Renin-Angiotensin System Modulation With Synthetic Angiotensin (1-7) and Angiotensin II Type 1 Receptor–Biased Ligand in Adults With COVID-19
et al., JAMA, doi:10.1001/jama.2023.3546, ACTIV-4, NCT04924660, Apr 2023
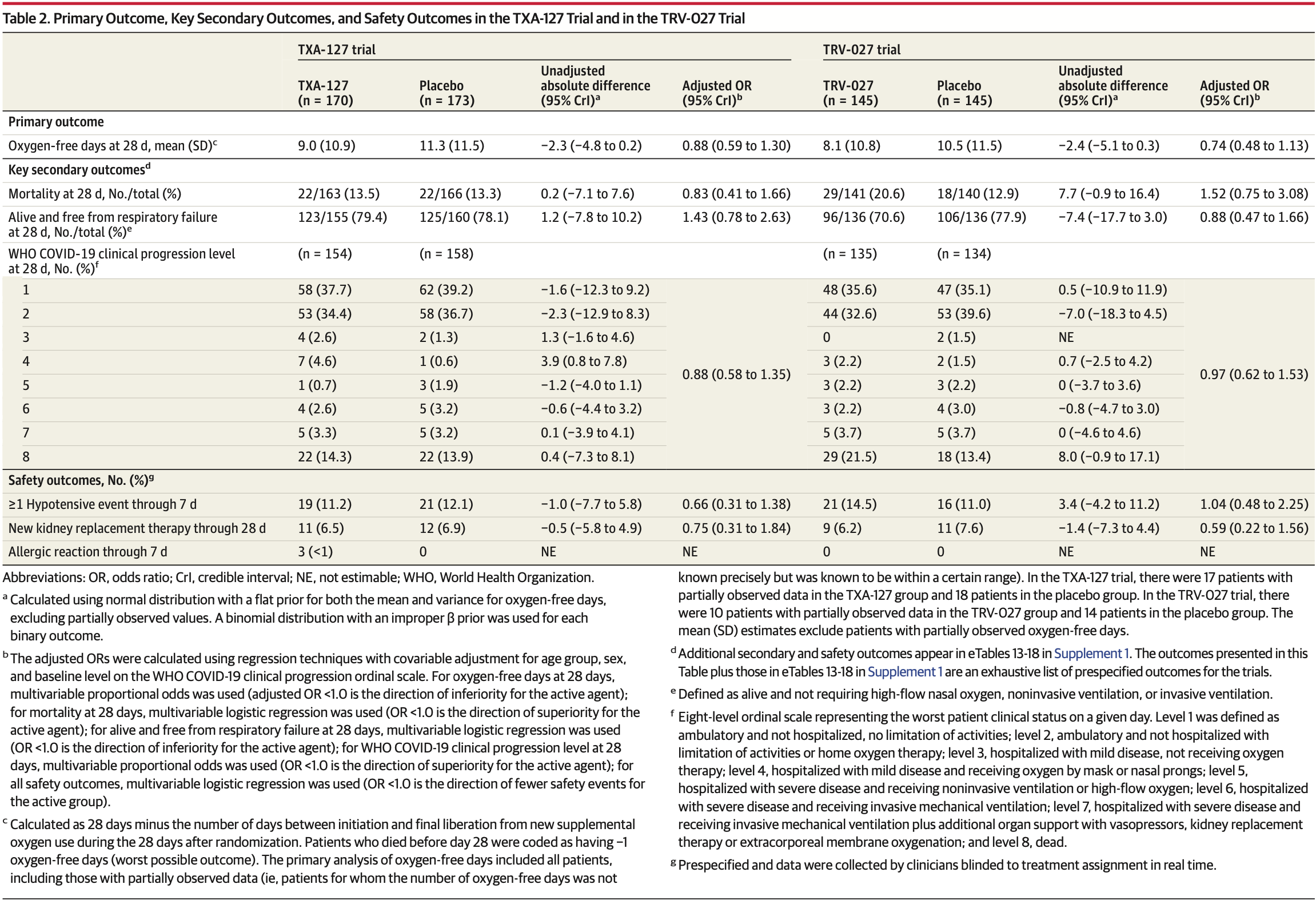
Two RCTs of 510 hospitalized COVID-19 patients showing no significant difference in mortality or days alive without supplemental oxygen with either TRV027 (angiotensin II type 1 receptor-biased ligand) or TXA127 (synthetic angiotensin 1-7) compared to placebo.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 52.0% higher, OR 1.52, p = 0.25, treatment 145, control 145, adjusted per study, multivariable, day 28, RR approximated with OR.
|
|
oxygen-free days, 35.1% higher, OR 1.35, p = 0.17, treatment 145, control 145, adjusted per study, inverted to make OR<1 favor treatment, oxygen-free days, multivariable, day 28, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Self et al., 11 Apr 2023, Double Blind Randomized Controlled Trial, placebo-controlled, USA, peer-reviewed, 46 authors, study period 22 July, 2021 - 20 April, 2022, trial NCT04924660 (history) (ACTIV-4).
Renin-Angiotensin System Modulation With Synthetic Angiotensin (1-7) and Angiotensin II Type 1 Receptor–Biased Ligand in Adults With COVID-19
JAMA, doi:10.1001/jama.2023.3546
IMPORTANCE Preclinical models suggest dysregulation of the renin-angiotensin system (RAS) caused by SARS-CoV-2 infection may increase the relative activity of angiotensin II compared with angiotensin (1-7) and may be an important contributor to COVID-19 pathophysiology. OBJECTIVE To evaluate the efficacy and safety of RAS modulation using 2 investigational RAS agents, TXA-127 (synthetic angiotensin [1-7]) and TRV-027 (an angiotensin II type 1 receptor-biased ligand), that are hypothesized to potentiate the action of angiotensin (1-7) and mitigate the action of the angiotensin II. DESIGN, SETTING, AND PARTICIPANTS Two randomized clinical trials including adults hospitalized with acute COVID-19 and new-onset hypoxemia were conducted at 35 sites in the US between July 22, 2021, and April 20, 2022; last follow-up visit: July 26, 2022. INTERVENTIONS A 0.5-mg/kg intravenous infusion of TXA-127 once daily for 5 days or placebo. A 12-mg/h continuous intravenous infusion of TRV-027 for 5 days or placebo.
MAIN OUTCOMES AND MEASURES The primary outcome was oxygen-free days, an ordinal outcome that classifies a patient's status at day 28 based on mortality and duration of supplemental oxygen use; an adjusted odds ratio (OR) greater than 1.0 indicated superiority of the RAS agent vs placebo. A key secondary outcome was 28-day all-cause mortality. Safety outcomes included allergic reaction, new kidney replacement therapy, and hypotension.
RESULTS Both trials met prespecified early stopping criteria for a low probability of efficacy. Of 343 patients in the TXA-127 trial (226 [65.9%] aged 31-64 years, 200 [58.3%] men, 225 [65.6%] White, and 274 [79.9%] not Hispanic), 170 received TXA-127 and 173 received placebo. Of 290 patients in the TRV-027 trial (199 [68.6%] aged 31-64 years, 168 [57.9%] men, 195 [67.2%] White, and 225 [77.6%] not Hispanic), 145 received TRV-027 and 145 received placebo. Compared with placebo, both TXA-127 (unadjusted mean difference, -2.3 [95% CrI, -4.8 to 0.2]; adjusted OR, 0.88 [95% CrI, 0.59 to 1.30]) and TRV-027 (unadjusted mean difference, -2.4 [95% CrI, -5.1 to 0.3]; adjusted OR, 0.74 [95% CrI, 0.48 to 1.13]) resulted in no difference in oxygen-free days. In the TXA-127 trial, 28-day all-cause mortality occurred in 22 of 163 patients (13.5%) in the TXA-127 group vs 22 of 166 patients (13.3%) in the placebo group (adjusted OR, 0.83 [95% CrI, 0.41 to 1.66]). In the TRV-027 trial, 28-day all-cause mortality occurred in 29 of 141 patients (20.6%) in the TRV-027 group vs 18 of 140 patients (12.9%) in the placebo group (adjusted OR, 1.52 [95% CrI, 0.75 to 3.08]). The frequency of the safety outcomes was similar with either TXA-127 or TRV-027 vs placebo.
CONCLUSIONS AND RELEVANCE In adults with severe COVID-19, RAS modulation (TXA-127 or TRV-027) did not improve oxygen-free days vs placebo. These results do not support the hypotheses that pharmacological interventions that selectively block the angiotensin II type 1 receptor or..
Author Contributions: Dr Shotwell had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Drs Self and Shotwell contributed equally to this work as co-lead authors. Concept and design: Self, Shotwell, Gibbs, de Wit, Files, Harkins, Hudock, Moskowitz, Javaheri, Busse, Ginde, Brown, Rosenberg, Lavieri, Orr, Pulley, Rice, Semler, Bernard, Collins. Acquisition, analysis, or interpretation of data: Self, Shotwell, Gibbs, de Wit, Files, Hudock, Merck, Moskowitz, Apodaca, Barksdale, Safdar, Javaheri, Sturek, Schrager, Iovine, Tiffany, Douglas, Levitt, Busse, Ginde, Brown, Hager, Boyle, Duggal, Khan, Lanspa, Chen, Puskarich, Vonderhaar, Venkateshaiah, Gentile,
Role of the Funder/Sponsor: The NHLBI supported the trial through funding, appointed a data and safety monitoring board, and reviewed the manuscript prior to submission for publication but did not have the power to veto publication or independently select a journal. Drs Rosenberg and Troendle, who are NHLBI employees, are included as authors and participated in the design and management of the study and interpretation of its results. Representatives from Constant Therapeutics, LLC and Trevena, Inc reviewed the manuscript prior to submission for publication but did not have the power to veto publication or select a journal. Representatives from Constant Therapeutics, LLC and Trevena, Inc had no role in the design and conduct of the study; no..
References
Adams, Rhoads, Surie, Influenza and Other Viruses in the Acutely Ill (IVY) Network. Vaccine effectiveness of primary series and booster doses against covid-19 associated hospital admissions in the United States: living test negative design study, BMJ, doi:10.1136/bmj-2022-072065
Bauer, Schreinlechner, Sappler, Discontinuation versus continuation of renin-angiotensin-system inhibitors in COVID-19 (ACEI-COVID): a prospective, parallel group, randomised, controlled, open-label trial, Lancet Respir Med, doi:10.1016/S2213-2600(21)00214-9
Beigel, Tomashek, Dodd, ACTT-1 Study Group Members. Remdesivir for the treatment of Covid-19-final report, N Engl J Med, doi:10.1056/NEJMoa2007764
Collins, Chappell, Files, The renin-angiotensin-aldosterone system in COVID-19-related and non-COVID-19-related acute respiratory distress syndrome: not so different after all?, Am J Respir Crit Care Med, doi:10.1164/rccm.202108-1904ED
Collins, Stoffels, Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV): an unprecedented partnership for unprecedented times, JAMA, doi:10.1001/jama.2020.8920?utm_campaign=articlePDF%26utm_medium=articlePDFlink%26utm_source=articlePDF%26utm_content=jama.2023.3546
Files, Gibbs, Schaich, A pilot study to assess the circulating renin-angiotensin system in COVID-19 acute respiratory failure, Am J Physiol Lung Cell Mol Physiol, doi:10.1152/ajplung.00129.2021
Gerard, Lecocq, Bouzin, Increased angiotensin-converting enzyme 2 and loss of alveolar type II cells in COVID-19-related acute respiratory distress syndrome, Am J Respir Crit Care Med, doi:10.1164/rccm.202012-4461OC
Harris, Taylor, Minor, The REDCap Consortium: building an international community of software platform partners, J Biomed Inform, doi:10.1016/j.jbi.2019.103208
Imai, Kuba, Penninger, The renin-angiotensin system in acute respiratory distress syndrome, Drug Discov Today Dis Mech, doi:10.1016/j.ddmec.2006.06.012
Imai, Kuba, Rao, Angiotensinconverting enzyme 2 protects from severe acute lung failure, Nature, doi:10.1038/nature03712
Kai, Kai, Interactions of coronaviruses with ACE2, angiotensin II, and RAS inhibitors-lessons from available evidence and insights into COVID-19, Hypertens Res, doi:10.1038/s41440-020-0455-8
Kalil, Patterson, Mehta, ACTT-2 Study Group Members. Baricitinib plus remdesivir for hospitalized adults with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2031994
Laterre, Berry, Blemings, Effect of selepressin vs placebo on ventilator-and vasopressor-free days in patients with septic shock: the SEPSIS-ACT randomized clinical trial, JAMA, doi:10.1001/jama.2019.14607?utm_campaign=articlePDF%26utm_medium=articlePDFlink%26utm_source=articlePDF%26utm_content=jama.2023.3546
Lauring, Tenforde, Chappell, Influenza and Other Viruses in the Acutely Ill (IVY) Network. Clinical severity of, and effectiveness of mRNA vaccines against, covid-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: prospective observational study, BMJ, doi:10.1136/bmj-2021-069761
Liu, Wang, Zhou, Zhao, Zhang et al., Potential role of ACE2 in coronavirus disease 2019 (COVID-19) prevention and management, J Transl Int Med, doi:10.2478/jtim-2020-0003
Monteil, Kwon, Prado, Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2, Cell, doi:10.1016/j.cell.2020.04.004
Moskowitz, Shotwell, Gibbs, Fourth Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-4) Host Tissue Investigators. Oxygen-free days as an outcome measure in clinical trials of therapies for COVID-19 and other causes of new-onset hypoxemia, Chest, doi:10.1016/j.chest.2022.04.145
Pang, Butler, Collins, Biased ligand of the angiotensin II type 1 receptor in patients with acute heart failure: a randomized, double-blind, placebo-controlled, phase IIB, dose ranging trial (BLAST-AHF), Eur Heart J, doi:10.1093/eurheartj/ehx196
Raman, Bluemke, Lüscher, Neubauer, Long COVID: post-acute sequelae of COVID-19 with a cardiovascular focus, Eur Heart J, doi:10.1093/eurheartj/ehac031
Robbins, Bakri, Toke-Bjolgerud, The effect of TRV027 on coagulation in COVID-19: a pilot randomized, placebo-controlled trial, Br J Clin Pharmacol, doi:10.1111/bcp.15618
Rosenberg, Bistran-Hall, Joly, Orr, Pulley et al., Statistical analysis: Shotwell
Saville, Berry, Efficiencies of platform clinical trials: a vision of the future, Clin Trials, doi:10.1177/1740774515626362
Self, Semler, Leither, Effect of hydroxychloroquine on clinical status at 14 days in hospitalized patients with COVID-19: a randomized clinical trial, JAMA, doi:10.1001/jama.2020.22240?utm_campaign=articlePDF%26utm_medium=articlePDFlink%26utm_source=articlePDF%26utm_content=jama.2023.3546
Self, Wheeler, Stewart, Bernard, Rice, Passive Immunity Trial for Our Nation (PassITON) Investigators. Neutralizing COVID-19 convalescent plasma in adults hospitalized with COVID-19: a blinded, randomized, placebo-controlled trial, Chest, doi:10.1016/j.chest.2022.06.029
Shotwell, Wit, Harkins, Hudock, Merck et al., Supervision: Self, Shotwell, de Wit
South, Diz, Chappell, COVID-19, ACE2, and the cardiovascular consequences, Am J Physiol Heart Circ Physiol, doi:10.1152/ajpheart.00217.2020
Sterne, Murthy, Diaz, WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis, JAMA, doi:10.1001/jama.2020.17023?utm_campaign=articlePDF%26utm_medium=articlePDFlink%26utm_source=articlePDF%26utm_content=jama.2023.3546
Tenforde, Self, Adams, Influenza and Other Viruses in the Acutely Ill (IVY) Network. Association between mRNA vaccination and COVID-19 hospitalization and disease severity, JAMA, doi:10.1001/jama.2021.19499?utm_campaign=articlePDF%26utm_medium=articlePDFlink%26utm_source=articlePDF%26utm_content=jama.2023.3546
Violin, Soergel, Boerrigter, Burnett, Jr et al., GPCR biased ligands as novel heart failure therapeutics, Trends Cardiovasc Med, doi:10.1016/j.tcm.2013.01.002
Wagener, Goldklang, Gerber, A randomized, placebo-controlled, double-blinded pilot study of angiotensin 1-7 (TXA-127) for the treatment of severe COVID-19, Crit Care, doi:10.1186/s13054-022-04096-9
Wang, Lagakos, Ware, Hunter, Drazen, Statistics in medicine-reporting of subgroup analyses in clinical trials, N Engl J Med, doi:10.1056/NEJMsr077003
Wösten-Van Asperen, Lutter, Specht, Acute respiratory distress syndrome leads to reduced ratio of ACE/ACE2 activities and is prevented by angiotensin (1-7) or an angiotensin II receptor antagonist, J Pathol, doi:10.1002/path.2987
Zhang, Li, Niu, ACE2 and COVID-19 and the resulting ARDS, Postgrad Med J, doi:10.1136/postgradmedj-2020-137935
Zoufaly, Poglitsch, Aberle, Human recombinant soluble ACE2 in severe COVID-19, Lancet Respir Med, doi:10.1016/S2213-2600(20)30418-5
DOI record:
{
"DOI": "10.1001/jama.2023.3546",
"ISSN": [
"0098-7484"
],
"URL": "http://dx.doi.org/10.1001/jama.2023.3546",
"abstract": "<jats:sec><jats:title>Importance</jats:title><jats:p>Preclinical models suggest dysregulation of the renin-angiotensin system (RAS) caused by SARS-CoV-2 infection may increase the relative activity of angiotensin II compared with angiotensin (1-7) and may be an important contributor to COVID-19 pathophysiology.</jats:p></jats:sec><jats:sec><jats:title>Objective</jats:title><jats:p>To evaluate the efficacy and safety of RAS modulation using 2 investigational RAS agents, TXA-127 (synthetic angiotensin [1-7]) and TRV-027 (an angiotensin II type 1 receptor–biased ligand), that are hypothesized to potentiate the action of angiotensin (1-7) and mitigate the action of the angiotensin II.</jats:p></jats:sec><jats:sec><jats:title>Design, Setting, and Participants</jats:title><jats:p>Two randomized clinical trials including adults hospitalized with acute COVID-19 and new-onset hypoxemia were conducted at 35 sites in the US between July 22, 2021, and April 20, 2022; last follow-up visit: July 26, 2022.</jats:p></jats:sec><jats:sec><jats:title>Interventions</jats:title><jats:p>A 0.5-mg/kg intravenous infusion of TXA-127 once daily for 5 days or placebo. A 12-mg/h continuous intravenous infusion of TRV-027 for 5 days or placebo.</jats:p></jats:sec><jats:sec><jats:title>Main Outcomes and Measures</jats:title><jats:p>The primary outcome was oxygen-free days, an ordinal outcome that classifies a patient’s status at day 28 based on mortality and duration of supplemental oxygen use; an adjusted odds ratio (OR) greater than 1.0 indicated superiority of the RAS agent vs placebo. A key secondary outcome was 28-day all-cause mortality. Safety outcomes included allergic reaction, new kidney replacement therapy, and hypotension.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>Both trials met prespecified early stopping criteria for a low probability of efficacy. Of 343 patients in the TXA-127 trial (226 [65.9%] aged 31-64 years, 200 [58.3%] men, 225 [65.6%] White, and 274 [79.9%] not Hispanic), 170 received TXA-127 and 173 received placebo. Of 290 patients in the TRV-027 trial (199 [68.6%] aged 31-64 years, 168 [57.9%] men, 195 [67.2%] White, and 225 [77.6%] not Hispanic), 145 received TRV-027 and 145 received placebo. Compared with placebo, both TXA-127 (unadjusted mean difference, −2.3 [95% CrI, −4.8 to 0.2]; adjusted OR, 0.88 [95% CrI, 0.59 to 1.30]) and TRV-027 (unadjusted mean difference, −2.4 [95% CrI, −5.1 to 0.3]; adjusted OR, 0.74 [95% CrI, 0.48 to 1.13]) resulted in no difference in oxygen-free days. In the TXA-127 trial, 28-day all-cause mortality occurred in 22 of 163 patients (13.5%) in the TXA-127 group vs 22 of 166 patients (13.3%) in the placebo group (adjusted OR, 0.83 [95% CrI, 0.41 to 1.66]). In the TRV-027 trial, 28-day all-cause mortality occurred in 29 of 141 patients (20.6%) in the TRV-027 group vs 18 of 140 patients (12.9%) in the placebo group (adjusted OR, 1.52 [95% CrI, 0.75 to 3.08]). The frequency of the safety outcomes was similar with either TXA-127 or TRV-027 vs placebo.</jats:p></jats:sec><jats:sec><jats:title>Conclusions and Relevance</jats:title><jats:p>In adults with severe COVID-19, RAS modulation (TXA-127 or TRV-027) did not improve oxygen-free days vs placebo. These results do not support the hypotheses that pharmacological interventions that selectively block the angiotensin II type 1 receptor or increase angiotensin (1-7) improve outcomes for patients with severe COVID-19.</jats:p></jats:sec><jats:sec><jats:title>Trial Registration</jats:title><jats:p>ClinicalTrials.gov Identifier: <jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"uri\" xlink:href=\"https://clinicaltrials.gov/ct2/show/NCT04924660?id=NCT04924660&amp;amp;draw=2&amp;amp;rank=1\">NCT04924660</jats:ext-link></jats:p></jats:sec>",
"author": [
{
"affiliation": [
{
"name": "Vanderbilt Institute for Clinical and Translational Research, Department of Emergency Medicine, Vanderbilt University Medical Center, Nashville, Tennessee"
}
],
"family": "Self",
"given": "Wesley H.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Biostatistics, Vanderbilt University Medical Center, Nashville, Tennessee"
}
],
"family": "Shotwell",
"given": "Matthew S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Wake Forest University, Winston-Salem, North Carolina"
}
],
"family": "Gibbs",
"given": "Kevin W.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Virginia Commonwealth University, Richmond"
}
],
"family": "de Wit",
"given": "Marjolein",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Wake Forest University, Winston-Salem, North Carolina"
}
],
"family": "Files",
"given": "D. Clark",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, University of New Mexico, Albuquerque"
}
],
"family": "Harkins",
"given": "Michelle",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, University of Cincinnati, Cincinnati, Ohio"
}
],
"family": "Hudock",
"given": "Kristin M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Emergency Medicine, Virginia Commonwealth University Health System, Richmond"
}
],
"family": "Merck",
"given": "Lisa H.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Montefiore Medical Center, Bronx, New York"
}
],
"family": "Moskowitz",
"given": "Ari",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, University of New Mexico, Albuquerque"
}
],
"family": "Apodaca",
"given": "Krystle D.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Emergency Medicine, University of Nebraska Medical Center, Omaha"
}
],
"family": "Barksdale",
"given": "Aaron",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Emergency Medicine, Yale University, New Haven, Connecticut"
}
],
"family": "Safdar",
"given": "Basmah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Washington University, St Louis, Missouri"
}
],
"family": "Javaheri",
"given": "Ali",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, University of Virginia, Charlottesville"
}
],
"family": "Sturek",
"given": "Jeffrey M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Tufts School of Medicine, Newton-Wellesley Hospital, Newton, Massachusetts"
}
],
"family": "Schrager",
"given": "Harry",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, University of Florida, Gainesville"
}
],
"family": "Iovine",
"given": "Nicole",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Dignity Health, Phoenix, Arizona"
}
],
"family": "Tiffany",
"given": "Brian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Denver Health Medical Center, Denver, Colorado"
}
],
"family": "Douglas",
"given": "Ivor S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Stanford University, Stanford, California"
}
],
"family": "Levitt",
"given": "Joseph",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Emory University, Atlanta, Georgia"
}
],
"family": "Busse",
"given": "Laurence W.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Emergency Medicine, School of Medicine, University of Colorado, Aurora"
}
],
"family": "Ginde",
"given": "Adit A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pulmonary/Critical Care Medicine, Intermountain Medical Center, Murray, Utah"
}
],
"family": "Brown",
"given": "Samuel M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Johns Hopkins University, Baltimore, Maryland"
}
],
"family": "Hager",
"given": "David N.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Emergency Medicine, Beth Israel Deaconess Medical Center, Boston, Massachusetts"
}
],
"family": "Boyle",
"given": "Katherine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Cleveland Clinic Foundation, Cleveland, Ohio"
}
],
"family": "Duggal",
"given": "Abhijit",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Oregon Health & Science University, Portland"
}
],
"family": "Khan",
"given": "Akram",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pulmonary/Critical Care Medicine, Intermountain Medical Center, Murray, Utah"
}
],
"family": "Lanspa",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Cedars-Sinai Medical Center, Los Angeles, California"
}
],
"family": "Chen",
"given": "Peter",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Emergency Medicine, University of Minnesota, Minneapolis"
}
],
"family": "Puskarich",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Ochsner Medical Center, New Orleans, Louisiana"
}
],
"family": "Vonderhaar",
"given": "Derek",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Cleveland Clinic Akron General, Akron, Ohio"
}
],
"family": "Venkateshaiah",
"given": "Lokesh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Emergency Medicine, Temple University, Philadelphia, Pennsylvania"
}
],
"family": "Gentile",
"given": "Nina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "National Heart, Lung, and Blood Institute, Bethesda, Maryland"
}
],
"family": "Rosenberg",
"given": "Yves",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "National Heart, Lung, and Blood Institute, Bethesda, Maryland"
}
],
"family": "Troendle",
"given": "James",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Vanderbilt Institute for Clinical and Translational Research, Vanderbilt University Medical Center, Nashville, Tennessee"
}
],
"family": "Bistran-Hall",
"given": "Amanda J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biostatistics, Vanderbilt University Medical Center, Nashville, Tennessee"
}
],
"family": "DeClercq",
"given": "Josh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Vanderbilt Institute for Clinical and Translational Research, Vanderbilt University Medical Center, Nashville, Tennessee"
}
],
"family": "Lavieri",
"given": "Robert",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Vanderbilt Institute for Clinical and Translational Research, Vanderbilt University Medical Center, Nashville, Tennessee"
}
],
"family": "Joly",
"given": "Meghan Morrison",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Vanderbilt Institute for Clinical and Translational Research, Vanderbilt University Medical Center, Nashville, Tennessee"
}
],
"family": "Orr",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Vanderbilt Institute for Clinical and Translational Research, Vanderbilt University Medical Center, Nashville, Tennessee"
}
],
"family": "Pulley",
"given": "Jill",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Vanderbilt Institute for Clinical and Translational Research, Department of Medicine, Vanderbilt University Medical Center, Nashville, Tennessee"
}
],
"family": "Rice",
"given": "Todd W.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biostatistics, Vanderbilt University Medical Center, Nashville, Tennessee"
}
],
"family": "Schildcrout",
"given": "Jonathan S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Vanderbilt University Medical Center, Nashville, Tennessee"
}
],
"family": "Semler",
"given": "Matthew W.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biostatistics, Vanderbilt University Medical Center, Nashville, Tennessee"
}
],
"family": "Wang",
"given": "Li",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Vanderbilt Institute for Clinical and Translational Research, Department of Medicine, Vanderbilt University Medical Center, Nashville, Tennessee"
}
],
"family": "Bernard",
"given": "Gordon R.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Vanderbilt Institute for Clinical and Translational Research, Department of Emergency Medicine, Vanderbilt University Medical Center, Nashville, Tennessee"
},
{
"name": "Geriatric Research, Education, and Clinical Center, Veterans Affairs Tennessee Valley Healthcare System, Nashville"
}
],
"family": "Collins",
"given": "Sean P.",
"sequence": "additional"
},
{
"affiliation": [],
"name": "ACTIV-4 Host Tissue Investigators",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Becker",
"given": "Richard C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "del Zoppo",
"given": "Gregory",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Henke",
"given": "Peter",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Holubkov",
"given": "Richard",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Johnson",
"given": "Maryl",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Kerr",
"given": "Kim",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Lipman",
"given": "Hannah I.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Lurie",
"given": "Fedor",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Pitt",
"given": "Bertram",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Vesely",
"given": "Sara K.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Fleg",
"given": "Jerome L.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Aamodt",
"given": "Dave",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Ayers",
"given": "J'Mario",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Clark",
"given": "Debra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Collins",
"given": "Jessica",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Cook",
"given": "Maya",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Dixon",
"given": "Sheri",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Graves",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Jordan",
"given": "Courtney",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Lindsell",
"given": "Christopher J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Lopez",
"given": "Itzel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "McKeel",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Orozco",
"given": "Dirk",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Prato",
"given": "Nelson",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Qi",
"given": "Ally",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Qutab",
"given": "Madiha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Stoughton",
"given": "Christa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Vermillion",
"given": "Krista",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Walsh",
"given": "Kelly",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Winchell",
"given": "Stephanie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Young",
"given": "Taylor",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Franklin",
"given": "Richard",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Wagner",
"given": "Elizabeth",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Walther",
"given": "Thomas",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Demitrack",
"given": "Mark",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Johnson",
"given": "Jakea",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Walsh",
"given": "Ryan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Bales",
"given": "Brian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Miller",
"given": "Karen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Torr",
"given": "Donna",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Barot",
"given": "Harsh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Landreth",
"given": "Leigha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "LaRose",
"given": "Mary",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Parks",
"given": "Lisa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Teixeira",
"given": "J. Pedro",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Cardenas",
"given": "Sandra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Ceniceros",
"given": "Juan A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Cunningham",
"given": "Amy G.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Kunkel",
"given": "Susan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Lovato",
"given": "Debbie M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Zimmerman",
"given": "Brooklin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Nguyen",
"given": "Thanh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Zeger",
"given": "Wesley",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Nichols",
"given": "Heather",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Wiedel",
"given": "Noah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Javaheri",
"given": "Ali",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Stilinovic",
"given": "Stephanie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Brokowski",
"given": "Carolyn",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Lu",
"given": "Jing",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Solberg",
"given": "Muriel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Lee",
"given": "Dana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Roach",
"given": "Kristopher",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Tiffany",
"given": "Brian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Tanner",
"given": "Charlotte",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Taylor",
"given": "Annette",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Zumbahl",
"given": "Jennine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Syed",
"given": "Aamer",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Mason",
"given": "Jessica",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Jackson",
"given": "Patrick E. H.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Coleman",
"given": "Rachael W.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Haughey",
"given": "Heather M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Cherabuddi",
"given": "Kartik",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "James",
"given": "Nastasia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Wakeman",
"given": "Rebecca",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Duncan",
"given": "Christopher",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Montero",
"given": "Cynthia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Rogers",
"given": "Angela J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Wilson",
"given": "Jennifer G.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Vojnik",
"given": "Rosemary",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Perez",
"given": "Cynthia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Wyles",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Hiller",
"given": "Terra D.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Oakes",
"given": "Judy L.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Garcia",
"given": "Ana Z.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Gong",
"given": "Michelle",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Mohamed",
"given": "Amira",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Andrea",
"given": "Luke",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Nair",
"given": "Rahul",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Nkemdirim",
"given": "William",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Lopez",
"given": "Brenda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Boujid",
"given": "Sabah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Torres",
"given": "Martha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Garcia",
"given": "Ofelia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Martinez",
"given": "Flora",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Baduashvili",
"given": "Amiran",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Bastman",
"given": "Jill",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Chauhan",
"given": "Lakshmi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Douin",
"given": "David J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Finck",
"given": "Lani",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Licursi",
"given": "Ashley",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "ten Lohuis",
"given": "Caitlin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Zhang",
"given": "Sophia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Bender",
"given": "William",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Tovar",
"given": "Santiago",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Hayes",
"given": "Sharon",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Kurtzman",
"given": "Nicholas",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Rosseto",
"given": "Elinita",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Scaffidi",
"given": "Douglas",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Shapiro",
"given": "Nathan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Pak",
"given": "Jonathan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Allada",
"given": "Gopal",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Briceno",
"given": "Genesis",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Peña",
"given": "Jose",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Oh",
"given": "Minn",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Ali",
"given": "Harith",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Beselman",
"given": "Sasha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Eby",
"given": "Yolanda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Klimov",
"given": "Vitaliy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Hite",
"given": "R. Duncan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Tanzeem",
"given": "Hammad",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Droege",
"given": "Chris",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Winter",
"given": "Jessica",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Jackman",
"given": "Susan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Caudill",
"given": "Antonina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Bayoumi",
"given": "Emad",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Pascual",
"given": "Ethan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Chen",
"given": "Po-En",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Mucha",
"given": "Simon",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Thiruchelvam",
"given": "Nirosshan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Siuba",
"given": "Matthew",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Mehkri",
"given": "Omar",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Driver",
"given": "Brian E.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Hendrickson",
"given": "Audrey F.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Kaus",
"given": "Olivia R.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Ontiveros",
"given": "Christina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Riehm",
"given": "Amy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Laudun",
"given": "Sylvia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Hudock",
"given": "Debra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Ensley",
"given": "Christopher",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Shaner",
"given": "Valerie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Gentile",
"given": "Nina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Isenberg",
"given": "Derek",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Reimer",
"given": "Hannah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Cincola",
"given": "Paul",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Harris",
"given": "Estelle S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Callahan",
"given": "Sean J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Yamane",
"given": "Misty B.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Barrios",
"given": "Macy AG",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Desai",
"given": "Neeraj",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Bharara",
"given": "Amit",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Keller",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Majumder",
"given": "Prat",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Dohe",
"given": "Carrie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "D’Armiento",
"given": "Jeanine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Goldklang",
"given": "Monica",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Wagener",
"given": "Gebhard",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Fonseca",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Valezquez-Sanchez",
"given": "Itzel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Johnson",
"given": "Nicholas J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Petersen",
"given": "Emily",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Fuentes",
"given": "Megan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Newton",
"given": "Maranda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Gundel",
"given": "Stephanie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Srinivasan",
"given": "Vasisht",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Steel",
"given": "Tessa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-4 Host Tissue Investigators"
}
],
"family": "Robinson",
"given": "Bryce",
"sequence": "additional"
}
],
"container-title": "JAMA",
"container-title-short": "JAMA",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
4,
11
]
],
"date-time": "2023-04-11T15:04:43Z",
"timestamp": 1681225483000
},
"deposited": {
"date-parts": [
[
2023,
4,
11
]
],
"date-time": "2023-04-11T15:04:47Z",
"timestamp": 1681225487000
},
"indexed": {
"date-parts": [
[
2024,
9,
15
]
],
"date-time": "2024-09-15T22:34:40Z",
"timestamp": 1726439680129
},
"is-referenced-by-count": 19,
"issue": "14",
"issued": {
"date-parts": [
[
2023,
4,
11
]
]
},
"journal-issue": {
"issue": "14",
"published-print": {
"date-parts": [
[
2023,
4,
11
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://jamanetwork.com/journals/jama/articlepdf/2803516/jama_self_2023_oi_230031_1680895597.6683.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "10",
"original-title": [],
"page": "1170",
"prefix": "10.1001",
"published": {
"date-parts": [
[
2023,
4,
11
]
]
},
"published-print": {
"date-parts": [
[
2023,
4,
11
]
]
},
"publisher": "American Medical Association (AMA)",
"reference": [
{
"DOI": "10.1001/jama.2021.19499",
"article-title": "Association between mRNA vaccination and COVID-19 hospitalization and disease severity.",
"author": "Tenforde",
"doi-asserted-by": "publisher",
"first-page": "2043",
"issue": "20",
"journal-title": "JAMA",
"key": "joi230031r1",
"volume": "326",
"year": "2021"
},
{
"DOI": "10.1136/bmj-2021-069761",
"article-title": "Clinical severity of, and effectiveness of mRNA vaccines against, covid-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: prospective observational study.",
"author": "Lauring",
"doi-asserted-by": "publisher",
"journal-title": "BMJ",
"key": "joi230031r2",
"volume": "376",
"year": "2022"
},
{
"DOI": "10.1136/bmj-2022-072065",
"article-title": "Vaccine effectiveness of primary series and booster doses against covid-19 associated hospital admissions in the United States: living test negative design study.",
"author": "Adams",
"doi-asserted-by": "publisher",
"journal-title": "BMJ",
"key": "joi230031r3",
"volume": "379",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the treatment of Covid-19—final report.",
"author": "Beigel",
"doi-asserted-by": "publisher",
"first-page": "1813",
"issue": "19",
"journal-title": "N Engl J Med",
"key": "joi230031r4",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.17023",
"article-title": "Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis.",
"author": "Sterne",
"doi-asserted-by": "publisher",
"first-page": "1330",
"issue": "13",
"journal-title": "JAMA",
"key": "joi230031r5",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2031994",
"article-title": "Baricitinib plus remdesivir for hospitalized adults with Covid-19.",
"author": "Kalil",
"doi-asserted-by": "publisher",
"first-page": "795",
"issue": "9",
"journal-title": "N Engl J Med",
"key": "joi230031r6",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1093/eurheartj/ehac031",
"article-title": "Long COVID: post-acute sequelae of COVID-19 with a cardiovascular focus.",
"author": "Raman",
"doi-asserted-by": "publisher",
"first-page": "1157",
"issue": "11",
"journal-title": "Eur Heart J",
"key": "joi230031r7",
"volume": "43",
"year": "2022"
},
{
"DOI": "10.1152/ajpheart.00217.2020",
"article-title": "COVID-19, ACE2, and the cardiovascular consequences.",
"author": "South",
"doi-asserted-by": "publisher",
"first-page": "H1084",
"issue": "5",
"journal-title": "Am J Physiol Heart Circ Physiol",
"key": "joi230031r8",
"volume": "318",
"year": "2020"
},
{
"DOI": "10.1038/nature03712",
"article-title": "Angiotensin-converting enzyme 2 protects from severe acute lung failure.",
"author": "Imai",
"doi-asserted-by": "publisher",
"first-page": "112",
"issue": "7047",
"journal-title": "Nature",
"key": "joi230031r9",
"volume": "436",
"year": "2005"
},
{
"DOI": "10.1002/path.2987",
"article-title": "Acute respiratory distress syndrome leads to reduced ratio of ACE/ACE2 activities and is prevented by angiotensin (1-7) or an angiotensin II receptor antagonist.",
"author": "Wösten-van Asperen",
"doi-asserted-by": "publisher",
"first-page": "618",
"issue": "4",
"journal-title": "J Pathol",
"key": "joi230031r10",
"volume": "225",
"year": "2011"
},
{
"DOI": "10.1016/S2213-2600(20)30418-5",
"article-title": "Human recombinant soluble ACE2 in severe COVID-19.",
"author": "Zoufaly",
"doi-asserted-by": "publisher",
"first-page": "1154",
"issue": "11",
"journal-title": "Lancet Respir Med",
"key": "joi230031r11",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.2478/jtim-2020-0003",
"article-title": "Potential role of ACE2 in coronavirus disease 2019 (COVID-19) prevention and management.",
"author": "Liu",
"doi-asserted-by": "publisher",
"first-page": "9",
"issue": "1",
"journal-title": "J Transl Int Med",
"key": "joi230031r12",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1152/ajplung.00129.2021",
"article-title": "A pilot study to assess the circulating renin-angiotensin system in COVID-19 acute respiratory failure.",
"author": "Files",
"doi-asserted-by": "publisher",
"first-page": "L213",
"issue": "1",
"journal-title": "Am J Physiol Lung Cell Mol Physiol",
"key": "joi230031r13",
"volume": "321",
"year": "2021"
},
{
"DOI": "10.1016/j.cell.2020.04.004",
"article-title": "Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2.",
"author": "Monteil",
"doi-asserted-by": "publisher",
"first-page": "905",
"issue": "4",
"journal-title": "Cell",
"key": "joi230031r14",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1001/jamanetworkopen.2022.2735",
"article-title": "Efficacy of losartan in hospitalized patients with COVID-19–induced lung injury: a randomized clinical trial.",
"author": "Puskarich",
"doi-asserted-by": "publisher",
"issue": "3",
"journal-title": "JAMA Netw Open",
"key": "joi230031r15",
"volume": "5",
"year": "2022"
},
{
"DOI": "10.1001/jama.2020.25864",
"article-title": "Effect of discontinuing vs continuing angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on days alive and out of the hospital in patients admitted with COVID-19: a randomized clinical trial.",
"author": "Lopes",
"doi-asserted-by": "publisher",
"first-page": "254",
"issue": "3",
"journal-title": "JAMA",
"key": "joi230031r16",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(20)30558-0",
"article-title": "Continuation versus discontinuation of renin-angiotensin system inhibitors in patients admitted to hospital with COVID-19: a prospective, randomised, open-label trial.",
"author": "Cohen",
"doi-asserted-by": "publisher",
"first-page": "275",
"issue": "3",
"journal-title": "Lancet Respir Med",
"key": "joi230031r17",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1016/j.eclinm.2021.101152",
"article-title": "Seven days treatment with the angiotensin II type 2 receptor agonist C21 in hospitalized COVID-19 patients; a placebo-controlled randomised multi-centre double-blind phase 2 trial.",
"author": "Tornling",
"doi-asserted-by": "publisher",
"journal-title": "EClinicalMedicine",
"key": "joi230031r18",
"volume": "41",
"year": "2021"
},
{
"DOI": "10.1111/bcp.v89.4",
"article-title": "The effect of TRV027 on coagulation in COVID-19: a pilot randomized, placebo-controlled trial.",
"author": "Robbins",
"doi-asserted-by": "publisher",
"first-page": "1495",
"issue": "4",
"journal-title": "Br J Clin Pharmacol",
"key": "joi230031r19",
"volume": "89",
"year": "2023"
},
{
"DOI": "10.1186/s13054-022-04096-9",
"article-title": "A randomized, placebo-controlled, double-blinded pilot study of angiotensin 1-7 (TXA-127) for the treatment of severe COVID-19.",
"author": "Wagener",
"doi-asserted-by": "publisher",
"first-page": "229",
"issue": "1",
"journal-title": "Crit Care",
"key": "joi230031r20",
"volume": "26",
"year": "2022"
},
{
"DOI": "10.1093/eurheartj/ehx196",
"article-title": "Biased ligand of the angiotensin II type 1 receptor in patients with acute heart failure: a randomized, double-blind, placebo-controlled, phase IIB, dose ranging trial (BLAST-AHF).",
"author": "Pang",
"doi-asserted-by": "publisher",
"first-page": "2364",
"issue": "30",
"journal-title": "Eur Heart J",
"key": "joi230031r21",
"volume": "38",
"year": "2017"
},
{
"DOI": "10.1001/jama.2020.8920",
"article-title": "Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV): an unprecedented partnership for unprecedented times.",
"author": "Collins",
"doi-asserted-by": "publisher",
"first-page": "2455",
"issue": "24",
"journal-title": "JAMA",
"key": "joi230031r22",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1016/j.jbi.2019.103208",
"article-title": "The REDCap Consortium: building an international community of software platform partners.",
"author": "Harris",
"doi-asserted-by": "publisher",
"journal-title": "J Biomed Inform",
"key": "joi230031r23",
"volume": "95",
"year": "2019"
},
{
"DOI": "10.1177/1740774515626362",
"article-title": "Efficiencies of platform clinical trials: a vision of the future.",
"author": "Saville",
"doi-asserted-by": "publisher",
"first-page": "358",
"issue": "3",
"journal-title": "Clin Trials",
"key": "joi230031r24",
"volume": "13",
"year": "2016"
},
{
"DOI": "10.1016/j.tcm.2013.01.002",
"article-title": "GPCR biased ligands as novel heart failure therapeutics.",
"author": "Violin",
"doi-asserted-by": "publisher",
"first-page": "242",
"issue": "7",
"journal-title": "Trends Cardiovasc Med",
"key": "joi230031r25",
"volume": "23",
"year": "2013"
},
{
"DOI": "10.1016/j.chest.2022.04.145",
"article-title": "Oxygen-free days as an outcome measure in clinical trials of therapies for COVID-19 and other causes of new-onset hypoxemia.",
"author": "Moskowitz",
"doi-asserted-by": "publisher",
"first-page": "804",
"issue": "4",
"journal-title": "Chest",
"key": "joi230031r26",
"volume": "162",
"year": "2022"
},
{
"DOI": "10.1016/S1473-3099(20)30483-7",
"article-title": "A minimal common outcome measure set for COVID-19 clinical research.",
"author": "WHO Working Group on the Clinical Characterisation and Management of COVID-19 Infection",
"doi-asserted-by": "publisher",
"first-page": "e192",
"issue": "8",
"journal-title": "Lancet Infect Dis",
"key": "joi230031r27",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1016/j.chest.2022.06.029",
"article-title": "Neutralizing COVID-19 convalescent plasma in adults hospitalized with COVID-19: a blinded, randomized, placebo-controlled trial.",
"author": "Self",
"doi-asserted-by": "publisher",
"first-page": "982",
"issue": "5",
"journal-title": "Chest",
"key": "joi230031r28",
"volume": "162",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa062200",
"article-title": "Comparison of two fluid-management strategies in acute lung injury.",
"author": "National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network",
"doi-asserted-by": "publisher",
"first-page": "2564",
"issue": "24",
"journal-title": "N Engl J Med",
"key": "joi230031r29",
"volume": "354",
"year": "2006"
},
{
"DOI": "10.1001/jama.2019.14607",
"article-title": "Effect of selepressin vs placebo on ventilator- and vasopressor-free days in patients with septic shock: the SEPSIS-ACT randomized clinical trial.",
"author": "Laterre",
"doi-asserted-by": "publisher",
"first-page": "1476",
"issue": "15",
"journal-title": "JAMA",
"key": "joi230031r30",
"volume": "322",
"year": "2019"
},
{
"DOI": "10.1001/jama.2020.22240",
"article-title": "Effect of hydroxychloroquine on clinical status at 14 days in hospitalized patients with COVID-19: a randomized clinical trial.",
"author": "Self",
"doi-asserted-by": "publisher",
"first-page": "2165",
"issue": "21",
"journal-title": "JAMA",
"key": "joi230031r31",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1056/NEJMsr077003",
"article-title": "Statistics in medicine–reporting of subgroup analyses in clinical trials.",
"author": "Wang",
"doi-asserted-by": "publisher",
"first-page": "2189",
"issue": "21",
"journal-title": "N Engl J Med",
"key": "joi230031r32",
"volume": "357",
"year": "2007"
},
{
"DOI": "10.1016/j.ddmec.2006.06.012",
"article-title": "The renin-angiotensin system in acute respiratory distress syndrome.",
"author": "Imai",
"doi-asserted-by": "publisher",
"first-page": "225",
"issue": "2",
"journal-title": "Drug Discov Today Dis Mech",
"key": "joi230031r33",
"volume": "3",
"year": "2006"
},
{
"DOI": "10.1136/postgradmedj-2020-137935",
"article-title": "ACE2 and COVID-19 and the resulting ARDS.",
"author": "Zhang",
"doi-asserted-by": "publisher",
"first-page": "403",
"issue": "1137",
"journal-title": "Postgrad Med J",
"key": "joi230031r34",
"volume": "96",
"year": "2020"
},
{
"DOI": "10.1038/s41440-020-0455-8",
"article-title": "Interactions of coronaviruses with ACE2, angiotensin II, and RAS inhibitors—lessons from available evidence and insights into COVID-19.",
"author": "Kai",
"doi-asserted-by": "publisher",
"first-page": "648",
"issue": "7",
"journal-title": "Hypertens Res",
"key": "joi230031r35",
"volume": "43",
"year": "2020"
},
{
"DOI": "10.1164/rccm.202108-1904ED",
"article-title": "The renin-angiotensin-aldosterone system in COVID-19-related and non-COVID-19-related acute respiratory distress syndrome: not so different after all?",
"author": "Collins",
"doi-asserted-by": "publisher",
"first-page": "1007",
"issue": "9",
"journal-title": "Am J Respir Crit Care Med",
"key": "joi230031r36",
"volume": "204",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(21)00214-9",
"article-title": "Discontinuation versus continuation of renin-angiotensin-system inhibitors in COVID-19 (ACEI-COVID): a prospective, parallel group, randomised, controlled, open-label trial.",
"author": "Bauer",
"doi-asserted-by": "publisher",
"first-page": "863",
"issue": "8",
"journal-title": "Lancet Respir Med",
"key": "joi230031r37",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1164/rccm.202012-4461OC",
"article-title": "Increased angiotensin-converting enzyme 2 and loss of alveolar type II cells in COVID-19-related acute respiratory distress syndrome.",
"author": "Gerard",
"doi-asserted-by": "publisher",
"first-page": "1024",
"issue": "9",
"journal-title": "Am J Respir Crit Care Med",
"key": "joi230031r38",
"volume": "204",
"year": "2021"
}
],
"reference-count": 38,
"references-count": 38,
"relation": {},
"resource": {
"primary": {
"URL": "https://jamanetwork.com/journals/jama/fullarticle/2803516"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [
"Two Randomized Clinical Trials"
],
"title": "Renin-Angiotensin System Modulation With Synthetic Angiotensin (1-7) and Angiotensin II Type 1 Receptor–Biased Ligand in Adults With COVID-19",
"type": "journal-article",
"volume": "329"
}