A randomized controlled trial to evaluate outcomes with Aggrenox in patients with SARS-CoV-2 infection
et al., PLOS ONE, doi:10.1371/journal.pone.0274243, NCT04410328, Jan 2023
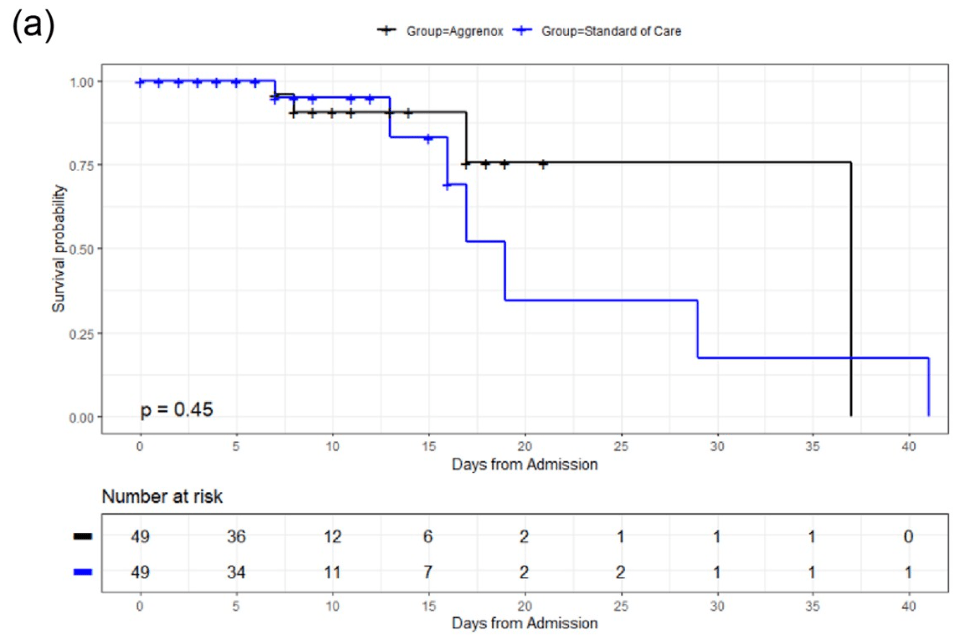
RCT 98 hospitalized patients in the USA, 49 treated with aspirin and dipyridamole, showing improved results with treatment, but without statistical significance.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 57.4% lower, RR 0.43, p = 0.44, treatment 3 of 49 (6.1%), control 5 of 49 (10.2%), adjusted per study, odds ratio converted to relative risk, multivariable, day 28.
|
|
risk of death, 15.0% lower, OR 0.85, p = 0.87, treatment 49, control 49, adjusted per study, multivariable, day 14, RR approximated with OR.
|
|
risk of mechanical ventilation, 20.0% lower, RR 0.80, p = 1.00, treatment 4 of 49 (8.2%), control 5 of 49 (10.2%), NNT 49.
|
|
risk of ICU admission, 28.6% lower, RR 0.71, p = 0.76, treatment 5 of 49 (10.2%), control 7 of 49 (14.3%), NNT 25.
|
|
risk of progression, 33.3% lower, RR 0.67, p = 0.74, treatment 4 of 49 (8.2%), control 6 of 49 (12.2%), NNT 24, day 28.
|
|
risk of progression, 76.3% lower, RR 0.24, p = 0.22, treatment 4 of 49 (8.2%), control 7 of 49 (14.3%), odds ratio converted to relative risk, respiratory failure, day 28.
|
|
risk of progression, 44.4% lower, RR 0.56, p = 0.39, treatment 5 of 49 (10.2%), control 9 of 49 (18.4%), NNT 12, AKI.
|
|
risk of progression, 85.7% lower, RR 0.14, p = 0.24, treatment 0 of 49 (0.0%), control 3 of 49 (6.1%), NNT 16, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), DIC.
|
|
risk of progression, 25.0% lower, RR 0.75, p = 0.62, treatment 9 of 49 (18.4%), control 12 of 49 (24.5%), NNT 16, liver dysfunction.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Singla et al., 30 Jan 2023, Randomized Controlled Trial, USA, peer-reviewed, 26 authors, study period 1 October, 2020 - 30 April, 2021, this trial uses multiple treatments in the treatment arm (combined with dipyridamole) - results of individual treatments may vary, trial NCT04410328 (history).
Contact: as3321@njms.rutgers.edu.
A randomized controlled trial to evaluate outcomes with Aggrenox in patients with SARS-CoV-2 infection
PLOS ONE, doi:10.1371/journal.pone.0274243
Background Coronavirus disease 2019 (COVID-19) is an immunoinflammatory and hypercoagulable state that contributes to respiratory distress, multi-organ dysfunction, and mortality. Dipyridamole, by increasing extracellular adenosine, has been postulated to be protective for COVID-19 patients through its immunosuppressive, anti-inflammatory, anti-coagulant, vasodilatory, and anti-viral actions. Likewise, low-dose aspirin has also demonstrated protective effects for COVID-19 patients. This study evaluated the effect of these two drugs formulated together as Aggrenox in hospitalized COVID-19 patients.
Methods In an open-label, single site randomized controlled trial (RCT), hospitalized COVID-19 patients were assigned to adjunctive Aggrenox (Dipyridamole ER 200mg/ Aspirin 25mg orally/enterally) with standard of care treatment compared to standard of care treatment alone. Primary endpoint was illness severity according to changes on the eight-point COVID ordinal scale, with levels of 1 to 8 where higher scores represent worse illness. Secondary endpoints included all-cause mortality and respiratory failure. Outcomes were measured through days 14, 28, and/or hospital discharge.
Supporting information
S1 File. (DOCX)
S2 File. (PDF)
Author Contributions Conceptualization: Amit Singla, Nicholas B. Dadario, Ashima Singla, Detlev Boison, Rakesh Malhotra, Yingda Xie, Steven K. Libutti. Data curation: Amit Singla, Nicholas B. Dadario, Patricia Greenberg, Rachel Yan, Detlev Boison, Sunil Patel, Suri Nipun, Kaur Maninderpal, Dorothy Castro, Sanaa Bdiiwi, Hala Boktor, Htay Htay Kyi, Anne Sutherland, Amee Patrawalla, Kevin Ly, Yingda Xie, Priyank Khandelwal, James Liu, Sara Subanna. Formal analysis: Amit Singla, Nicholas B. Dadario, Patricia Greenberg. Methodology: Amit Singla, Patricia Greenberg, Detlev Boison. Project administration: Amit Singla, Anil Nanda, Sunil Patel, Anne Sutherland, Steven K. Libutti. Resources: Amit Singla, Anil Nanda, Yingda Xie, Ashish Sonig, James Liu, Joseph Koziol, Diana Finkle, Steven K. Libutti. Software: Amit Singla, Patricia Greenberg. Supervision: Amit Singla, Anne Sutherland, Steven K. Libutti. Validation: Amit Singla, Patricia Greenberg, Ashish Sonig. Visualization: Amit Singla, Sunil Patel, Kaur Maninderpal. Writing -original draft: Amit Singla, Nicholas B. Dadario, Ashima Singla, Patricia Greenberg. Writing -review & editing: Amit Singla, Nicholas B. Dadario, Ashima Singla, Patricia Greenberg, Rachel Yan, Anil Nanda, Detlev Boison, Rakesh Malhotra, Sunil Patel, Suri Nipun, Kaur Maninderpal, Dorothy Castro, Sanaa Bdiiwi, Hala Boktor, Htay Htay Kyi, Anne Sutherland, Amee Patrawalla, Kevin Ly, Yingda Xie, Ashish..
References
Aliter, Ra, Potential Therapeutic Benefits of Dipyridamole in COVID-19 Patients, Curr Pharm Des, doi:10.2174/1381612826666201001125604
Aly, Ibrahim, Should aspirin be used for prophylaxis of COVID-19-induced coagulopathy?, Med Hypotheses, doi:10.1016/j.mehy.2020.109975
Beigel, Tomashek, Dodd, Remdesivir for the Treatment of Covid-19-Final Report, New England Journal of Medicine, doi:10.1056/NEJMoa2007764
Bernal, Da Silva, Musungaie, Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients, New England Journal of Medicine, doi:10.1056/NEJMoa2116044
Bikdeli, Madhavan, Jimenez, COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review, J Am Coll Cardiol, doi:10.1016/j.jacc.2020.04.031
Cavalcanti, Zampieri, Rosa, Hydroxychloroquine with or without Azithromycin in Mildto-Moderate Covid-19, New England Journal of Medicine, doi:10.1056/NEJMoa2019014
Chow, Khanna, Kethireddy, Aspirin Use Is Associated With Decreased Mechanical Ventilation, Intensive Care Unit Admission, and In-Hospital Mortality in Hospitalized Patients With Coronavirus Disease 2019, Anesthesia & Analgesia, doi:10.1213/ANE.0000000000005292
Diener, Cunha, Forbes, Sivenius, Smets et al., European Stroke Prevention Study
Fata-Hartley, Palmenberg, Dipyridamole reversibly inhibits mengovirus RNA replication, J Virol, doi:10.1128/JVI.79.17.11062-11070.2005
Firth, Bias reduction of maximum likelihood estimates, Biometrika
Geiger, Khan, Murugan, Boison, Possible Role of Adenosine in COVID-19 Pathogenesis and Therapeutic Opportunities, Front Pharmacol, doi:10.3389/fphar.2020.594487
Gresele, Momi, Falcinelli, Anti-platelet therapy: phosphodiesterase inhibitors, British Journal of Clinical Pharmacology, doi:10.1111/j.1365-2125.2011.04034.x
Group, Horby, Pessoa-Amorim, Aspirin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial, doi:10.1016/S0140-6736%2821%2901825-0
Guchev, Klochkov, Dipyridamole prevention of outbreaks of respiratory infections in the homogeneous population
Guimaraes, Quirk, Furtado, Tofacitinib in Patients Hospitalized with Covid-19 Pneumonia, N Engl J Med, doi:10.1056/NEJMoa2101643
Huang, Chen, Geng, Mucosal Profiling of Pediatric-Onset Colitis and IBD Reveals Common Pathogenics and Therapeutic Pathways, Cell, doi:10.1016/j.cell.2019.10.027
Investigators, Effect of Intermediate-Dose vs Standard-Dose Prophylactic Anticoagulation on Thrombotic Events, Extracorporeal Membrane Oxygenation Treatment, or Mortality Among Patients With COVID-19 Admitted to the Intensive Care Unit: The INSPIRATION Randomized Clinical Trial, JAMA, doi:10.1001/jama.2021.4152
Ionescu, Jaiyesimi, Petrescu, Association of anticoagulation dose and survival in hospitalized COVID-19 patients: A retrospective propensity score-weighted analysis, Eur J Haematol, doi:10.1111/ejh.13533
Jime ´nez, Garcı ´a-Sanchez, Rali, Incidence of VTE and Bleeding Among Hospitalized Patients With Coronavirus Disease 2019: A Systematic Review and Meta-analysis, Chest, doi:10.1016/j.chest.2020.11.005
Kalil, Patterson, Mehta, Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19, New England Journal of Medicine, doi:10.1056/NEJMoa2031994
Langer, Kluge, Klamroth, Oldenburg, Coagulopathy in COVID-19 and Its Implication for Safe and Efficacious Thromboprophylaxis, Hamostaseologie, doi:10.1055/a-1178-3551
Li, Li, Huang, FEP-based screening prompts drug repositioning against COVID-19
Liu, Li, Liu, Potential therapeutic effects of dipyridamole in the severely ill patients with COVID-19, Acta Pharm Sin B, doi:10.1016/j.apsb.2020.04.008
Logette, Lorin, Favreau, A Machine-Generated View of the Role of Blood Glucose Levels in the Severity of COVID-19, Frontiers in Public Health, doi:10.3389/fpubh.2021.695139
Mahase, Covid-19: Pfizer's paxlovid is 89% effective in patients at risk of serious illness, company reports, BMJ, doi:10.1136/bmj.n2713
Marconi, Ramanan, De Bono, Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebocontrolled phase 3 trial, Lancet Respir Med, doi:10.1016/S2213-2600%2821%2900331-3
Meizlish, Goshua, Liu, Intermediate-dose anticoagulation, aspirin, and in-hospital mortality in COVID-19: A propensity score-matched analysis, Am J Hematol, doi:10.1002/ajh.26102
Miller, Brody, What makes placebo-controlled trials unethical?, Am J Bioeth, doi:10.1162/152651602317533523
Nadkarni, Bagiella, Anticoagulation, Bleeding, Mortality, and Pathology in Hospitalized Patients With COVID-19, J Am Coll Cardiol, doi:10.1016/j.jacc.2020.08.041
Omarjee, Meilhac, Perrot, Janin, Mahe, Can Ticagrelor be used to prevent sepsis-induced coagulopathy in COVID-19?, Clin Immunol, doi:10.1016/j.clim.2020.108468
Osborne, Veigulis, Arreola, Mahajan, ¨o ¨sli et al., Association of mortality and aspirin prescription for COVID-19 patients at the Veterans Health Administration, PLOS ONE, doi:10.1371/journal.pone.0246825
Petrilli, Jones, Yang, Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study, BMJ, doi:10.1136/bmj.m1966
Qin, Zhou, Lu, Effectiveness of glucocorticoid therapy in patients with severe coronavirus disease 2019: protocol of a randomized controlled trial, Chinese Medical Journal, doi:10.1097/CM9.0000000000000791
Sammartino, Jafri, Cook, Predictors for inpatient mortality during the first wave of the SARS-CoV-2 pandemic: A retrospective analysis, PLoS One, doi:10.1371/journal.pone.0251262
Song, Rocha, ´nior, Treatment of severe COVID-19 patients with either low-or high-volume of convalescent plasma versus standard of care: A multicenter Bayesian randomized open-label clinical trial (COOP-COVID-19-MCTI), The Lancet Regional Health-Americas
Tang, Bai, Chen, Gong, Li et al., Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy, J Thromb Haemost, doi:10.1111/jth.14817
Tartof, Slezak, Fischer, Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study, The Lancet
Tenser, Gaydos, Hay, Inhibition of herpes simplex virus reactivation by dipyridamole, Antimicrobial Agents and Chemotherapy, doi:10.1128/AAC.45.12.3657-3659.2001
Thachil, Tang, Gando, ISTH interim guidance on recognition and management of coagulopathy in COVID-19, J Thromb Haemost, doi:10.1111/jth.14810
Tonew, Indulen, Dzeguze, Antiviral action of dipyridamole and its derivatives against influenza virus A, Acta Virol
Walters, Sample size and power estimation for studies with health related quality of life outcomes: a comparison of four methods using the SF-36, Health and Quality of Life Outcomes, doi:10.1186/1477-7525-2-26
Weiss, Murdoch, Clinical course and mortality risk of severe COVID-19, Lancet, doi:10.1016/S0140-6736%2820%2930633-4
Who, WHO Timeline-COVID-19
Zhang, Xiang, Huo, Molecular mechanism of interaction between SARS-CoV-2 and host cells and interventional therapy, Signal Transduction and Targeted Therapy, doi:10.1038/s41392-021-00653-w
DOI record:
{
"DOI": "10.1371/journal.pone.0274243",
"ISSN": [
"1932-6203"
],
"URL": "http://dx.doi.org/10.1371/journal.pone.0274243",
"abstract": "<jats:sec id=\"sec001\">\n<jats:title>Background</jats:title>\n<jats:p>Coronavirus disease 2019 (COVID-19) is an immunoinflammatory and hypercoagulable state that contributes to respiratory distress, multi-organ dysfunction, and mortality. Dipyridamole, by increasing extracellular adenosine, has been postulated to be protective for COVID-19 patients through its immunosuppressive, anti-inflammatory, anti-coagulant, vasodilatory, and anti-viral actions. Likewise, low-dose aspirin has also demonstrated protective effects for COVID-19 patients. This study evaluated the effect of these two drugs formulated together as <jats:italic>Aggrenox</jats:italic> in hospitalized COVID-19 patients.</jats:p>\n</jats:sec>\n<jats:sec id=\"sec002\">\n<jats:title>Methods</jats:title>\n<jats:p>In an open-label, single site randomized controlled trial (RCT), hospitalized COVID-19 patients were assigned to adjunctive Aggrenox (Dipyridamole ER 200mg/ Aspirin 25mg orally/enterally) with standard of care treatment compared to standard of care treatment alone. Primary endpoint was illness severity according to changes on the eight-point COVID ordinal scale, with levels of 1 to 8 where higher scores represent worse illness. Secondary endpoints included all-cause mortality and respiratory failure. Outcomes were measured through days 14, 28, and/or hospital discharge.</jats:p>\n</jats:sec>\n<jats:sec id=\"sec003\">\n<jats:title>Results</jats:title>\n<jats:p>From October 1, 2020 to April 30, 2021, a total of 98 patients, who had a median [IQR] age of 57 [47, 62] years and were 53.1% (n = 52) female, were randomized equally between study groups (n = 49 Aggrenox plus standard of care versus n = 49 standard of care alone). No clinically significant differences were found between those who received adjunctive Aggrenox and the control group in terms of illness severity (COVID ordinal scale) at days 14 and 28. The overall mortality through day 28 was 6.1% (3 patients, n = 49) in the Aggrenox group and 10.2% (5 patients, n = 49) in the control group (OR [95% CI]: 0.40 [0.04, 4.01], p = 0.44). Respiratory failure through day 28 occurred in 4 (8.3%, n = 48) patients in the Aggrenox group and 7 (14.6%, n = 48) patients in the standard of care group (OR [95% CI]: 0.21 [0.02, 2.56], p = 0.22). A larger decrease in the platelet count and blood glucose levels, and larger increase in creatinine and sodium levels within the first 7 days of hospital admission were each independent predictors of 28-day mortality (p < 0.05).</jats:p>\n</jats:sec>\n<jats:sec id=\"sec004\">\n<jats:title>Conclusion</jats:title>\n<jats:p>In this study of hospitalized patients with COVID-19, while the outcomes of COVID illness severity, odds of mortality, and chance of respiratory failure were better in the Aggrenox group compared to standard of care alone, the data did not reach statistical significance to support the standard use of adjuvant Aggrenox in such patients.</jats:p>\n</jats:sec>",
"author": [
{
"ORCID": "http://orcid.org/0000-0002-4162-4691",
"affiliation": [],
"authenticated-orcid": true,
"family": "Singla",
"given": "Amit",
"sequence": "first"
},
{
"affiliation": [],
"family": "Dadario",
"given": "Nicholas B.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Singla",
"given": "Ashima",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Greenberg",
"given": "Patricia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yan",
"given": "Rachel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nanda",
"given": "Anil",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Boison",
"given": "Detlev",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Malhotra",
"given": "Rakesh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Patel",
"given": "Sunil",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nipun",
"given": "Suri",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Maninderpal",
"given": "Kaur",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Castro",
"given": "Dorothy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bdiiwi",
"given": "Sanaa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Boktor",
"given": "Hala",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kyi",
"given": "Htay Htay",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sutherland",
"given": "Anne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Patrawalla",
"given": "Amee",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ly",
"given": "Kevin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Xie",
"given": "Yingda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sonig",
"given": "Ashish",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Khandelwal",
"given": "Priyank",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liu",
"given": "James",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Koziol",
"given": "Joseph",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Finkle",
"given": "Diana",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1222-9980",
"affiliation": [],
"authenticated-orcid": true,
"family": "Subanna",
"given": "Sara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Libutti",
"given": "Steven K.",
"sequence": "additional"
}
],
"container-title": "PLOS ONE",
"container-title-short": "PLoS ONE",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"www.plosone.org"
]
},
"created": {
"date-parts": [
[
2023,
1,
30
]
],
"date-time": "2023-01-30T19:36:19Z",
"timestamp": 1675107379000
},
"deposited": {
"date-parts": [
[
2023,
1,
30
]
],
"date-time": "2023-01-30T19:36:54Z",
"timestamp": 1675107414000
},
"editor": [
{
"affiliation": [],
"family": "Vousden",
"given": "George",
"sequence": "first"
}
],
"funder": [
{
"DOI": "10.13039/100001003",
"doi-asserted-by": "publisher",
"name": "Boehringer Ingelheim"
},
{
"DOI": "10.13039/100000002",
"award": [
"UL1TR003017"
],
"doi-asserted-by": "publisher",
"name": "National Institutes of Health"
},
{
"DOI": "10.13039/100000002",
"award": [
"NS065957 and NS103740"
],
"doi-asserted-by": "publisher",
"name": "National Institutes of Health"
}
],
"indexed": {
"date-parts": [
[
2023,
1,
31
]
],
"date-time": "2023-01-31T06:13:56Z",
"timestamp": 1675145636995
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2023,
1,
30
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2023,
1,
30
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
1,
30
]
],
"date-time": "2023-01-30T00:00:00Z",
"timestamp": 1675036800000
}
}
],
"link": [
{
"URL": "https://dx.plos.org/10.1371/journal.pone.0274243",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "340",
"original-title": [],
"page": "e0274243",
"prefix": "10.1371",
"published": {
"date-parts": [
[
2023,
1,
30
]
]
},
"published-online": {
"date-parts": [
[
2023,
1,
30
]
]
},
"publisher": "Public Library of Science (PLoS)",
"reference": [
{
"key": "pone.0274243.ref001",
"unstructured": "WHO. Archived: WHO Timeline—COVID-19. 2020. https://www.who.int/news/item/27-04-2020-who-timeline---covid-19 (accessed September 28, 2021."
},
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the Treatment of Covid-19—Final Report",
"author": "JH Beigel",
"doi-asserted-by": "crossref",
"first-page": "1813",
"issue": "19",
"journal-title": "New England Journal of Medicine",
"key": "pone.0274243.ref002",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2031994",
"article-title": "Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19",
"author": "AC Kalil",
"doi-asserted-by": "crossref",
"first-page": "795",
"issue": "9",
"journal-title": "New England Journal of Medicine",
"key": "pone.0274243.ref003",
"volume": "384",
"year": "2020"
},
{
"DOI": "10.1016/S2213-2600(21)00331-3",
"article-title": "Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial",
"author": "VC Marconi",
"doi-asserted-by": "crossref",
"first-page": "1407",
"issue": "12",
"journal-title": "Lancet Respir Med",
"key": "pone.0274243.ref004",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2116044",
"article-title": "Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients",
"author": "A Jayk Bernal",
"doi-asserted-by": "crossref",
"first-page": "509",
"issue": "6",
"journal-title": "New England Journal of Medicine",
"key": "pone.0274243.ref005",
"volume": "386",
"year": "2021"
},
{
"DOI": "10.1136/bmj.n2713",
"article-title": "Covid-19: Pfizer’s paxlovid is 89% effective in patients at risk of serious illness, company reports",
"author": "E. Mahase",
"doi-asserted-by": "crossref",
"first-page": "n2713",
"journal-title": "BMJ",
"key": "pone.0274243.ref006",
"volume": "375",
"year": "2021"
},
{
"DOI": "10.1038/s41392-021-00653-w",
"article-title": "Molecular mechanism of interaction between SARS-CoV-2 and host cells and interventional therapy",
"author": "Q Zhang",
"doi-asserted-by": "crossref",
"first-page": "233",
"issue": "1",
"journal-title": "Signal Transduction and Targeted Therapy",
"key": "pone.0274243.ref007",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(20)30633-4",
"article-title": "Clinical course and mortality risk of severe COVID-19",
"author": "P Weiss",
"doi-asserted-by": "crossref",
"first-page": "1014",
"issue": "10229",
"journal-title": "Lancet",
"key": "pone.0274243.ref008",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/j.chest.2020.11.005",
"article-title": "Incidence of VTE and Bleeding Among Hospitalized Patients With Coronavirus Disease 2019: A Systematic Review and Meta-analysis",
"author": "D Jiménez",
"doi-asserted-by": "crossref",
"first-page": "1182",
"issue": "3",
"journal-title": "Chest",
"key": "pone.0274243.ref009",
"volume": "159",
"year": "2021"
},
{
"DOI": "10.1016/j.jacc.2020.04.031",
"article-title": "COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review",
"author": "B Bikdeli",
"doi-asserted-by": "crossref",
"first-page": "2950",
"issue": "23",
"journal-title": "J Am Coll Cardiol",
"key": "pone.0274243.ref010",
"volume": "75",
"year": "2020"
},
{
"DOI": "10.1016/j.jacc.2020.08.041",
"article-title": "Anticoagulation, Bleeding, Mortality, and Pathology in Hospitalized Patients With COVID-19",
"author": "GN Nadkarni",
"doi-asserted-by": "crossref",
"first-page": "1815",
"issue": "16",
"journal-title": "J Am Coll Cardiol",
"key": "pone.0274243.ref011",
"volume": "76",
"year": "2020"
},
{
"DOI": "10.1111/jth.14810",
"article-title": "ISTH interim guidance on recognition and management of coagulopathy in COVID-19",
"author": "J Thachil",
"doi-asserted-by": "crossref",
"first-page": "1023",
"issue": "5",
"journal-title": "J Thromb Haemost",
"key": "pone.0274243.ref012",
"volume": "18",
"year": "2020"
},
{
"article-title": "Coagulopathy in COVID-19 and Its Implication for Safe and Efficacious Thromboprophylaxis",
"author": "F Langer",
"journal-title": "Hamostaseologie",
"key": "pone.0274243.ref013",
"year": "2020"
},
{
"DOI": "10.1111/jth.14817",
"article-title": "Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy",
"author": "N Tang",
"doi-asserted-by": "crossref",
"first-page": "1094",
"issue": "5",
"journal-title": "J Thromb Haemost",
"key": "pone.0274243.ref014",
"volume": "18",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2103417",
"article-title": "Therapeutic Anticoagulation with Heparin in Critically Ill Patients with Covid-19",
"doi-asserted-by": "crossref",
"first-page": "777",
"issue": "9",
"journal-title": "New England Journal of Medicine",
"key": "pone.0274243.ref015",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1001/jama.2021.4152",
"article-title": "Effect of Intermediate-Dose vs Standard-Dose Prophylactic Anticoagulation on Thrombotic Events, Extracorporeal Membrane Oxygenation Treatment, or Mortality Among Patients With COVID-19 Admitted to the Intensive Care Unit: The INSPIRATION Randomized Clinical Trial",
"author": "I. Investigators",
"doi-asserted-by": "crossref",
"first-page": "1620",
"issue": "16",
"journal-title": "JAMA",
"key": "pone.0274243.ref016",
"volume": "325",
"year": "2021"
},
{
"article-title": "Aspirin Use Is Associated With Decreased Mechanical Ventilation, Intensive Care Unit Admission, and In-Hospital Mortality in Hospitalized Patients With Coronavirus Disease 2019",
"author": "JH Chow",
"issue": "4",
"journal-title": "Anesthesia & Analgesia",
"key": "pone.0274243.ref017",
"volume": "132",
"year": "2021"
},
{
"DOI": "10.1002/ajh.26102",
"article-title": "Intermediate-dose anticoagulation, aspirin, and in-hospital mortality in COVID-19: A propensity score-matched analysis",
"author": "ML Meizlish",
"doi-asserted-by": "crossref",
"first-page": "471",
"issue": "4",
"journal-title": "Am J Hematol",
"key": "pone.0274243.ref018",
"volume": "96",
"year": "2021"
},
{
"article-title": "Aspirin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial",
"author": "RC Group",
"journal-title": "medRxiv",
"key": "pone.0274243.ref019",
"year": "2021"
},
{
"DOI": "10.1128/JVI.79.17.11062-11070.2005",
"article-title": "Dipyridamole reversibly inhibits mengovirus RNA replication",
"author": "CL Fata-Hartley",
"doi-asserted-by": "crossref",
"first-page": "11062",
"issue": "17",
"journal-title": "J Virol",
"key": "pone.0274243.ref020",
"volume": "79",
"year": "2005"
},
{
"DOI": "10.1016/j.apsb.2020.04.008",
"article-title": "Potential therapeutic effects of dipyridamole in the severely ill patients with COVID-19",
"author": "X Liu",
"doi-asserted-by": "crossref",
"first-page": "1205",
"issue": "7",
"journal-title": "Acta Pharm Sin B",
"key": "pone.0274243.ref021",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2019.10.027",
"article-title": "Mucosal Profiling of Pediatric-Onset Colitis and IBD Reveals Common Pathogenics and Therapeutic Pathways",
"author": "B Huang",
"doi-asserted-by": "crossref",
"first-page": "1160",
"issue": "5",
"journal-title": "Cell",
"key": "pone.0274243.ref022",
"volume": "179",
"year": "2019"
},
{
"DOI": "10.1111/j.1365-2125.2011.04034.x",
"article-title": "Anti-platelet therapy: phosphodiesterase inhibitors",
"author": "P Gresele",
"doi-asserted-by": "crossref",
"first-page": "634",
"issue": "4",
"journal-title": "British Journal of Clinical Pharmacology",
"key": "pone.0274243.ref023",
"volume": "72",
"year": "2011"
},
{
"article-title": "FEP-based screening prompts drug repositioning against COVID-19",
"author": "Z Li",
"journal-title": "bioRxiv",
"key": "pone.0274243.ref024",
"year": "2020"
},
{
"DOI": "10.1162/152651602317533523",
"article-title": "What makes placebo-controlled trials unethical?",
"author": "FG Miller",
"doi-asserted-by": "crossref",
"first-page": "3",
"issue": "2",
"journal-title": "Am J Bioeth",
"key": "pone.0274243.ref025",
"volume": "2",
"year": "2002"
},
{
"DOI": "10.1056/NEJMoa2101643",
"article-title": "Tofacitinib in Patients Hospitalized with Covid-19 Pneumonia",
"author": "PO Guimaraes",
"doi-asserted-by": "crossref",
"first-page": "406",
"issue": "5",
"journal-title": "N Engl J Med",
"key": "pone.0274243.ref026",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1093/biomet/80.1.27",
"article-title": "Bias reduction of maximum likelihood estimates",
"author": "D. Firth",
"doi-asserted-by": "crossref",
"first-page": "27",
"issue": "1",
"journal-title": "Biometrika",
"key": "pone.0274243.ref027",
"volume": "80",
"year": "1993"
},
{
"DOI": "10.3389/fphar.2020.594487",
"article-title": "Possible Role of Adenosine in COVID-19 Pathogenesis and Therapeutic Opportunities",
"author": "JD Geiger",
"doi-asserted-by": "crossref",
"first-page": "594487",
"journal-title": "Front Pharmacol",
"key": "pone.0274243.ref028",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1111/ejh.13533",
"article-title": "Association of anticoagulation dose and survival in hospitalized COVID-19 patients: A retrospective propensity score-weighted analysis",
"author": "F Ionescu",
"doi-asserted-by": "crossref",
"first-page": "165",
"issue": "2",
"journal-title": "Eur J Haematol",
"key": "pone.0274243.ref029",
"volume": "106",
"year": "2021"
},
{
"DOI": "10.1016/j.clim.2020.108468",
"article-title": "Can Ticagrelor be used to prevent sepsis-induced coagulopathy in COVID-19?",
"author": "L Omarjee",
"doi-asserted-by": "crossref",
"first-page": "108468",
"journal-title": "Clin Immunol",
"key": "pone.0274243.ref030",
"volume": "216",
"year": "2020"
},
{
"DOI": "10.2174/1381612826666201001125604",
"article-title": "Potential Therapeutic Benefits of Dipyridamole in COVID-19 Patients",
"author": "KF Aliter",
"doi-asserted-by": "crossref",
"first-page": "866",
"issue": "6",
"journal-title": "Curr Pharm Des",
"key": "pone.0274243.ref031",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1371/journal.pone.0246825",
"article-title": "Association of mortality and aspirin prescription for COVID-19 patients at the Veterans Health Administration",
"author": "TF Osborne",
"doi-asserted-by": "crossref",
"first-page": "e0246825",
"issue": "2",
"journal-title": "PLOS ONE",
"key": "pone.0274243.ref032",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1016/j.mehy.2020.109975",
"article-title": "Should aspirin be used for prophylaxis of COVID-19-induced coagulopathy?",
"author": "AAR Mohamed-Hussein",
"doi-asserted-by": "crossref",
"first-page": "109975",
"journal-title": "Med Hypotheses",
"key": "pone.0274243.ref033",
"volume": "144",
"year": "2020"
},
{
"article-title": "Antiviral action of dipyridamole and its derivatives against influenza virus A",
"author": "E Tonew",
"first-page": "125",
"issue": "3",
"journal-title": "Acta Virol",
"key": "pone.0274243.ref034",
"volume": "26",
"year": "1982"
},
{
"DOI": "10.1128/AAC.45.12.3657-3659.2001",
"article-title": "Inhibition of herpes simplex virus reactivation by dipyridamole",
"author": "RB Tenser",
"doi-asserted-by": "crossref",
"first-page": "3657",
"issue": "12",
"journal-title": "Antimicrobial Agents and Chemotherapy",
"key": "pone.0274243.ref035",
"volume": "45",
"year": "2001"
},
{
"article-title": "Dipyridamole prevention of outbreaks of respiratory infections in the homogeneous population",
"author": "IA Guchev",
"first-page": "45",
"issue": "11",
"journal-title": "Klin Med (Mosk)",
"key": "pone.0274243.ref036",
"volume": "82",
"year": "2004"
},
{
"DOI": "10.1016/S0022-510X(96)00308-5",
"article-title": "European Stroke Prevention Study. 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke",
"author": "HC Diener",
"doi-asserted-by": "crossref",
"first-page": "1",
"issue": "1–2",
"journal-title": "J Neurol Sci",
"key": "pone.0274243.ref037",
"volume": "143",
"year": "1996"
},
{
"article-title": "Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study",
"author": "SY Tartof",
"journal-title": "The Lancet",
"key": "pone.0274243.ref038"
},
{
"DOI": "10.1136/bmj.m1966",
"article-title": "Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study",
"author": "CM Petrilli",
"doi-asserted-by": "crossref",
"first-page": "m1966",
"journal-title": "BMJ",
"key": "pone.0274243.ref039",
"volume": "369",
"year": "2020"
},
{
"article-title": "A Machine-Generated View of the Role of Blood Glucose Levels in the Severity of COVID-19",
"author": "E Logette",
"issue": "1068",
"journal-title": "Frontiers in Public Health",
"key": "pone.0274243.ref040",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1097/CM9.0000000000000791",
"article-title": "Effectiveness of glucocorticoid therapy in patients with severe coronavirus disease 2019: protocol of a randomized controlled trial",
"author": "Y-Y Qin",
"doi-asserted-by": "crossref",
"first-page": "1080",
"issue": "9",
"journal-title": "Chinese Medical Journal",
"key": "pone.0274243.ref041",
"volume": "133",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2019014",
"article-title": "Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19",
"author": "AB Cavalcanti",
"doi-asserted-by": "crossref",
"first-page": "2041",
"issue": "21",
"journal-title": "New England Journal of Medicine",
"key": "pone.0274243.ref042",
"volume": "383",
"year": "2020"
},
{
"article-title": "Treatment of severe COVID-19 patients with either low- or high-volume of convalescent plasma versus standard of care: A multicenter Bayesian randomized open-label clinical trial (COOP-COVID-19-MCTI)",
"author": "ATW Song",
"journal-title": "The Lancet Regional Health—Americas",
"key": "pone.0274243.ref043",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1186/1477-7525-2-26",
"article-title": "Sample size and power estimation for studies with health related quality of life outcomes: a comparison of four methods using the SF-36",
"author": "SJ Walters",
"doi-asserted-by": "crossref",
"first-page": "26",
"issue": "1",
"journal-title": "Health and Quality of Life Outcomes",
"key": "pone.0274243.ref044",
"volume": "2",
"year": "2004"
},
{
"DOI": "10.1371/journal.pone.0251262",
"article-title": "Predictors for inpatient mortality during the first wave of the SARS-CoV-2 pandemic: A retrospective analysis",
"author": "D Sammartino",
"doi-asserted-by": "crossref",
"first-page": "e0251262",
"issue": "5",
"journal-title": "PLoS One",
"key": "pone.0274243.ref045",
"volume": "16",
"year": "2021"
}
],
"reference-count": 45,
"references-count": 45,
"relation": {},
"resource": {
"primary": {
"URL": "https://dx.plos.org/10.1371/journal.pone.0274243"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Multidisciplinary"
],
"subtitle": [],
"title": "A randomized controlled trial to evaluate outcomes with Aggrenox in patients with SARS-CoV-2 infection",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1371/journal.pone.corrections_policy",
"volume": "18"
}