Neutralizing COVID-19 Convalescent Plasma in Adults Hospitalized With COVID-19
et al., Chest, doi:10.1016/j.chest.2022.06.029, PassItOn, NCT04362176, Nov 2022
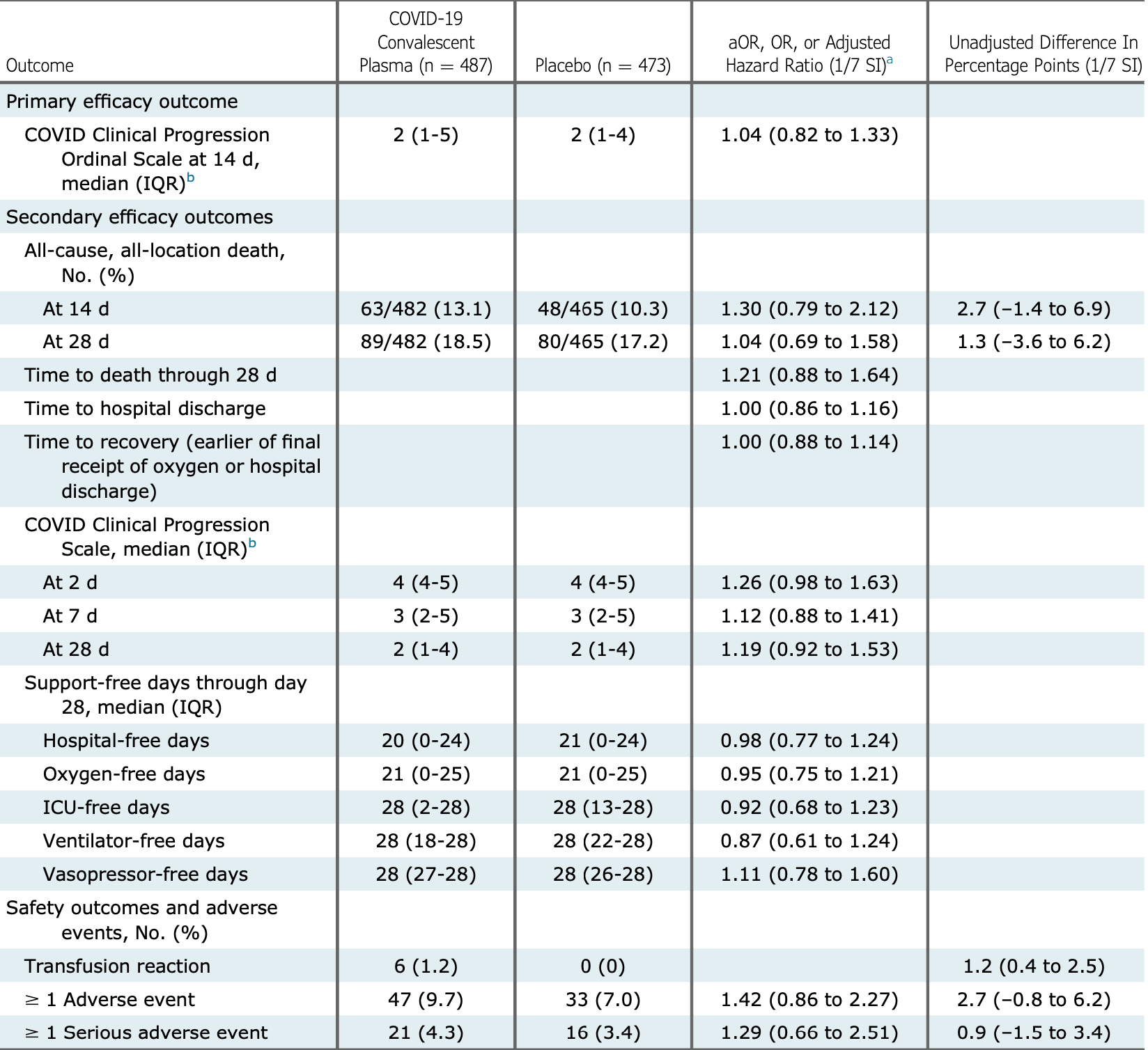
RCT 947 hospitalized patients in the USA, showing no signficant difference with convalescent plasma treatment.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 3.3% higher, RR 1.03, p = 0.86, treatment 89 of 482 (18.5%), control 80 of 465 (17.2%), odds ratio converted to relative risk, day 28.
|
|
risk of death, 26.1% higher, RR 1.26, p = 0.29, treatment 63 of 482 (13.1%), control 48 of 465 (10.3%), odds ratio converted to relative risk, day 14.
|
|
risk of 7-point scale, 4.0% higher, OR 1.04, p = 0.76, treatment 487, control 473, day 14, primary outcome, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Self et al., 30 Nov 2022, Double Blind Randomized Controlled Trial, placebo-controlled, USA, peer-reviewed, 51 authors, study period 28 April, 2020 - 1 June, 2021, average treatment delay 8.0 days, trial NCT04362176 (history) (PassItOn).
Contact: wesley.self@vumc.org.
Neutralizing COVID-19 Convalescent Plasma in Adults Hospitalized With COVID-19
Chest, doi:10.1016/j.chest.2022.06.029
BACKGROUND: Convalescent plasma has been one of the most common treatments for COVID-19, but most clinical trial data to date have not supported its efficacy. RESEARCH QUESTION: Is rigorously selected COVID-19 convalescent plasma with neutralizing anti-SARS-CoV-2 antibodies an efficacious treatment for adults hospitalized with COVID-19? STUDY DESIGN AND METHODS: This was a multicenter, blinded, placebo-controlled randomized clinical trial among adults hospitalized with SARS-CoV-2 infection and acute respiratory symptoms for < 14 days. Enrolled patients were randomly assigned to receive one unit of COVID-19 convalescent plasma (n ¼ 487) or placebo (n ¼ 473). The primary outcome was clinical status (disease severity) 14 days following study infusion measured with a seven-category ordinal scale ranging from discharged from the hospital with resumption of normal activities (lowest score) to death (highest score). The primary outcome was analyzed with a multivariable ordinal regression model, with an adjusted odds ratio (aOR) < 1.0 indicating more favorable outcomes with convalescent plasma than with placebo. In secondary analyses, trial participants were stratified according to the presence of endogenous anti-SARS-CoV-2 antibodies ("serostatus") at randomization. The trial included 13 secondary efficacy outcomes, including 28-day mortality. RESULTS: Among 974 randomized patients, 960 were included in the primary analysis. Clinical status on the ordinal outcome scale at 14 days did not differ between the convalescent plasma and placebo groups in the overall population (aOR, 1.04; one-seventh support interval [1/7 SI], 0.82-1.33), in patients without endogenous antibodies (aOR, 1.15; 1/7 SI, 0.74-1.80), or in patients with endogenous antibodies (aOR, 0.96; 1/7 SI, 0.72-1.30). None of the 13 secondary efficacy outcomes were different between groups. At 28 days, 89 of 482 (18.5%) patients in the convalescent plasma group and 80 of 465 (17.2%) patients in the placebo group had died (aOR, 1.04; 1/7 SI, 0.69-1.58). INTERPRETATION: Among adults hospitalized with COVID-19, including those seronegative for anti-SARS-CoV-2 antibodies, treatment with convalescent plasma did not improve clinical outcomes.
In each plot, the convalescent plasma group is represented by blue lines and the placebo group by red lines. The top set of lines are Kaplan-Meier survival plots. The bottom set of lines denote the proportion of participants alive and discharged from the hospital. Patient disposition is represented by the three locations within the plot area: dead, represented by the area above the survival lines; alive and still in the hospital, represented by the area between the survival and discharge lines; and discharged from the hospital alive, represented by the area under the discharge lines. The proportion in each disposition state is denoted by the relative height of the region for each day. On study day 1, the vast majority of participants were alive and in the hospital (middle region). Over time, the proportion of participants in the alive and discharged state (lower region) and dead state (upper region) increases, which gives rise to the "funnel" shape of the plot. Participants could move from either in-hospital or discharged states to the dead state. Patients were followed up via medical records and telephone follow-up until 28 days following study infusion. Patients lost to follow-up were included in the risk-set for the portion of days for which disposition was known. A patient was considered discharged from the hospital once discharged from the index hospitalization; re-hospitalizations were not considered in this analysis. In model-based estimates of treatment..
References
Abraham, Passive antibody therapy in COVID-19, Nat Rev Immunol
Agarwal, Mukherjee, Kumar, Convalescent plasma in the management of moderate Covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial), BMJ
Beigel, Tomashek, Dodd, Remdesivir for the treatment of Covid-19-final report, N Engl J Med
Blume, Likelihood methods for measuring statistical evidence, Statist Med
Bégin, Callum, Jamula, Convalescent plasma for hospitalized patients with COVID-19: an open-label, randomized controlled trial, Nat Med
Casadevall, Pirofski, The convalescent sera option for containing COVID-19, J Clin Invest
Dashboard, None
Estcourt, Turgeon, Effect of convalescent plasma on organ support-free days in critically ill patients with COVID-19: a randomized clinical trial, JAMA
Gilchuk, Thomsen, Yoder, Standardized two-step testing of antibody activity in COVID-19 convalescent plasma, iScience
Group, EuroQol-a new facility for the measurement of health-related quality of life, Health Policy
Harris, Taylor, Minor, The REDCap consortium: building an international community of software platform partners, J Biomed Informatics
Herdman, Gudex, Lloyd, Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L), Qual Life Res
Janiaud, Axfors, Schmitt, Association of convalescent plasma treatment with clinical outcomes in patients with COVID-19: a systematic review and meta-analysis, JAMA
Joyner, Carter, Senefeld, Convalescent plasma antibody levels and the risk of death from Covid-19, N Engl J Med
Korley, Durkalski-Mauldin, Yeatts, Early convalescent plasma for high-risk outpatients with Covid-19
Kunze, Johnson, Van Helmond, Mortality in individuals treated with COVID-19 convalescent plasma varies with the geographic provenance of donors, Infectious Diseases, doi:10.1101/2021.03.19.21253975
Ly-Cov555 Study, Group, Lundgren, Grund, A neutralizing monoclonal antibody for hospitalized patients with Covid-19
Roback, Guarner, Convalescent plasma to treat COVID-19: possibilities and challenges, JAMA
Royall, Statistical Evidence: A Likelihood Paradigm
Self, Semler, Leither, Effect of hydroxychloroquine on clinical status at 14 days in hospitalized patients with COVID-19: a randomized clinical trial, JAMA
Self, Stewart, Wheeler, Passive Immunity Trial for Our Nation (PassITON): study protocol for a randomized placebo-control clinical trial evaluating COVID-19 convalescent plasma in hospitalized adults, Trials
Simonovich, Pratx, Scibona, A randomized trial of convalescent plasma in Covid-19 severe pneumonia, N Engl J Med
Singer, Deutschman, Seymour, The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3), JAMA
Spinner, Gottlieb, Criner, Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial, JAMA
Tico Bamlanivimab, Group, Lundgren, Grund, Responses to a neutralizing monoclonal antibody for hospitalized patients with COVID-19 according to baseline antibody and antigen levels: a randomized controlled trial, Ann Intern Med
Wang, Blume, An evidential approach to non-inferiority clinical trials, Pharmaceut Statist
DOI record:
{
"DOI": "10.1016/j.chest.2022.06.029",
"ISSN": [
"0012-3692"
],
"URL": "http://dx.doi.org/10.1016/j.chest.2022.06.029",
"alternative-id": [
"S0012369222012016"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Neutralizing COVID-19 Convalescent Plasma in Adults Hospitalized With COVID-19"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Chest"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.chest.2022.06.029"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2022 American College of Chest Physicians. Published by Elsevier Inc. All rights reserved."
}
],
"author": [
{
"affiliation": [],
"family": "Self",
"given": "Wesley H.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Wheeler",
"given": "Allison P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Stewart",
"given": "Thomas G.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schrager",
"given": "Harry",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mallada",
"given": "Jason",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thomas",
"given": "Christopher B.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cataldo",
"given": "Vince D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "O’Neal",
"given": "Hollis R.",
"sequence": "additional",
"suffix": "Jr."
},
{
"affiliation": [],
"family": "Shapiro",
"given": "Nathan I.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Higgins",
"given": "Conor",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ginde",
"given": "Adit A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chauhan",
"given": "Lakshmi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Johnson",
"given": "Nicholas J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Henning",
"given": "Daniel J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jaiswal",
"given": "Stuti J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mammen",
"given": "Manoj J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Harris",
"given": "Estelle S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pannu",
"given": "Sonal R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Laguio-Vila",
"given": "Maryrose",
"sequence": "additional"
},
{
"affiliation": [],
"family": "El Atrouni",
"given": "Wissam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "de Wit",
"given": "Marjolein",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hoda",
"given": "Daanish",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cohn",
"given": "Claudia S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McWilliams",
"given": "Carla",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shanholtz",
"given": "Carl",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jones",
"given": "Alan E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Raval",
"given": "Jay S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mucha",
"given": "Simon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ipe",
"given": "Tina S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Qiao",
"given": "Xian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schrantz",
"given": "Stephen J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shenoy",
"given": "Aarthi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fremont",
"given": "Richard D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Brady",
"given": "Eric J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Carnahan",
"given": "Robert H.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chappell",
"given": "James D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Crowe",
"given": "James E.",
"sequence": "additional",
"suffix": "Jr."
},
{
"affiliation": [],
"family": "Denison",
"given": "Mark R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gilchuk",
"given": "Pavlo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Stevens",
"given": "Laura J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sutton",
"given": "Rachel E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thomsen",
"given": "Isaac",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yoder",
"given": "Sandra M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bistran-Hall",
"given": "Amanda J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Casey",
"given": "Jonathan D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lindsell",
"given": "Christopher J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Li",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pulley",
"given": "Jill M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rhoads",
"given": "Jillian P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bernard",
"given": "Gordon R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rice",
"given": "Todd W.",
"sequence": "additional"
}
],
"container-title": "Chest",
"container-title-short": "Chest",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.com.au",
"clinicalkey.es",
"clinicalkey.com",
"journal.chestnet.org",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2022,
7,
1
]
],
"date-time": "2022-07-01T07:59:26Z",
"timestamp": 1656662366000
},
"deposited": {
"date-parts": [
[
2023,
4,
7
]
],
"date-time": "2023-04-07T17:05:56Z",
"timestamp": 1680887156000
},
"funder": [
{
"DOI": "10.13039/100006108",
"award": [
"3UL1TR002243-04S3"
],
"doi-asserted-by": "publisher",
"name": "National Center for Advancing Translational Sciences"
},
{
"DOI": "10.13039/100000002",
"doi-asserted-by": "publisher",
"name": "National Institutes of Health"
},
{
"DOI": "10.13039/100006483",
"doi-asserted-by": "publisher",
"name": "AbbVie"
},
{
"DOI": "10.13039/100000005",
"doi-asserted-by": "publisher",
"name": "U.S. Department of Defense"
},
{
"DOI": "10.13039/100013017",
"doi-asserted-by": "publisher",
"name": "Vanderbilt University Medical Center"
},
{
"DOI": "10.13039/100000030",
"doi-asserted-by": "publisher",
"name": "Centers for Disease Control and Prevention"
}
],
"indexed": {
"date-parts": [
[
2023,
4,
12
]
],
"date-time": "2023-04-12T09:51:51Z",
"timestamp": 1681293111280
},
"is-referenced-by-count": 8,
"issue": "5",
"issued": {
"date-parts": [
[
2022,
11
]
]
},
"journal-issue": {
"issue": "5",
"published-print": {
"date-parts": [
[
2022,
11
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
11,
1
]
],
"date-time": "2022-11-01T00:00:00Z",
"timestamp": 1667260800000
}
},
{
"URL": "http://www.elsevier.com/open-access/userlicense/1.0/",
"content-version": "am",
"delay-in-days": 242,
"start": {
"date-parts": [
[
2023,
7,
1
]
],
"date-time": "2023-07-01T00:00:00Z",
"timestamp": 1688169600000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S0012369222012016?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S0012369222012016?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "982-994",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022,
11
]
]
},
"published-print": {
"date-parts": [
[
2022,
11
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1001/jama.2020.4940",
"article-title": "Convalescent plasma to treat COVID-19: possibilities and challenges",
"author": "Roback",
"doi-asserted-by": "crossref",
"first-page": "1561",
"issue": "16",
"journal-title": "JAMA",
"key": "10.1016/j.chest.2022.06.029_bib3",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1172/JCI138003",
"article-title": "The convalescent sera option for containing COVID-19",
"author": "Casadevall",
"doi-asserted-by": "crossref",
"first-page": "1545",
"issue": "4",
"journal-title": "J Clin Invest",
"key": "10.1016/j.chest.2022.06.029_bib4",
"volume": "130",
"year": "2020"
},
{
"DOI": "10.1038/s41577-020-0365-7",
"article-title": "Passive antibody therapy in COVID-19",
"author": "Abraham",
"doi-asserted-by": "crossref",
"first-page": "401",
"issue": "7",
"journal-title": "Nat Rev Immunol",
"key": "10.1016/j.chest.2022.06.029_bib5",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2031893",
"article-title": "Convalescent plasma antibody levels and the risk of death from Covid-19",
"author": "Joyner",
"doi-asserted-by": "crossref",
"first-page": "1015",
"issue": "11",
"journal-title": "N Engl J Med",
"key": "10.1016/j.chest.2022.06.029_bib6",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1136/bmj.m3939",
"article-title": "Convalescent plasma in the management of moderate Covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial)",
"author": "Agarwal",
"doi-asserted-by": "crossref",
"first-page": "m3939",
"journal-title": "BMJ",
"key": "10.1016/j.chest.2022.06.029_bib8",
"volume": "371",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2031304",
"article-title": "A randomized trial of convalescent plasma in Covid-19 severe pneumonia",
"author": "Simonovich",
"doi-asserted-by": "crossref",
"first-page": "619",
"issue": "7",
"journal-title": "N Engl J Med",
"key": "10.1016/j.chest.2022.06.029_bib9",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(21)00897-7",
"article-title": "Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial",
"doi-asserted-by": "crossref",
"first-page": "2049",
"issue": "10289",
"journal-title": "Lancet",
"key": "10.1016/j.chest.2022.06.029_bib10",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1001/jama.2021.2747",
"article-title": "Association of convalescent plasma treatment with clinical outcomes in patients with COVID-19: a systematic review and meta-analysis",
"author": "Janiaud",
"doi-asserted-by": "crossref",
"first-page": "1185",
"issue": "12",
"journal-title": "JAMA",
"key": "10.1016/j.chest.2022.06.029_bib11",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2103784",
"article-title": "Early convalescent plasma for high-risk outpatients with Covid-19",
"author": "Korley",
"doi-asserted-by": "crossref",
"first-page": "1951",
"issue": "21",
"journal-title": "N Engl J Med",
"key": "10.1016/j.chest.2022.06.029_bib12",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1038/s41591-021-01488-2",
"article-title": "Convalescent plasma for hospitalized patients with COVID-19: an open-label, randomized controlled trial",
"author": "Bégin",
"doi-asserted-by": "crossref",
"first-page": "2012",
"issue": "11",
"journal-title": "Nat Med",
"key": "10.1016/j.chest.2022.06.029_bib13",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1001/jama.2021.18178",
"article-title": "Effect of convalescent plasma on organ support-free days in critically ill patients with COVID-19: a randomized clinical trial",
"author": "Estcourt",
"doi-asserted-by": "crossref",
"first-page": "1690",
"issue": "17",
"journal-title": "JAMA",
"key": "10.1016/j.chest.2022.06.029_bib14",
"volume": "326",
"year": "2021"
},
{
"DOI": "10.1016/j.isci.2021.103602",
"article-title": "Standardized two-step testing of antibody activity in COVID-19 convalescent plasma",
"author": "Gilchuk",
"doi-asserted-by": "crossref",
"issue": "1",
"journal-title": "iScience",
"key": "10.1016/j.chest.2022.06.029_bib15",
"volume": "25",
"year": "2022"
},
{
"article-title": "Efficacy and safety of two neutralising monoclonal antibody therapies, sotrovimab and BRII-196 plus BRII-198, for adults hospitalised with COVID-19 (TICO): a randomised controlled trial",
"first-page": "622",
"issue": "5",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/j.chest.2022.06.029_bib16",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.7326/M21-3507",
"article-title": "Responses to a neutralizing monoclonal antibody for hospitalized patients with COVID-19 according to baseline antibody and antigen levels: a randomized controlled trial",
"author": "Lundgren",
"doi-asserted-by": "crossref",
"first-page": "234",
"issue": "2",
"journal-title": "Ann Intern Med",
"key": "10.1016/j.chest.2022.06.029_bib17",
"volume": "175",
"year": "2022"
},
{
"DOI": "10.1186/s13063-021-05171-2",
"article-title": "Passive Immunity Trial for Our Nation (PassITON): study protocol for a randomized placebo-control clinical trial evaluating COVID-19 convalescent plasma in hospitalized adults",
"author": "Self",
"doi-asserted-by": "crossref",
"first-page": "221",
"issue": "1",
"journal-title": "Trials",
"key": "10.1016/j.chest.2022.06.029_bib18",
"volume": "22",
"year": "2021"
},
{
"article-title": "The REDCap consortium: building an international community of software platform partners",
"author": "Harris",
"journal-title": "J Biomed Informatics",
"key": "10.1016/j.chest.2022.06.029_bib19",
"volume": "95",
"year": "2019"
},
{
"DOI": "10.1016/0168-8510(90)90421-9",
"article-title": "EuroQol—a new facility for the measurement of health-related quality of life",
"doi-asserted-by": "crossref",
"first-page": "199",
"issue": "3",
"journal-title": "Health Policy",
"key": "10.1016/j.chest.2022.06.029_bib20",
"volume": "16",
"year": "1990"
},
{
"DOI": "10.1007/s11136-011-9903-x",
"article-title": "Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L)",
"author": "Herdman",
"doi-asserted-by": "crossref",
"first-page": "1727",
"issue": "10",
"journal-title": "Qual Life Res",
"key": "10.1016/j.chest.2022.06.029_bib21",
"volume": "20",
"year": "2011"
},
{
"key": "10.1016/j.chest.2022.06.029_bib22",
"unstructured": "World Health Organization (WHO). WHO R&D Blueprint-Novel Coronavirus, COVID-19 Therapeutic Trial Synopsis. 2020. Accessed July 5, 2021. WHO R&D Blueprint-Novel Coronavirus, COVID-19 Therapeutic Trial Synopsis. https://www.who.int/teams/blueprint/covid-19"
},
{
"DOI": "10.1001/jama.2020.22240",
"article-title": "Effect of hydroxychloroquine on clinical status at 14 days in hospitalized patients with COVID-19: a randomized clinical trial",
"author": "Self",
"doi-asserted-by": "crossref",
"first-page": "2165",
"issue": "21",
"journal-title": "JAMA",
"key": "10.1016/j.chest.2022.06.029_bib23",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2033130",
"article-title": "A neutralizing monoclonal antibody for hospitalized patients with Covid-19",
"author": "Lundgren",
"doi-asserted-by": "crossref",
"first-page": "905",
"issue": "10",
"journal-title": "N Engl J Med",
"key": "10.1016/j.chest.2022.06.029_bib24",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the treatment of Covid-19—final report",
"author": "Beigel",
"doi-asserted-by": "crossref",
"first-page": "1813",
"issue": "19",
"journal-title": "N Engl J Med",
"key": "10.1016/j.chest.2022.06.029_bib25",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.16349",
"article-title": "Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial",
"author": "Spinner",
"doi-asserted-by": "crossref",
"first-page": "1048",
"issue": "11",
"journal-title": "JAMA",
"key": "10.1016/j.chest.2022.06.029_bib26",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1214/aoms/1177731118",
"article-title": "Sequential tests of statistical hypotheses",
"author": "Wald",
"doi-asserted-by": "crossref",
"first-page": "117",
"issue": "2",
"journal-title": "Ann Math Statist",
"key": "10.1016/j.chest.2022.06.029_bib27",
"volume": "16",
"year": "1945"
},
{
"DOI": "10.1002/pst.513",
"article-title": "An evidential approach to non-inferiority clinical trials",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "440",
"issue": "5",
"journal-title": "Pharmaceut Statist",
"key": "10.1016/j.chest.2022.06.029_bib28",
"volume": "10",
"year": "2011"
},
{
"author": "Royall",
"key": "10.1016/j.chest.2022.06.029_bib29",
"series-title": "Statistical Evidence: A Likelihood Paradigm",
"year": "1997"
},
{
"DOI": "10.1002/sim.1216",
"article-title": "Likelihood methods for measuring statistical evidence",
"author": "Blume",
"doi-asserted-by": "crossref",
"first-page": "2563",
"issue": "17",
"journal-title": "Statist Med",
"key": "10.1016/j.chest.2022.06.029_bib30",
"volume": "21",
"year": "2002"
},
{
"DOI": "10.1001/jama.2016.0287",
"article-title": "The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3)",
"author": "Singer",
"doi-asserted-by": "crossref",
"first-page": "801",
"issue": "8",
"journal-title": "JAMA",
"key": "10.1016/j.chest.2022.06.029_bib31",
"volume": "315",
"year": "2016"
},
{
"author": "Kunze",
"key": "10.1016/j.chest.2022.06.029_bib32"
}
],
"reference-count": 29,
"references-count": 29,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S0012369222012016"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Cardiology and Cardiovascular Medicine",
"Critical Care and Intensive Care Medicine",
"Pulmonary and Respiratory Medicine"
],
"subtitle": [],
"title": "Neutralizing COVID-19 Convalescent Plasma in Adults Hospitalized With COVID-19",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "162"
}