Targeting GPVI with glenzocimab in COVID-19 patients: Results from a randomized clinical trial
et al., PLOS ONE, doi:10.1371/journal.pone.0302897, NCT04659109, Jun 2024
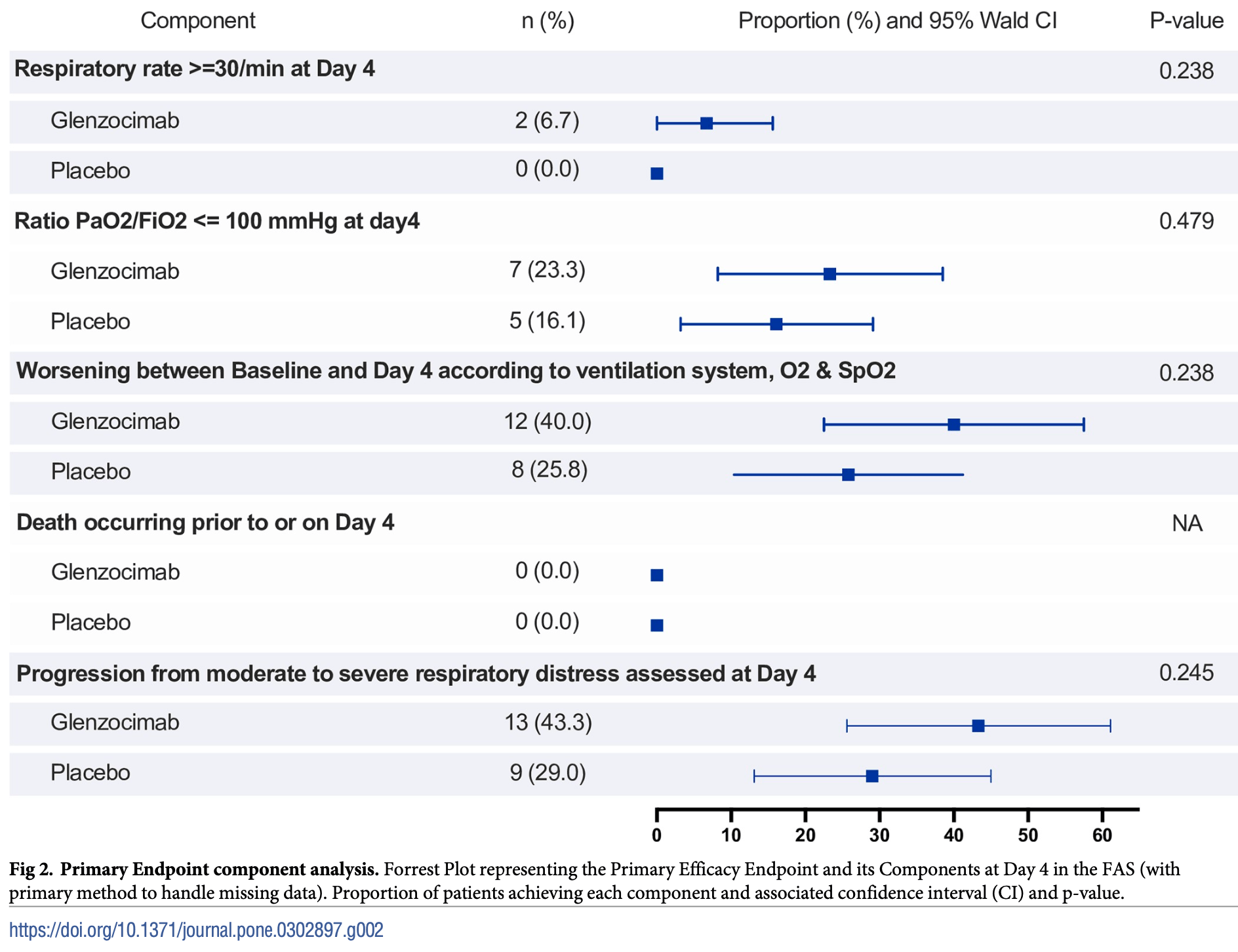
RCT 61 hospitalized COVID-19 patients showing no significant difference in primary outcomes with glenzocimab (an anti-GPVI antibody) vs. placebo. Authors report glenzocimab showed a significant improvement in the NEWS-2 category at day 4, however the actual results are not provided. There were no deaths and no significant safety signals in either group. The results suggest targeting GPVI at this stage of disease severity may not be sufficient to prevent progression to ARDS.
|
worsening according to ventilation system, O2, and SpO2, 60.0% higher, RR 1.60, p = 0.28, treatment 12 of 30 (40.0%), control 8 of 32 (25.0%).
|
|
progression from moderate to severe, 54.1% higher, RR 1.54, p = 0.29, treatment 13 of 30 (43.3%), control 9 of 32 (28.1%).
|
|
PaO2/FiO2 ≤ 100, 49.3% higher, RR 1.49, p = 0.53, treatment 7 of 30 (23.3%), control 5 of 32 (15.6%).
|
|
respiratory rate ≥ 30/min, 413.3% higher, RR 5.13, p = 0.23, treatment 2 of 30 (6.7%), control 0 of 32 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm).
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Pottecher et al., 17 Jun 2024, Double Blind Randomized Controlled Trial, placebo-controlled, multiple countries, peer-reviewed, 15 authors, study period December 2020 - August 2021, trial NCT04659109 (history).
Contact: elie.toledano@acticor-biotech.com, garden.study@acticor-biotech.com.
Targeting GPVI with glenzocimab in COVID-19 patients: Results from a randomized clinical trial
PLOS ONE, doi:10.1371/journal.pone.0302897
Background Glenzocimab is a novel antithrombotic agent which targets platelet glycoprotein VI (GPVI) and does not induce haemorrhage. SARS-CoV-2 triggers a prothrombotic state and lung injury whose mechanisms include coagulopathy, endothelial dysfunction, and inflammation with dysregulated platelets.
Methods and patients GARDEN was a randomised double-blind, exploratory phase II study of glenzocimab in SARS-CoV-2 respiratory failure (NCT04659109). PCR+ adults in Brazil and France (7 centres) were randomized to standard-of-care (SOC) plus glenzocimab (1000 mg/dayx3 days) or placebo, followed for 40 days. Primary efficacy endpoint was clinical progression at Day 4. All analyses concerned the intention-to-treat population.
Results Between December 2020 and August 2021, 61 patients received at least one dose (30 glenzocimab vs 32 placebo) and 58 completed the study (29 vs 29). Clinical progression of COVID-19 ARDS was not statistically different between glenzocimab and placebo arms (43.3% and 29.0%, respectively; p = 0.245). Decrease in the NEWS-2 category at D4 was statistically significant (p = 0.0290) in the glenzocimab arm vs placebo. No Serious Adverse Event (SAE) was deemed related to study drug; bleeding related events were reported in 6 patients (7 events) and 4 patients (4 events) in glenzocimab and placebo arms, respectively.
References
Ackermann, Verleden, Kuehnel, Haverich, Welte et al., Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19, N Engl J Med, doi:10.1056/NEJMoa2015432
Barrett, Lee, Xia, Lin, Black et al., Platelet and vascular biomarkers associate with thrombosis and death in coronavirus disease, Circ Res, doi:10.1161/CIRCRESAHA.120.317803
Berger, Kornblith, Gong, Reynolds, Cushman et al., Effect of P2Y12 Inhibitors on Survival Free of Organ Support Among Non-Critically Ill Hospitalized Patients With COVID-19: A Randomized Clinical Trial, JAMA, doi:10.1001/jama.2021.23605
Billiald, Slater, Welin, Clark, Loyau et al., Targeting platelet GPVI with glenzocimab: a novel mechanism for inhibition, Blood Advances, doi:10.1182/bloodadvances.2022007863
Bradbury, Lawler, Stanworth, Mcverry, Mcquilten, Effect of Antiplatelet Therapy on Survival and Organ Support-Free Days in Critically Ill Patients With COVID-19: A Randomized Clinical Trial, JAMA, doi:10.1001/jama.2022.2910
Chow, Yin, Yamane, Davison, Keneally et al., Association of prehospital antiplatelet therapy with survival in patients hospitalized with COVID-19: A propensity score-matched analysis, J Thromb Haemost, doi:10.1111/jth.15517
Cobb, Sathe, Duan, Seitz, Thau et al., Comparison of Clinical Features and Outcomes in Critically Ill Patients Hospitalized with COVID-19 versus Influenza, Ann Am Thorac Soc, doi:10.1513/AnnalsATS.202007-805OC
Conway, Mackman, Warren, Wolberg, Mosnier et al., Understanding COVID-19-associated coagulopathy, Nat Rev Immunol, doi:10.1038/s41577-022-00762-9
Coudroy, Frat, Girault, Thille, Reliability of methods to estimate the fraction of inspired oxygen in patients with acute respiratory failure breathing through non-rebreather reservoir bag oxygen mask, Thorax, doi:10.1136/thoraxjnl-2020-214863
Denorme, Ajanel, Campbell, Shining a light on platelet activation in COVID-19, J Thromb Haemost, doi:10.1111/jth.15678
Denorme, Rondina, Targeting glycoprotein VI for thromboembolic disorders, Arterioscler Thromb Vasc Biol, doi:10.1161/ATVBAHA.119.312621
Garcia, Duong, Poe ¨tte, Ribes, Payre et al., Platelet activation and partial desensitization are associated with viral xenophagy in patients with severe COVID-19, Blood Adv, doi:10.1182/bloodadvances.2022007143
Hoffmann, Kleine-Weber, Schroeder, Kru ¨ger, Herrler et al., SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor, Cell, doi:10.1016/j.cell.2020.02.052
Iba, Levy, Levi, Connors, Thachil, Coagulopathy of coronavirus disease 2019, Crit Care Med, doi:10.1097/CCM.0000000000004458
Jandrot-Perrus, Hermans, Mezzano, Platelet glycoprotein VI genetic quantitative and qualitative defects, Platelets, doi:10.1080/09537104.2019.1610166
Le ´opold, Pereverzeva, Schuurman, Reijnders, Saris et al., Platelets are Hyperactivated but Show Reduced Glycoprotein VI Reactivity in COVID-19 Patients, Thromb Haemost, doi:10.1055/a-1347-5555
Lebozec, Jandrot-Perrus, Avenard, Favre-Bulle, Billiald, Design, development and characterization of ACT017, a humanized Fab that blocks platelet's glycoprotein VI function without causing bleeding risks, mAbs, doi:10.1080/19420862.2017.1336592
Ludwig, Jacob, Basedow, Andersohn, Walker, Clinical outcomes and characteristics of patients hospitalized for Influenza or COVID-19 in Germany, Int J Infect Dis, doi:10.1016/j.ijid.2020.11.204
Mangin, Gardiner, ¨ns, Jandrot-Perrus, GPVI interplay with fibrin(ogen) in thrombosis, J Thromb Haemost, doi:10.1016/j.jtha.2023.03.022
Martos, Machkour, Vali, Venisse, Idir et al., Critical role of platelets during lung fibrosis, ECTH
Nicolai, Leunig, Brambs, Kaiser, Weinberger et al., Immunothrombotic Dysregulation in COVID-19 Pneumonia Is Associated With Respiratory Failure and Coagulopathy, Circulation, doi:10.1161/CIRCULATIONAHA.120.048488
O'sullivan, Gonagle, Ward, Preston, Donnell, Endothelial cells orchestrate COVID-19 coagulopathy, Lancet Haematol, doi:10.1016/S2352-3026%2820%2930215-5
Rapkiewicz, Mai, Carsons, Pittaluga, Kleiner et al., Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: A case series, ECli-nicalMedicine, doi:10.1016/j.eclinm.2020.100434
Rayes, Watson, Nieswandt, Functional significance of the platelet immune receptors GPVI and CLEC-2, J Clin Invest, doi:10.1172/JCI122955
Recovery Collaborative, Aspirin in patients admitted to hospital with COVID-19 (RECOV-ERY): a randomised, controlled, open-label, platform trial, Lancet, doi:10.1016/S0140-6736%2821%2901825-0
Renaud, Lebozec, Voors-Pette, Dogterom, Billiald et al., Population Pharmacokinetic/Pharmacodynamic Modeling of Glenzocimab (ACT017) a Glycoprotein VI Inhibitor of Collagen-Induced Platelet Aggregation, J Clin Pharmacol, doi:10.1002/jcph.1616
Rondina, Brewster, Grissom, Zimmerman, Kastendieck et al., In vivo platelet activation in critically ill patients with primary 2009 influenza A(H1N1), Chest, doi:10.1378/chest.11-2860
Santoro, Nuñez-Gil, Vitale, Viana-Llamas, Mc et al., Antiplatelet therapy and outcome in COVID-19: the Health Outcome Predictive Evaluation Registry, Heart, doi:10.1136/heartjnl-2021-319552
Tang, Du, Wang, Cao, Guan et al., Comparison of Hospitalized Patients With ARDS Caused by COVID-19 and H1N1, Chest, doi:10.1016/j.chest.2020.03.032
Tang, Li, Wang, Sun, Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia, J Thromb Haemost, doi:10.1111/jth.14768
Taus, Salvagno, Canè, Fava, Mazzaferri et al., Platelets Promote Thromboinflammation in SARS-CoV-2 Pneumonia, Arterioscler Thromb Vasc Biol, doi:10.1161/ATVBAHA.120.315175
Thachil, Tang, Gando, Falanga, Cattaneo et al., ISTH interim guidance on recognition and management of coagulopathy in COVID-19, J Thromb Haemost, doi:10.1111/jth.14810
Voors-Pette, Lebozec, Dogterom, Jullien, Billiald et al., Safety and tolerability, pharmacokinetics, and pharmacodynamics of ACT017, an antiplatelet GPVI (glycoprotein VI) fab. Arterioscler, Thromb Vasc Biol, doi:10.1161/ATVBAHA.118.312314
Wang, Yang, Liu, Guo, Zhang et al., SARS coronavirus entry into host cells through a novel clathrin-and caveolae-independent endocytic pathway, Cell Res, doi:10.1038/cr.2008.15
Xu, Shi, Wang, Zhang, Huang et al., Pathological findings of COVID-19 associated with acute respiratory distress syndrome, Lancet Respir Med, doi:10.1016/S2213-2600%2820%2930076-X
Xu, Yu, Qu, Zhang, Jiang et al., Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2, Eur J Nucl Med Mol Imaging, doi:10.1007/s00259-020-04735-9
Zaid, Puhm, Allaeys, Naya, Oudghiri et al., Platelets Can Associate with SARS-Cov-2 RNA and Are Hyperactivated in COVID-19, Circ Res, doi:10.1161/CIRCRESAHA.120.317703
DOI record:
{
"DOI": "10.1371/journal.pone.0302897",
"ISSN": [
"1932-6203"
],
"URL": "http://dx.doi.org/10.1371/journal.pone.0302897",
"abstract": "<jats:sec id=\"sec001\">\n<jats:title>Background</jats:title>\n<jats:p>Glenzocimab is a novel antithrombotic agent which targets platelet glycoprotein VI (GPVI) and does not induce haemorrhage. SARS-CoV-2 triggers a prothrombotic state and lung injury whose mechanisms include coagulopathy, endothelial dysfunction, and inflammation with dysregulated platelets.</jats:p>\n</jats:sec>\n<jats:sec id=\"sec002\">\n<jats:title>Methods and patients</jats:title>\n<jats:p>GARDEN was a randomised double-blind, exploratory phase II study of glenzocimab in SARS-CoV-2 respiratory failure (NCT04659109). PCR+ adults in Brazil and France (7 centres) were randomized to standard-of-care (SOC) plus glenzocimab (1000 mg/dayx3 days) or placebo, followed for 40 days. Primary efficacy endpoint was clinical progression at Day 4. All analyses concerned the intention-to-treat population.</jats:p>\n</jats:sec>\n<jats:sec id=\"sec003\">\n<jats:title>Results</jats:title>\n<jats:p>Between December 2020 and August 2021, 61 patients received at least one dose (30 glenzocimab vs 32 placebo) and 58 completed the study (29 <jats:italic>vs</jats:italic> 29). Clinical progression of COVID-19 ARDS was not statistically different between glenzocimab and placebo arms (43.3% and 29.0%, respectively; <jats:italic>p = 0</jats:italic>.<jats:italic>245</jats:italic>). Decrease in the NEWS-2 category at D4 was statistically significant (<jats:italic>p = 0</jats:italic>.<jats:italic>0290</jats:italic>) in the glenzocimab arm vs placebo. No Serious Adverse Event (SAE) was deemed related to study drug; bleeding related events were reported in 6 patients (7 events) and 4 patients (4 events) in glenzocimab and placebo arms, respectively.</jats:p>\n</jats:sec>\n<jats:sec id=\"sec004\">\n<jats:title>Conclusions</jats:title>\n<jats:p>Therapeutic GPVI inhibition assessment during COVID-19 was conducted in response to a Public Health emergency. Glenzocimab in coagulopathic patients under therapeutic heparin was neither associated with increased bleeding, nor SAE. Clinical impact of glenzocimab on COVID-19 ARDS was not demonstrated. A potential role for GPVI inhibition in other types of ARDS deserves further experimentation. Glenzocimab is currently studied in stroke (ACTISAVE: NCT05070260) and cardiovascular indications.</jats:p>\n</jats:sec>",
"author": [
{
"ORCID": "http://orcid.org/0000-0001-6073-4354",
"affiliation": [],
"authenticated-orcid": true,
"family": "Pottecher",
"given": "Julien",
"sequence": "first"
},
{
"affiliation": [],
"family": "Raffi",
"given": "Francois",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jandrot-Perrus",
"given": "Martine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Binay",
"given": "Sophie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Comenducci",
"given": "Andrea",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Desort-Henin",
"given": "Violaine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "François",
"given": "Déborah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gharakhanian",
"given": "Shahin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Labart",
"given": "Marilyn",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Meilhoc",
"given": "Adeline",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7190-0052",
"affiliation": [],
"authenticated-orcid": true,
"family": "Toledano",
"given": "Elie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pletan",
"given": "Yannick",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Avenard",
"given": "Gilles",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sato",
"given": "Victor H.",
"sequence": "additional"
},
{
"affiliation": [],
"name": "the GARDEN Investigators",
"sequence": "additional"
}
],
"container-title": "PLOS ONE",
"container-title-short": "PLoS ONE",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"www.plosone.org"
]
},
"created": {
"date-parts": [
[
2024,
6,
17
]
],
"date-time": "2024-06-17T17:59:51Z",
"timestamp": 1718647191000
},
"deposited": {
"date-parts": [
[
2024,
6,
17
]
],
"date-time": "2024-06-17T18:00:20Z",
"timestamp": 1718647220000
},
"editor": [
{
"affiliation": [],
"family": "Nannini",
"given": "Esteban",
"sequence": "first"
}
],
"funder": [
{
"name": "Acticor Biotech"
}
],
"indexed": {
"date-parts": [
[
2024,
6,
18
]
],
"date-time": "2024-06-18T00:22:44Z",
"timestamp": 1718670164739
},
"is-referenced-by-count": 0,
"issue": "6",
"issued": {
"date-parts": [
[
2024,
6,
17
]
]
},
"journal-issue": {
"issue": "6",
"published-online": {
"date-parts": [
[
2024,
6,
17
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
6,
17
]
],
"date-time": "2024-06-17T00:00:00Z",
"timestamp": 1718582400000
}
}
],
"link": [
{
"URL": "https://dx.plos.org/10.1371/journal.pone.0302897",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "340",
"original-title": [],
"page": "e0302897",
"prefix": "10.1371",
"published": {
"date-parts": [
[
2024,
6,
17
]
]
},
"published-online": {
"date-parts": [
[
2024,
6,
17
]
]
},
"publisher": "Public Library of Science (PLoS)",
"reference": [
{
"DOI": "10.1016/j.cell.2020.02.052",
"article-title": "SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor",
"author": "M Hoffmann",
"doi-asserted-by": "crossref",
"first-page": "271",
"journal-title": "Cell",
"key": "pone.0302897.ref001",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1038/cr.2008.15",
"article-title": "SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway",
"author": "H Wang",
"doi-asserted-by": "crossref",
"first-page": "290",
"journal-title": "Cell Res",
"key": "pone.0302897.ref002",
"volume": "18",
"year": "2008"
},
{
"DOI": "10.1016/S2352-3026(20)30215-5",
"article-title": "Endothelial cells orchestrate COVID-19 coagulopathy",
"author": "JM O’Sullivan",
"doi-asserted-by": "crossref",
"first-page": "e553",
"journal-title": "Lancet Haematol",
"key": "pone.0302897.ref003",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2015432",
"article-title": "Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19",
"author": "M Ackermann",
"doi-asserted-by": "crossref",
"first-page": "120",
"journal-title": "N Engl J Med",
"key": "pone.0302897.ref004",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1097/CCM.0000000000004458",
"article-title": "Coagulopathy of coronavirus disease 2019",
"author": "T Iba",
"doi-asserted-by": "crossref",
"first-page": "1358",
"journal-title": "Crit Care Med",
"key": "pone.0302897.ref005",
"volume": "48",
"year": "2020"
},
{
"DOI": "10.1111/jth.14768",
"article-title": "Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia",
"author": "N Tang",
"doi-asserted-by": "crossref",
"first-page": "844",
"journal-title": "J Thromb Haemost",
"key": "pone.0302897.ref006",
"volume": "18",
"year": "2020"
},
{
"article-title": "Platelets Can Associate with SARS-Cov-2 RNA and Are Hyperactivated in COVID-19",
"author": "Y Zaid",
"journal-title": "Circ Res",
"key": "pone.0302897.ref007",
"year": "2020"
},
{
"DOI": "10.1161/ATVBAHA.120.315175",
"article-title": "Platelets Promote Thromboinflammation in SARS-CoV-2 Pneumonia",
"author": "F Taus",
"doi-asserted-by": "crossref",
"first-page": "2975",
"journal-title": "Arterioscler Thromb Vasc Biol",
"key": "pone.0302897.ref008",
"volume": "40",
"year": "2020"
},
{
"DOI": "10.1111/jth.15678",
"article-title": "Shining a light on platelet activation in COVID-19",
"author": "F Denorme",
"doi-asserted-by": "crossref",
"first-page": "1286",
"journal-title": "J Thromb Haemost",
"key": "pone.0302897.ref009",
"volume": "20",
"year": "2022"
},
{
"DOI": "10.1007/s00259-020-04735-9",
"article-title": "Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2",
"author": "X Xu",
"doi-asserted-by": "crossref",
"first-page": "1275",
"journal-title": "Eur J Nucl Med Mol Imaging",
"key": "pone.0302897.ref010",
"volume": "47",
"year": "2020"
},
{
"DOI": "10.1378/chest.11-2860",
"article-title": "In vivo platelet activation in critically ill patients with primary 2009 influenza A(H1N1)",
"author": "MT Rondina",
"doi-asserted-by": "crossref",
"first-page": "1490",
"journal-title": "Chest",
"key": "pone.0302897.ref011",
"volume": "141",
"year": "2012"
},
{
"DOI": "10.1016/j.chest.2020.03.032",
"article-title": "Comparison of Hospitalized Patients With ARDS Caused by COVID-19 and H1N1",
"author": "X Tang",
"doi-asserted-by": "crossref",
"first-page": "195",
"journal-title": "Chest",
"key": "pone.0302897.ref012",
"volume": "158",
"year": "2020"
},
{
"DOI": "10.1016/j.ijid.2020.11.204",
"article-title": "Clinical outcomes and characteristics of patients hospitalized for Influenza or COVID-19 in Germany",
"author": "M Ludwig",
"doi-asserted-by": "crossref",
"first-page": "316",
"journal-title": "Int J Infect Dis",
"key": "pone.0302897.ref013",
"volume": "103",
"year": "2021"
},
{
"DOI": "10.1513/AnnalsATS.202007-805OC",
"article-title": "Comparison of Clinical Features and Outcomes in Critically Ill Patients Hospitalized with COVID-19 versus Influenza",
"author": "NL Cobb",
"doi-asserted-by": "crossref",
"first-page": "632",
"journal-title": "Ann Am Thorac Soc",
"key": "pone.0302897.ref014",
"volume": "18",
"year": "2021"
},
{
"DOI": "10.1111/jth.14810",
"article-title": "ISTH interim guidance on recognition and management of coagulopathy in COVID-19",
"author": "J Thachil",
"doi-asserted-by": "crossref",
"first-page": "1023",
"journal-title": "J Thromb Haemost",
"key": "pone.0302897.ref015",
"volume": "18",
"year": "2020"
},
{
"article-title": "GPVI interplay with fibrin(ogen) in thrombosis",
"author": "PH Mangin",
"journal-title": "J Thromb Haemost",
"key": "pone.0302897.ref016",
"year": "2023"
},
{
"DOI": "10.1080/09537104.2019.1610166",
"article-title": "Platelet glycoprotein VI genetic quantitative and qualitative defects",
"author": "M Jandrot-Perrus",
"doi-asserted-by": "crossref",
"first-page": "708",
"journal-title": "Platelets",
"key": "pone.0302897.ref017",
"volume": "30",
"year": "2019"
},
{
"DOI": "10.1172/JCI122955",
"article-title": "Functional significance of the platelet immune receptors GPVI and CLEC-2",
"author": "J Rayes",
"doi-asserted-by": "crossref",
"first-page": "12",
"journal-title": "J Clin Invest",
"key": "pone.0302897.ref018",
"volume": "129",
"year": "2019"
},
{
"DOI": "10.1161/ATVBAHA.119.312621",
"article-title": "Targeting glycoprotein VI for thromboembolic disorders",
"author": "F Denorme",
"doi-asserted-by": "crossref",
"first-page": "839",
"journal-title": "Arterioscler Thromb Vasc Biol",
"key": "pone.0302897.ref019",
"volume": "39",
"year": "2019"
},
{
"article-title": "Critical role of platelets during lung fibrosis",
"author": "R Martos",
"first-page": "37",
"journal-title": "ECTH",
"key": "pone.0302897.ref020",
"year": "2019"
},
{
"DOI": "10.1080/19420862.2017.1336592",
"article-title": "Design, development and characterization of ACT017, a humanized Fab that blocks platelet’s glycoprotein VI function without causing bleeding risks",
"author": "K Lebozec",
"doi-asserted-by": "crossref",
"first-page": "945",
"journal-title": "mAbs",
"key": "pone.0302897.ref021",
"volume": "9",
"year": "2017"
},
{
"article-title": "Targeting platelet GPVI with glenzocimab: a novel mechanism for inhibition",
"author": "P Billiald",
"journal-title": "Blood Advances",
"key": "pone.0302897.ref022",
"year": "2022"
},
{
"DOI": "10.1161/ATVBAHA.118.312314",
"article-title": "Safety and tolerability, pharmacokinetics, and pharmacodynamics of ACT017, an antiplatelet GPVI (glycoprotein VI) fab",
"author": "C Voors-Pette",
"doi-asserted-by": "crossref",
"first-page": "956",
"journal-title": "Arterioscler Thromb Vasc Biol",
"key": "pone.0302897.ref023",
"volume": "39",
"year": "2019"
},
{
"DOI": "10.1136/thoraxjnl-2020-214863",
"article-title": "Reliability of methods to estimate the fraction of inspired oxygen in patients with acute respiratory failure breathing through non-rebreather reservoir bag oxygen mask",
"author": "R Coudroy",
"doi-asserted-by": "crossref",
"first-page": "805",
"journal-title": "Thorax",
"key": "pone.0302897.ref024",
"volume": "75",
"year": "2020"
},
{
"DOI": "10.1161/CIRCRESAHA.120.317803",
"article-title": "Platelet and vascular biomarkers associate with thrombosis and death in coronavirus disease",
"author": "TJ Barrett",
"doi-asserted-by": "crossref",
"first-page": "945",
"journal-title": "Circ Res",
"key": "pone.0302897.ref025",
"volume": "127",
"year": "2020"
},
{
"DOI": "10.1001/jama.2022.2910",
"article-title": "Effect of Antiplatelet Therapy on Survival and Organ Support-Free Days in Critically Ill Patients With COVID-19: A Randomized Clinical Trial",
"author": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators",
"doi-asserted-by": "crossref",
"first-page": "1247",
"journal-title": "JAMA",
"key": "pone.0302897.ref026",
"volume": "327",
"year": "2022"
},
{
"DOI": "10.1182/bloodadvances.2022007143",
"article-title": "Platelet activation and partial desensitization are associated with viral xenophagy in patients with severe COVID-19",
"author": "C Garcia",
"doi-asserted-by": "crossref",
"first-page": "3884",
"journal-title": "Blood Adv",
"key": "pone.0302897.ref027",
"volume": "6",
"year": "2022"
},
{
"DOI": "10.1161/CIRCULATIONAHA.120.048488",
"article-title": "Immunothrombotic Dysregulation in COVID-19 Pneumonia Is Associated With Respiratory Failure and Coagulopathy",
"author": "L Nicolai",
"doi-asserted-by": "crossref",
"first-page": "1176",
"journal-title": "Circulation",
"key": "pone.0302897.ref028",
"volume": "142",
"year": "2020"
},
{
"DOI": "10.1016/j.eclinm.2020.100434",
"article-title": "Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: A case series",
"author": "AV Rapkiewicz",
"doi-asserted-by": "crossref",
"first-page": "100434",
"journal-title": "EClinicalMedicine",
"key": "pone.0302897.ref029",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1016/S2213-2600(20)30076-X",
"article-title": "Pathological findings of COVID-19 associated with acute respiratory distress syndrome",
"author": "Z Xu",
"doi-asserted-by": "crossref",
"first-page": "420",
"journal-title": "Lancet Respir Med",
"key": "pone.0302897.ref030",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1055/a-1347-5555",
"article-title": "Platelets are Hyperactivated but Show Reduced Glycoprotein VI Reactivity in COVID-19",
"author": "V Léopold",
"doi-asserted-by": "crossref",
"first-page": "1258",
"journal-title": "Patients. Thromb Haemost",
"key": "pone.0302897.ref031",
"volume": "121",
"year": "2021"
},
{
"DOI": "10.1002/jcph.1616",
"article-title": "Population Pharmacokinetic/Pharmacodynamic Modeling of Glenzocimab (ACT017) a Glycoprotein VI Inhibitor of Collagen-Induced Platelet Aggregation",
"author": "L Renaud",
"doi-asserted-by": "crossref",
"first-page": "1198",
"journal-title": "J Clin Pharmacol",
"key": "pone.0302897.ref032",
"volume": "60",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(21)01825-0",
"article-title": "Aspirin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial",
"author": "RECOVERY Collaborative Group",
"doi-asserted-by": "crossref",
"first-page": "143",
"journal-title": "Lancet",
"key": "pone.0302897.ref033",
"volume": "399",
"year": "2022"
},
{
"DOI": "10.1001/jama.2021.23605",
"article-title": "Effect of P2Y12 Inhibitors on Survival Free of Organ Support Among Non-Critically Ill Hospitalized Patients With COVID-19: A Randomized Clinical Trial",
"author": "JS Berger",
"doi-asserted-by": "crossref",
"first-page": "227",
"journal-title": "JAMA",
"key": "pone.0302897.ref034",
"volume": "327",
"year": "2022"
},
{
"DOI": "10.1038/s41577-022-00762-9",
"article-title": "Understanding COVID-19-associated coagulopathy",
"author": "EM Conway",
"doi-asserted-by": "crossref",
"first-page": "639",
"journal-title": "Nat Rev Immunol",
"key": "pone.0302897.ref035",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1111/jth.15517",
"article-title": "Association of prehospital antiplatelet therapy with survival in patients hospitalized with COVID-19: A propensity score-matched analysis",
"author": "JH Chow",
"doi-asserted-by": "crossref",
"first-page": "2814",
"journal-title": "J Thromb Haemost",
"key": "pone.0302897.ref036",
"volume": "19",
"year": "2021"
},
{
"DOI": "10.1136/heartjnl-2021-319552",
"article-title": "Antiplatelet therapy and outcome in COVID-19: the Health Outcome Predictive Evaluation Registry",
"author": "F Santoro",
"doi-asserted-by": "crossref",
"first-page": "130",
"journal-title": "Heart",
"key": "pone.0302897.ref037",
"volume": "108",
"year": "2022"
}
],
"reference-count": 37,
"references-count": 37,
"relation": {},
"resource": {
"primary": {
"URL": "https://dx.plos.org/10.1371/journal.pone.0302897"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Targeting GPVI with glenzocimab in COVID-19 patients: Results from a randomized clinical trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1371/journal.pone.corrections_policy",
"volume": "19"
}