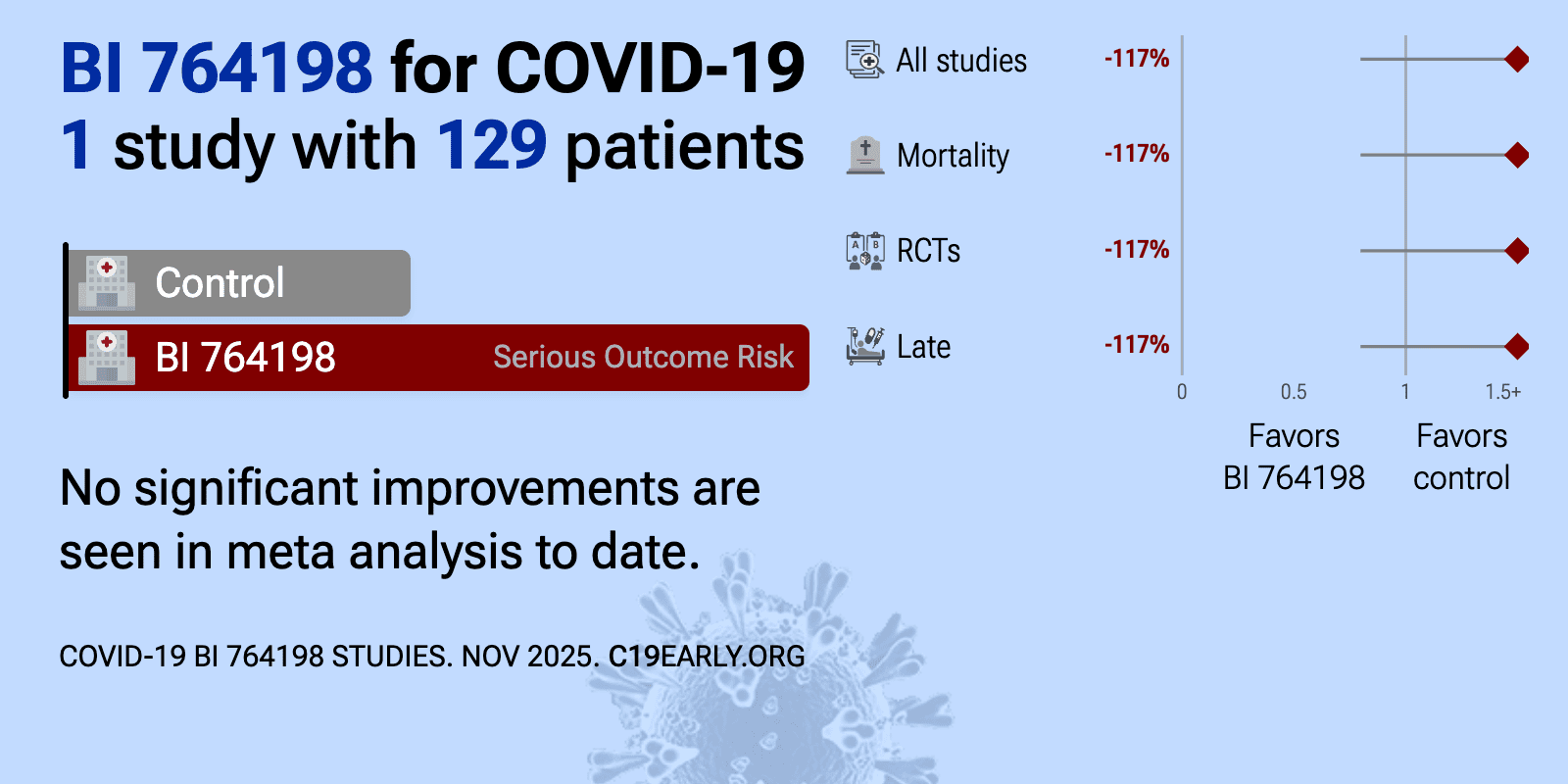
BI 764198 is an oral, small molecule inhibitor of the transient receptor potential channel 6 (TRPC6), investigated for the potential to reduce COVID-19-related lung injury and progression to ARDS.
Recent:Ware.
Apr 6 2023 |
et al., Thorax, doi:10.1136/thorax-2022-219668 | TRPC6 inhibitor (BI 764198) to reduce risk and severity of ARDS due to COVID-19: a phase II randomised controlled trial |
| 117% higher mortality (p=0.18), 12% higher progression (p=0.84), and 42% worse recovery (p=0.48). RCT 129 hospitalized COVID-19 patients requiring non-invasive oxygen support showing no benefit and potential harm with BI 764198 (TRPC6 inhibitor). The study was terminated early due to lack of efficacy and an imbalance of fatal events (.. | ||