Bamlanivimab for the Prevention of Hospitalizations and Emergency Department Visits in SARS-CoV-2–Positive Patients in a Regional Health Care System
et al., Infectious Diseases in Clinical Practice, doi:10.1097/IPC.0000000000001130, Jan 2022
25th treatment shown to reduce risk in
May 2021, now with p = 0.00049 from 22 studies, recognized in 11 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 379 bamlanivimab patients and 379 matched controls in the USA, showing no significant differences with treatment.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments6.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, no change, RR 1.00, p = 1.00, treatment 6 of 379 (1.6%), control 6 of 379 (1.6%).
|
|
risk of hospitalization, 3.9% higher, RR 1.04, p = 0.86, treatment 79 of 379 (20.8%), control 76 of 379 (20.1%), all-cause hospital revisit.
|
|
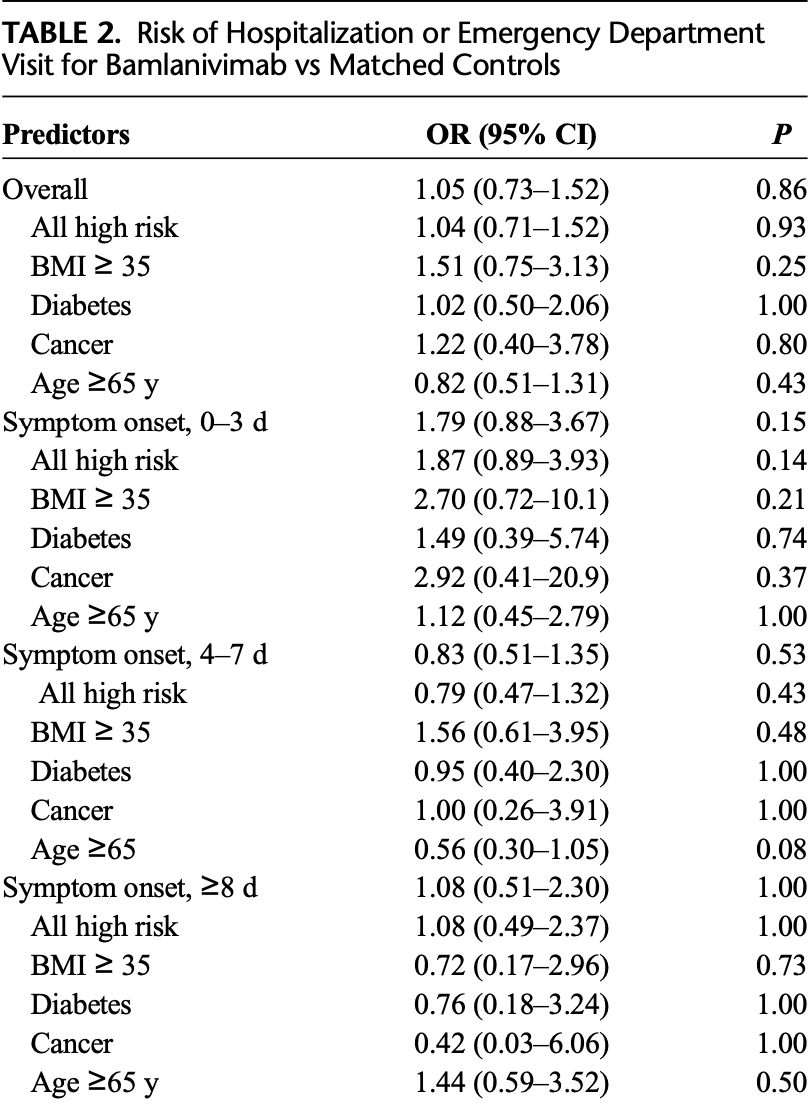
risk of hospitalization/ER, 5.0% higher, OR 1.05, p = 0.86, treatment 379, control 379, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
2.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
3.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
4.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
Priest et al., 27 Jan 2022, retrospective, propensity score matching, USA, peer-reviewed, 5 authors, study period October 2020 - March 2021, average treatment delay 6.0 days.
Bamlanivimab for the Prevention of Hospitalizations and Emergency Department Visits in SARS-CoV-2-Positive Patients in a Regional Health Care System
Introduction: Bamlanivimab (LY-CoV555) was approved by Emergency Use Authorization by the United States Food and Drug Administration in the ambulatory setting to prevent hospitalizations and emergency department visits. We report a retrospective, case-control study of bamlanivimab use in a regional health care system. Methods: A retrospective case-control study for SARS-CoV-2-positive patients receiving bamlanivimab and matched controls between October 2020 and March 2021 was performed. End points included all-cause hospitalization, emergency department visits, and mortality. Results: No statistically significant difference was noted in all-cause hospitalization, emergency department visits, or mortality, including patients 65 years or older, body mass index of 35 or higher, diagnosis of diabetes mellitus, or cancer (high-risk patients). No difference was seen based on timing of bamlanivimab infusion relative to symptom onset or timing of infusion within the study period.
Conclusions: Based on the evaluated endpoints, there was no benefit from bamlanivimab, regardless of when it was received in a patient's clinical course or when during the study period it was received. A lack of efficacy of monoclonal antibodies in patients infected with COVID-19 variants has been noted, but the impact of local variants on these results could not be assessed given a lack of available variant diagnostic tools. These findings do not support bamlanivimab for the prevention of hospitalization or emergency department visits for patients with mild to moderate SARS-CoV-2 infection.
References
Chen, Nirula, Heller, SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2029849
Dhand, Lobo, Wolfe, Bamlanivimab for treatment of COVID-19 in solid organ transplant recipients: early single-center experience, Clin Transpl, doi:10.1111/ctr.14245
Focosi, Maggi, Neutralising antibody escape of SARS-CoV-2 spike protein: risk assessment for antibody-based Covid-19 therapeutics and vaccines, Rev Med Virol, doi:10.1002/rmv.2231
Gottlieb, Nirula, Chen, Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial, JAMA
Kaplon, Reichert, Antibodies to watch in 2021, MAbs, doi:10.1080/19420862.2020.1860476
Liu, Wei, Zhang, V2 and 501Y.V3 variants of SARS-CoV-2 lose binding to Bamlanivimab in vitro, MAbs, doi:10.1080/19420862.2021.1919285
Ly-Cov555 Study Grouplundgren, Grund, Barkauskas, A neutralizing monoclonal antibody for hospitalized patients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2033130
Mcginley, Only one Covid-19 treatment is designed to keep people out of the hospital
Pallotta, Kim, Gordon, Monoclonal antibodies for treating COVID-19, Cleve Clin J Med, doi:10.3949/ccjm.88a.ccc074
Starr, Greaney, Dingens, Complete map of SARS-CoV-2 RBD mutations that escape the monoclonal antibody LY-CoV555 and its cocktail with LY-CoV016, Cell Rep Med
Tuccori, Ferraro, Convertino, Anti-SARS-CoV-2 neutralizing monoclonal antibodies: clinical pipeline, MAbs, doi:10.1080/19420862.2020.1854149
Tulledge-Scheitel, Bell, Larsen, A mobile unit overcomes the challenges to monoclonal antibody infusion for COVID-19 in skilled care facilities, J Am Geriatr Soc, doi:10.1111/jgs.17090
DOI record:
{
"DOI": "10.1097/ipc.0000000000001130",
"ISSN": [
"1536-9943",
"1056-9103"
],
"URL": "http://dx.doi.org/10.1097/ipc.0000000000001130",
"author": [
{
"affiliation": [],
"family": "Priest",
"given": "David H.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Blanchette",
"given": "Lisa M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hekman",
"given": "Aliza L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Maddikunta",
"given": "Rahul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Burleson",
"given": "Paula E.",
"sequence": "additional"
}
],
"container-title": [
"Infectious Diseases in Clinical Practice"
],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
1,
26
]
],
"date-time": "2022-01-26T18:00:09Z",
"timestamp": 1643220009000
},
"deposited": {
"date-parts": [
[
2022,
1,
27
]
],
"date-time": "2022-01-27T09:00:22Z",
"timestamp": 1643274022000
},
"indexed": {
"date-parts": [
[
2022,
1,
28
]
],
"date-time": "2022-01-28T06:56:24Z",
"timestamp": 1643352984074
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "electronic",
"value": "1536-9943"
},
{
"type": "print",
"value": "1056-9103"
}
],
"issue": "2",
"issued": {
"date-parts": [
[
2022,
1,
27
]
]
},
"journal-issue": {
"issue": "2",
"published-print": {
"date-parts": [
[
2022
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://journals.lww.com/10.1097/IPC.0000000000001130",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "276",
"original-title": [],
"page": "1-4",
"prefix": "10.1097",
"published": {
"date-parts": [
[
2022,
1,
27
]
]
},
"published-online": {
"date-parts": [
[
2022,
1,
27
]
]
},
"published-print": {
"date-parts": [
[
2022,
3
]
]
},
"publisher": "Ovid Technologies (Wolters Kluwer Health)",
"reference": [
{
"DOI": "10.1080/19420862.2020.1854149",
"article-title": "Anti-SARS-CoV-2 neutralizing monoclonal antibodies: clinical pipeline",
"doi-asserted-by": "crossref",
"first-page": "1854149",
"issue": "1",
"journal-title": "MAbs",
"key": "bib3-20220127",
"volume": "12",
"year": "2020"
},
{
"article-title": "Monoclonal antibodies for treating COVID-19",
"journal-title": "Cleve Clin J Med",
"key": "bib4-20220127",
"year": "2021"
},
{
"article-title": "An EUA for bamlanivimab - a monoclonal antibody for COVID-19",
"first-page": "185",
"issue": "1612",
"journal-title": "Med Lett Drugs Ther",
"key": "bib5-20220127",
"volume": "62",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2029849",
"article-title": "SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19",
"doi-asserted-by": "crossref",
"first-page": "229",
"issue": "3",
"journal-title": "N Engl J Med",
"key": "bib7-20220127",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2033130",
"article-title": "A neutralizing monoclonal antibody for hospitalized patients with Covid-19",
"doi-asserted-by": "crossref",
"first-page": "905",
"issue": "10",
"journal-title": "N Engl J Med",
"key": "bib8-20220127",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1001/jama.2021.0202",
"article-title": "Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial",
"doi-asserted-by": "crossref",
"first-page": "632",
"issue": "7",
"journal-title": "JAMA",
"key": "bib9-20220127",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1080/19420862.2020.1860476",
"article-title": "Antibodies to watch in 2021",
"doi-asserted-by": "crossref",
"first-page": "1860476",
"issue": "1",
"journal-title": "MAbs",
"key": "bib12-20220127",
"volume": "13",
"year": "2021"
},
{
"article-title": "Bamlanivimab for treatment of COVID-19 in solid organ transplant recipients: early single-center experience",
"first-page": "e14245",
"journal-title": "Clin Transpl",
"key": "bib13-20220127",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1111/jgs.17090",
"article-title": "A mobile unit overcomes the challenges to monoclonal antibody infusion for COVID-19 in skilled care facilities",
"doi-asserted-by": "crossref",
"first-page": "868",
"journal-title": "J Am Geriatr Soc",
"key": "bib14-20220127",
"volume": "69",
"year": "2021"
},
{
"article-title": "Neutralising antibody escape of SARS-CoV-2 spike protein: risk assessment for antibody-based Covid-19 therapeutics and vaccines",
"first-page": "e2231",
"journal-title": "Rev Med Virol",
"key": "bib15-20220127",
"volume": "31",
"year": "2021"
},
{
"DOI": "10.1016/j.xcrm.2021.100255",
"article-title": "Complete map of SARS-CoV-2 RBD mutations that escape the monoclonal antibody LY-CoV555 and its cocktail with LY-CoV016",
"doi-asserted-by": "crossref",
"first-page": "100255",
"issue": "4",
"journal-title": "Cell Rep Med",
"key": "bib16-20220127",
"volume": "2",
"year": "2021"
},
{
"DOI": "10.1080/19420862.2021.1919285",
"article-title": "501Y.V2 and 501Y.V3 variants of SARS-CoV-2 lose binding to Bamlanivimab in vitro",
"doi-asserted-by": "crossref",
"first-page": "1919285",
"issue": "1",
"journal-title": "MAbs",
"key": "bib17-20220127",
"volume": "13",
"year": "2021"
}
],
"reference-count": 12,
"references-count": 12,
"relation": {},
"score": 1,
"short-container-title": [
"Infect Dis Clin Pract"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Microbiology (medical)"
],
"subtitle": [],
"title": [
"Bamlanivimab for the Prevention of Hospitalizations and Emergency Department Visits in SARS-CoV-2–Positive Patients in a Regional Health Care System"
],
"type": "journal-article",
"volume": "30"
}
