Effect of Antiplatelet Therapy on Survival and Organ Support–Free Days in Critically Ill Patients With COVID-19: A Randomized Clinical Trial
et al., JAMA, doi:10.1001/jama.2022.2910, REMAP-CAP, NCT02735707, Mar 2022
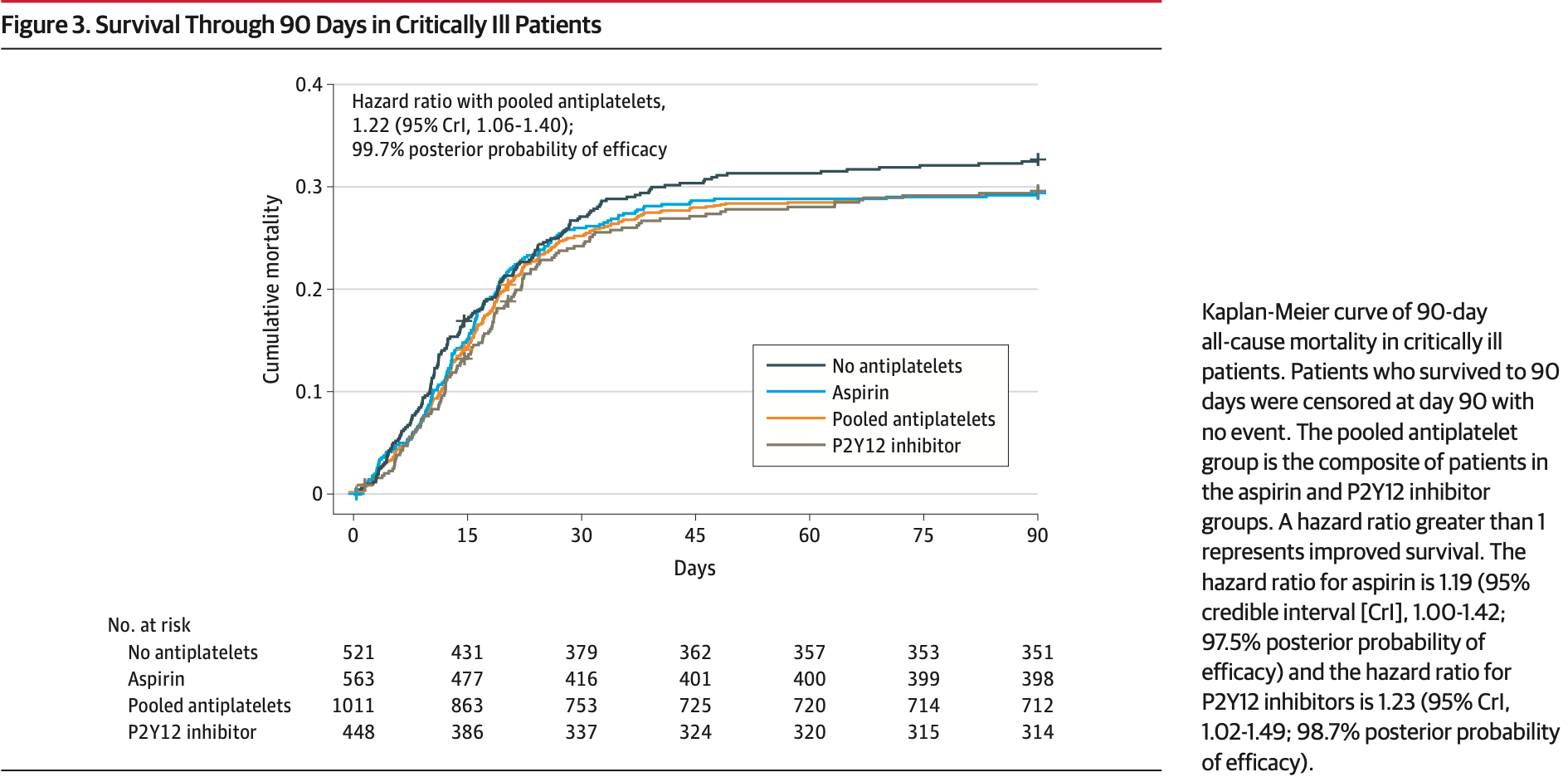
RCT 1,557 critical patients, showing significantly lower mortality with aspirin, with 97.5% posterior probability of efficacy.
|
risk of death, 16.0% lower, HR 0.84, p = 0.05, treatment 165 of 563 (29.3%), control 170 of 521 (32.6%), NNT 30, inverted to make HR<1 favor treatment, Kaplan-Meier, day 90.
|
|
risk of no hospital discharge, 16.9% lower, RR 0.83, p = 0.08, treatment 161 of 563 (28.6%), control 167 of 521 (32.1%), NNT 29, adjusted per study, inverted to make RR<1 favor treatment, odds ratio converted to relative risk.
|
|
risk of progression, 21.0% lower, RR 0.79, p = 0.02, treatment 204 of 563 (36.2%), control 212 of 521 (40.7%), adjusted per study, odds ratio converted to relative risk, combined death/thrombosis.
|
|
risk of progression, 4.8% lower, OR 0.95, p = 0.67, treatment 563, control 521, adjusted per study, inverted to make OR<1 favor treatment, support-free days, primary outcome, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Bradbury et al., 22 Mar 2022, Randomized Controlled Trial, multiple countries, peer-reviewed, 73 authors, study period 30 October, 2020 - 23 June, 2021, trial NCT02735707 (history) (REMAP-CAP).
Effect of Antiplatelet Therapy on Survival and Organ Support–Free Days in Critically Ill Patients With COVID-19
JAMA, doi:10.1001/jama.2022.2910
IMPORTANCE The efficacy of antiplatelet therapy in critically ill patients with COVID-19 is uncertain. OBJECTIVE To determine whether antiplatelet therapy improves outcomes for critically ill adults with COVID-19.
DESIGN, SETTING, AND PARTICIPANTS In an ongoing adaptive platform trial (REMAP-CAP) testing multiple interventions within multiple therapeutic domains, 1557 critically ill adult patients with COVID-19 were enrolled between October 30, 2020, and June 23, 2021, from 105 sites in 8 countries and followed up for 90 days (final follow-up date: July 26, 2021). INTERVENTIONS Patients were randomized to receive either open-label aspirin (n = 565), a P2Y12 inhibitor (n = 455), or no antiplatelet therapy (control; n = 529). Interventions were continued in the hospital for a maximum of 14 days and were in addition to anticoagulation thromboprophylaxis.
MAIN OUTCOMES AND MEASURES The primary end point was organ support-free days (days alive and free of intensive care unit-based respiratory or cardiovascular organ support) within 21 days, ranging from −1 for any death in hospital (censored at 90 days) to 22 for survivors with no organ support. There were 13 secondary outcomes, including survival to discharge and major bleeding to 14 days. The primary analysis was a bayesian cumulative logistic model. An odds ratio (OR) greater than 1 represented improved survival, more organ support-free days, or both. Efficacy was defined as greater than 99% posterior probability of an OR greater than 1. Futility was defined as greater than 95% posterior probability of an OR less than 1.2 vs control. Intervention equivalence was defined as greater than 90% probability that the OR (compared with each other) was between 1/1.2 and 1.2 for 2 noncontrol interventions.
RESULTS The aspirin and P2Y12 inhibitor groups met the predefined criteria for equivalence at an adaptive analysis and were statistically pooled for further analysis. Enrollment was discontinued after the prespecified criterion for futility was met for the pooled antiplatelet group compared with control. Among the 1557 critically ill patients randomized, 8 patients withdrew consent and 1549 completed the trial (median age, 57 years; 521 [33.6%] female). The median for organ support-free days was 7 (IQR, −1 to 16) in both the antiplatelet and control groups (median-adjusted OR, 1.02 [95% credible interval {CrI}, 0.86-1.23]; 95.7% posterior probability of futility). The proportions of patients surviving to hospital discharge were 71.5% (723/1011) and 67.9% (354/521) in the antiplatelet and control groups, respectively (median-adjusted OR, 1.27 [95% CrI, 0.99-1.62]; adjusted absolute difference, 5% [95% CrI, −0.2% to 9.5%]; 97% posterior probability of efficacy). Among survivors, the median for organ support-free days was 14 in both groups. Major bleeding occurred in 2.1% and 0.4% of patients in the antiplatelet and control groups (adjusted OR, 2.97 [95% CrI, 1.23-8.28]; adjusted absolute risk increase,..
Role of the Funder/Sponsor: The study funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. The platform trial has 4 regional nonprofit sponsors: Monash University, Melbourne, Victoria, Australia (Australasian sponsor); Utrecht Medical Center, Utrecht, the Netherlands (European sponsor); St Michael's Hospital, Toronto, Ontario, Canada (Canadian sponsor); and the Global Coalition for Adaptive Research, San Francisco, California (US sponsor). Several authors are employees of these organizations. However, beyond the declared author contributions, the sponsors had no additional role.
Disclaimer: The views expressed in this publication are those of the author(s) and not necessarily those of the National Health Service, the NIHR, or the Department of Health and Social Care. Dr Seymour is Associate Editor of JAMA, Dr Angus is Senior Editor of JAMA, and Dr Lewis is Statistical Editor of JAMA, but none were involved in any of the decisions regarding review of the manuscript or its acceptance.
Group Information: The REMAP-CAP Investigators are listed in Supplement 3.
Data Sharing Statement: See Supplement 4. Meeting Presentation: This study was presented at the International Symposium on Intensive Care and Emergency Medicine (ISICEM); March 22, 2022; Brussels, Belgium.
Additional Contributions: We are..
References
Al-Beidh, None, PhD
Al-Samkari, Leaf, Dzik, COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection, Blood, doi:10.1182/blood.2020006520
Alistair, Nichol, Md, None
Angus, Berry, Lewis, The REMAP-CAP (Randomized Embedded Multifactorial Adaptive Platform for Community-Acquired Pneumonia) study: rationale and design, Ann Am Thorac Soc, doi:10.1513/AnnalsATS.202003-192SD
Angus, Derde, Al-Beidh, Writing Committee for the REMAP-CAP Investigators. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial, JAMA, doi:10.1001/jama.2020.17022?utm_campaign=articlePDF%26utm_medium=articlePDFlink%26utm_source=articlePDF%26utm_content=jama.2022.2910
Angus, Md, Mph, None
Annane, Md, None
Arabi, Gordon, Derde, None
Attacc Investigators, Therapeutic anticoagulation with heparin in critically ill patients with Covid-19
Author, Webb, Bradbury, Lawler, Mcverry et al., Drs Bradbury and Lewis had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis
Beane, Wilma Van Bentum-Puijk, None, MSc
Berry, None
Berry, None, PhD
Bhimani, Mph, None
Bihari, Phd, None
Bikdeli, Madhavan, Jimenez, Global COVID-19 Thrombosis Collaborative Group. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review, J Am Coll Cardiol, doi:10.1016/j.jacc.2020.04.031
Bilaloglu, Aphinyanaphongs, Jones, Iturrate, Hochman et al., Thrombosis in hospitalized patients with COVID-19 in a New York City health system, JAMA, doi:10.1001/jama.2020.13372?utm_campaign=articlePDF%26utm_medium=articlePDFlink%26utm_source=articlePDF%26utm_content=jama.2022.2910
Buxton, Carrier, None
Buzgau, None, MSc
Cheng, Mbbs, None
Christopher, Horvat, Md;, Hunt, Md, None
Consultants, Austin, Texas, Berry, Lorenzi et al., None
Cove, Mbbs, None
Daniel, Mcauley, Md, None
Detry, None, PhD
Escher, Breakey, Lämmle, Severe COVID-19 infection associated with endothelial activation, Thromb Res, doi:10.1016/j.thromres.2020.04.014
Estcourt, Mbbch, None
Estcourt, Turgeon, Mcquilten, Writing Committee for the REMAP-CAP Investigators. Effect of convalescent plasma on organ support-free days in critically ill patients with COVID-19: a randomized clinical trial, JAMA, doi:10.1001/jama.2021.18178?utm_campaign=articlePDF%26utm_medium=articlePDFlink%26utm_source=articlePDF%26utm_content=jama.2022.2910
Fitzgerald, None
Frank, Brunkhorst, Md, None
Frantzeskaki, Armaganidis, Orfanos, Immunothrombosis in acute respiratory distress syndrome: cross talks between inflammation and coagulation, Respiration, doi:10.1159/000453002
Girard, Md, Msci, None
Godoy, Goligher, Lawler, Slutsky, Zarychanski, Anticipating and managing coagulopathy and thrombotic manifestations of severe COVID-19, CMAJ, doi:10.1503/cmaj.201240
Goligher, Bradbury, Mcverry, None
Goligher, Md, None
Gomez, Laffan, Bradbury, Debate: should the dose or duration of anticoagulants for the prevention of venous thrombosis be increased in patients with COVID-19 while we are awaiting the results of clinical trials?, Br J Haematol, doi:10.1111/bjh.17241
Goossens, Phd, None
Gordon, Mbbs, Md, Affiliations of Authors/Writing Committee
Gordon, Mouncey, Al-Beidh, None
Goshua, Pine, Meizlish, Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study, Lancet Haematol, doi:10.1016/S2352-3026(20)30216-7
Group, Horby, Pessoa-Amorim, Aspirin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial, Lancet, doi:10.1016/S0140-6736(21)01825-0
Haniffa, Phd, None
Health, None
Helms, Tacquard, Severac, Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study, Intensive Care Med, doi:10.1007/s00134-020-06062-x
Higgins, None, PhD
Hills, Mbbs, None
Huang, Md, Mph, None
Ichihara, Md, Mph, None
Investigators, Interleukin-6 receptor antagonists in critically ill patients with Covid-19
Investigators, Lopinavir-ritonavir and hydroxychloroquine for critically ill patients with COVID-19: REMAP-CAP randomized controlled trial, Intensive Care Med, doi:10.1007/s00134-021-06448-5
Kelsey, Linstrum, Ms, None
Klok, Kruip, Van Der Meer, Incidence of thrombotic complications in critically ill ICU patients with COVID-19, Thromb Res, doi:10.1016/j.thromres.2020.04.013
Lamontagne, Md, None
Lawler, Goligher, Berger, Therapeutic anticoagulation with heparin in noncritically ill patients with Covid-19
Lax, Skok, Zechner, Pulmonary arterial thrombosis in COVID-19 with fatal outcome: results from a prospective, single-center, clinicopathologic case series, Ann Intern Med, doi:10.7326/M20-2566
Leavis, Md, None
Lennie, Derde, Md, None
Lewis, Md, None
Litton, Md, None
Loo, School, Medicine, None
Lorenzi, Phd, None
Manne, Denorme, Middleton, Platelet gene expression and function in patients with COVID-19, Blood, doi:10.1182/blood.2020007214
Marc, Bonten, Md, None
Marshall, Md, None
Mcarthur, Md, None
Mcglothlin, Phd, None
Mcquilten, Phd, None
Mcverry, Md, None
Medical, World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects, JAMA, doi:10.1001/jama.2013.281053?utm_campaign=articlePDF%26utm_medium=articlePDFlink%26utm_source=articlePDF%26utm_content=jama.2022.2910
Middeldorp, Coppens, Van Haaps, Incidence of venous thromboembolism in hospitalized patients with COVID-19, J Thromb Haemost, doi:10.1111/jth.14888
Middeldorp, Md, None
Middleton, He, Denorme, Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome, Blood, doi:10.1182/blood.2020007008
Montgomery, None, MSc
Morpeth, Md, Murthy, Md, None
Morris, Bortolasci, Puri, Preventing the development of severe COVID-19 by modifying immunothrombosis, Life Sci, doi:10.1016/j.lfs.2020.118617
Munk, Cardiac Centre at University Health Network
Neal, Md, None
O'sullivan, Gonagle, Ward, Preston, Donnell, Endothelial cells orchestrate COVID-19 coagulopathy, Lancet Haematol, doi:10.1016/S2352-3026(20)30215-5
Panigada, Bottino, Tagliabue, Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis, J Thromb Haemost, doi:10.1111/jth.14850
Parke, None, PhD
Parker, Bn, None
Patrick, Lawler, Md, Mph, None
Paul, Mouncey, None, MSc
Ranucci, Ballotta, Dedda, The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome, J Thromb Haemost, doi:10.1111/jth.14854
Rapkiewicz, Mai, Carsons, Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: a case series, EClinicalMedicine, doi:10.1016/j.eclinm.2020.100434
Reyes, Md, None
Rowan, None, PhD
Saito, Santos, Md, Mshs, None
Saud Bin Abdulaziz, None
Saunders, None, PhD
Serpa-Neto, Phd, Msc, Md; Christopher, Seymour et al., None, MSc
Shah, Donovan, Mchugh, Thrombotic and haemorrhagic complications in critically ill patients with COVID-19: a multicentre observational study, Crit Care, doi:10.1186/s13054-020-03260-3
Shankar-Hari, Md, None
Shaw, Bradbury, Abrams, Wang, Toh, COVID-19 and immunothrombosis: emerging understanding and clinical management, Br J Haematol, doi:10.1111/bjh.17664
Shay, Mcguinness, Md, None
Singh, None
Singh, None
Singh, Turgeon, Turner, Green, Lewis et al., None
Stanley, None
Stanworth, Md, None
Tang, Li, Wang, Sun, Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia, J Thromb Haemost, doi:10.1111/jth.14768
Tolppa, Mbbs, None
Turgeon, Md, None
Turner, Mph; Frank, Van De Veerdonk, Green, None, MSc
Varga, Flammer, Steiger, Endothelial cell infection and endotheliitis in COVID-19, Lancet, doi:10.1016/S0140-6736(20)30937-5
Webb, Md, None
Wichmann, Sperhake, Lütgehetmann, Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study, Ann Intern Med, doi:10.7326/M20-2003
Yaseen, Arabi, Md, None
Zaid, Puhm, Allaeys, Platelets can associate with SARS-CoV-2 RNA and are hyperactivated in COVID-19, Circ Res, doi:10.1161/CIRCRESAHA.120.317703
Zarychanski, Md, None
DOI record:
{
"DOI": "10.1001/jama.2022.2910",
"ISSN": [
"0098-7484"
],
"URL": "http://dx.doi.org/10.1001/jama.2022.2910",
"author": [
{
"affiliation": [],
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators",
"sequence": "first"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Florescu",
"given": "Simin ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Stanciu",
"given": "Delia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Zaharia",
"given": "Mihaela ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": " Kosa",
"given": "Alma",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Codreanu",
"given": "Daniel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kidwai",
"given": "Aneela",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Masood",
"given": "Sobia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kaye",
"given": "Callum",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Coutts",
"given": "Amanda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "MacKay",
"given": "Lynn",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Summers",
"given": "Charlotte",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Polgarova",
"given": "Petra ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Farahi",
"given": "Neda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Fox",
"given": "Eleonore",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "McWilliam",
"given": "Stephen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hawcutt",
"given": "Daniel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Rad",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "O’Malley",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Whitbread",
"given": "Jennifer",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Jones",
"given": "Dawn",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Dore",
"given": "Rachael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Saunderson",
"given": "Paula ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kelsall",
"given": "Olivia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Cowley",
"given": "Nicholas",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Wild",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Thrush",
"given": "Jessica",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Wood",
"given": "Hannah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Austin",
"given": "Karen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bélteczki",
"given": "János",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Magyar",
"given": "István",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Fazekas",
"given": "Ágnes",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kovács",
"given": "Sándor",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Szőke",
"given": "Viktória",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Donnelly",
"given": "Adrian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kelly",
"given": "Martin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Smyth",
"given": "Naoise",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "O’Kane",
"given": "Sinéad",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "McClintock",
"given": "Declan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Warnock",
"given": "Majella",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Campbell",
"given": "Ryan ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "McCallion",
"given": "Edmund",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": " Azaiz",
"given": "Amine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Charron",
"given": "Cyril",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Godement",
"given": "Mathieu",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Geri",
"given": "Guillaume",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Vieillard-Baron",
"given": "Antoine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Johnson",
"given": "Paul",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "McKenna",
"given": "Shirley",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hanley",
"given": "Joanne",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Currie",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Allen",
"given": "Barbara",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "McGoldrick",
"given": "Clare ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "McMaster",
"given": "Moyra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Mani",
"given": "Ashwin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Mathew",
"given": "Meghena",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kandeepan",
"given": "Revathi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Vignesh",
"given": "C",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "TV",
"given": "Bharath",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ramakrishnan",
"given": "N",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "James",
"given": "Augustian ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Elvira ",
"given": "Evangeline",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Jayakumar",
"given": "Devachandran",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Pratheema",
"given": " Ramachandran",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Babu",
"given": " Suresh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ebenezer",
"given": "R",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Krishnaoorthy",
"given": "S",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ranganathan",
"given": "Lakshmi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ganesan",
"given": "Manisha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Shree",
"given": "Madhu",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Guilder",
"given": "Eileen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Butler",
"given": "Magdalena",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Cowdrey",
"given": "Keri-Anne",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Robertson",
"given": "Melissa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ali",
"given": "Farisha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "McMahon",
"given": "Ellie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Duffy",
"given": "Eamon",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Chen",
"given": "Yan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Simmonds",
"given": "Catherine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "McConnochie",
"given": "Rachael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "O’Connor",
"given": "Caroline",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "El-Khawas",
"given": "Khaled",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Richardson",
"given": "Angus",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hill",
"given": "Dianne",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Commons",
"given": "Robert",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Abdelkharim",
"given": "Hussam",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Jha",
"given": "Rajeev",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kalogirou",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ellis",
"given": "Christine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Krishnamurthy",
"given": "Vinodh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "O’Connor",
"given": "Aibhilin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Thurairatnam",
"given": "Saranya",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Mukherjee",
"given": "Dipak",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kaliappan",
"given": "Agilan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Vertue",
"given": "Mark",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Nicholson",
"given": "Anne",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Riches",
"given": "Joanne",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Maloney",
"given": "Gracie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kittridge",
"given": "Lauren",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Solesbury",
"given": "Amanda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ramos",
"given": "Angelo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Collins",
"given": "Daniel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Brickell",
"given": "Kathy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Reid",
"given": "Liadain",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Smyth",
"given": "Michelle",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Breen",
"given": "Patrick",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Spain",
"given": "Sandra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Curley",
"given": "Gerard",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "McEvoy",
"given": "Natalie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Geoghegan",
"given": "Pierce",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Clarke ",
"given": "Jennifer",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Silversides",
"given": "Jon",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "McGuigan",
"given": "Peter",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ward",
"given": "Kathryn",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "O’Neill",
"given": "Aisling",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Finn",
"given": "Stephanie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Wright",
"given": "Chris",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Green",
"given": "Jackie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Collins",
"given": "Érin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Knott",
"given": "Cameron",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Smith",
"given": "Julie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Boschert",
"given": "Catherine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Slieker",
"given": "Kitty",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ewalds",
"given": "Esther",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Sanders",
"given": "Arnate",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Wittenberg",
"given": "Wendy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Geurts",
"given": "Heidi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Reeve",
"given": "Brenda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Dechert ",
"given": "William",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Phillips",
"given": "Barbara ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Oritz-Ruiz de Gordoa",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Affleck",
"given": "Julia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Apte",
"given": "Yogesh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Subbanna",
"given": "Umesh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bartholdy",
"given": "Roland",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Frakking",
"given": "Thuy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Pinnell",
"given": "Jez",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Robinson",
"given": "Matt ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Gledhill",
"given": "Lisa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Wood",
"given": "Tracy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Keat",
"given": "Karuna",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bhonagiri",
"given": "Deepak",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Sanghavi",
"given": "Ritesh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Nema",
"given": "Jodie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ford",
"given": "Megan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Parikh",
"given": "Harshel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Avard",
"given": "Bronwyn",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Nourse",
"given": "Mary",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hoiting",
"given": "Oscar",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Peters",
"given": "Marco",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Rengers",
"given": "Els",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Evers",
"given": "Mirjam",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Prinssen",
"given": "Anton",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Morgan",
"given": "Matt",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Cole",
"given": "Jade ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hill",
"given": "Helen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Davies",
"given": "Michelle",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Williams",
"given": "Angharad ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Thomas",
"given": "Emma",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Davies",
"given": "Rhys",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Wise",
"given": "Matt",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Grimm",
"given": "Patrick",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Soukup",
"given": "Jens",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Wetzold",
"given": "Richard",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Löbel",
"given": "Madlen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Starke",
"given": "Lisa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Lellouche",
"given": "Francois ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Lizotte",
"given": "Patricia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Declerq",
"given": "Pierre",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Antoine",
"given": "Marchalot ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Stephanie",
"given": "Gelinotte",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Jean-Pierre",
"given": "Eraldi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "François",
"given": "Bourgerol ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Marion",
"given": "Beuzelin ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Philippe",
"given": "Rigaud",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Pourcine",
"given": "Franck",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Monchi",
"given": "Mehran",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Luis",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Mercier",
"given": "Romain",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Sagnier",
"given": "Anne",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Verrier",
"given": "Nathalie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Caplin",
"given": "Cecile",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Richecoeu",
"given": "Jack",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Combaux",
"given": "Daniele",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Siami",
"given": "Shidasp",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Aparicio",
"given": "Christelle",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Vautier",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Jeblaoui",
"given": "Asma",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Lemaire-Brunel",
"given": "Delphine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Carbonneau",
"given": "Frédérick",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Leblond",
"given": "Julie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Plantefeve",
"given": "Gaetan ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Leparco",
"given": "Cécile",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Contou",
"given": "Damien",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Fartoukh",
"given": "Muriel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Courtin",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Labbe",
"given": "Vincent",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Voiriot",
"given": "Guillaume",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Salhi",
"given": "Sara",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Chassé",
"given": "Michaël",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Carrier",
"given": "François",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Boumahni",
"given": "Dounia ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Benettaib",
"given": "Fatna",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ghamraoui",
"given": "Ali",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Sement",
"given": "Arnaud",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Gachet",
"given": "Alexandre",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hanisch",
"given": "Alexis",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Haffiane",
"given": "Abdelmagid",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Boivin",
"given": "Anne-Hélène",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Barreau",
"given": "Amelie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Guerineau",
"given": "Elodie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Poupblanc",
"given": "Séverine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Egreteau",
"given": "Pierre",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Lefevre",
"given": "Montaine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bocher",
"given": "Simon",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Le Loup",
"given": "Guillaume",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Le Guen",
"given": "Lenaïg",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Carn",
"given": "Vanessa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bertel",
"given": "Melanie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Antcliffe",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Templeton",
"given": "Maie ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Rojo",
"given": "Roceld",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Coghlan",
"given": "Phoebe",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Smee",
"given": "Joanna ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Barker",
"given": "Gareth",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Finn",
"given": "André",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kreß",
"given": "Gabriele",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hoff",
"given": "Uwe",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hinrichs",
"given": "Carl",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Nee",
"given": "Jens",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Mackay",
"given": "Euan ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Cort",
"given": "Jon",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Whileman",
"given": "Amanda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Spencer",
"given": "Thomas",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Spittle",
"given": "Nick",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Beavis",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Padmakumar",
"given": "Anand",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Dale",
"given": "Katie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hawes",
"given": "Joanne",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Moakes",
"given": "Emma",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Gascoyne",
"given": "Rachel ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Pritchard",
"given": "Kelly",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Stevenson",
"given": "Lesley",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Cooke",
"given": "Justin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Nemeth-Roszpopa",
"given": "Karolina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Gauli",
"given": "Basanta",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bastola",
"given": "Sirjana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Muller",
"given": "Grégoire",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Nay",
"given": "Mai-Anh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kamel",
"given": "Toufik",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Benzekri",
"given": "Dalila",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Jacquier",
"given": "Sophie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Runge",
"given": "Isabelle",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Mathonnet",
"given": "Armelle",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Barbier",
"given": "François",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bretagnol",
"given": "Anne",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Carter",
"given": "Jay",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Van Der Heyden",
"given": "Kymbalee",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Mehrtens",
"given": "Jan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Morris",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Morgan",
"given": "Stacey",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Burke",
"given": "Tara",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Mercier",
"given": "Emmanuelle",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Chartier",
"given": "Delphine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Salmon",
"given": "Charlotte",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Dequin",
"given": "Pierre-François",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Garot",
"given": "Denis",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bellemare",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Cloutier",
"given": "Ève ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Daher",
"given": "Rana ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Costerousse",
"given": "Olivier",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Boulanger",
"given": "Marie-Claude",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Couillard-Chénard",
"given": "Émilie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Francoeur ",
"given": "François",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Francois",
"given": "Bruno",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Gay",
"given": "Alexandra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Anne-Laure",
"given": "Fedou",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ramali",
"given": "Mohamed",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "HC",
"given": "Ooi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ghosh",
"given": "Alison",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Osagie",
"given": "Rawlings",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Arachchige",
"given": "Malka",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hartley",
"given": "Melissa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Cheung",
"given": "Winston",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kol",
"given": "Mark",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Wong",
"given": "Helen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Shah",
"given": "Asim",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Wagh ",
"given": "Atul",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bamford",
"given": "Peter",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Reid",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Cawley",
"given": "Kathryn",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Faulkner",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Pickering",
"given": "Charlotte",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Raj",
"given": "Ashok ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Tsinaslanidis",
"given": "Georgios",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Khade",
"given": "Reena ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Agha",
"given": "Gloria",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Sekiwala",
"given": "Rose",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Smith",
"given": "Tim",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Brewer",
"given": "Chris",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Gregory",
"given": "Jane",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Limb",
"given": "James",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Cowton",
"given": "Amanda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "O’Brien",
"given": "Julie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Postlethwaite",
"given": "Kelly",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Malakouti ",
"given": "Salim",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Music",
"given": "Edvin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ricketts",
"given": "Dan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "King",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Clermont",
"given": "Gilles",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bart",
"given": "Robert",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Mayr",
"given": "Florian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Schoenling",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Andreae",
"given": "Mark",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Shetty",
"given": "Varun",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Brant",
"given": "Emily",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Malley",
"given": "Brian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Donadee",
"given": "Chenell",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Sackrowitz",
"given": "Rachel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Weissman",
"given": "Alexandra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Yealy",
"given": "Donald",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Barton",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Talia",
"given": "Nadine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Nikitas",
"given": "Nikitas",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Wells",
"given": "Colin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Lankester",
"given": "Liana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "McMillan",
"given": "Helen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "van den Oever",
"given": "Huub",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kruisdijk-Gerritsen",
"given": "Arriette",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Haidar",
"given": "Ghady ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bain",
"given": "William",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Barbash",
"given": "Ian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Fitzpatrick",
"given": "Meghan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Franz",
"given": "Christopher",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kitsios",
"given": "Georgios",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Moghbeli",
"given": "Kaveh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Rosborough",
"given": "Brian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Shah",
"given": "Faraaz",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Suber",
"given": "Tomeka",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Pulletz",
"given": "Mark",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Williams",
"given": "Patricia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Birch",
"given": "Jenny",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Wiseman",
"given": "Sophie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Horton",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Alegria",
"given": "Ana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Turki",
"given": "Salah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Elsefi",
"given": "Tarek",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Crisp",
"given": "Nikki",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Allen",
"given": "Louise",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Truman",
"given": "Nicholas",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Smith",
"given": "Matthew",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Chukkambotla",
"given": "Sri",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Goddard",
"given": "Wendy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Duberley",
"given": "Stephen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Khan",
"given": "Meherunnisa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kazi",
"given": "Aayesha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Simpson",
"given": "Joanna",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Duke",
"given": "Graeme",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Chan",
"given": "Peter",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Carter",
"given": "Brittney",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hunter",
"given": "Stephanie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Voigt",
"given": "Ingo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Schueler",
"given": "Robert",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Blank",
"given": "Elisabeth",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hüning",
"given": "Vanessa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Steffen",
"given": "Melanie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Goralski",
"given": "Patricia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Regli",
"given": "Adrian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Pellicano",
"given": "Susan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Palermo",
"given": "Annamaria",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Eroglu ",
"given": "Ege",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Laver",
"given": "Russell",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Shrestha",
"given": "Tapaswi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Jin ",
"given": "Xia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "French",
"given": "Craig",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bates",
"given": "Samantha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Towns",
"given": "Miriam",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Yang",
"given": "Yang",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "McGain",
"given": "Forbes",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "McCullagh",
"given": "Iain",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Cairns",
"given": "Tom",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hanson",
"given": "Helen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Patel",
"given": "Bijal",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Clement",
"given": "Ian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Evetts",
"given": "George",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Touma",
"given": "Omar ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Holland",
"given": "Susan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hodge",
"given": "Christopher",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Taylor",
"given": "Holly",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Alderman",
"given": "Meera",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Barnes",
"given": "Nicky",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Da Rocha",
"given": "Joana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Smith",
"given": "Catherine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Brooks",
"given": "Nicole",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Weerasinghe",
"given": "Thanuja",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Sinclair",
"given": "Julie-Ann",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Abusamra",
"given": "Yousuf",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Doherty",
"given": "Ronan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Cudlipp",
"given": "Joanna ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Singh",
"given": "Rajeev",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Yu",
"given": "Haili",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Daebis",
"given": "Admad ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "NG",
"given": "Christopher",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kendrick",
"given": "Sara ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Saran",
"given": "Anita",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Makky",
"given": "Ahmed ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Greener",
"given": "Danni",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Rowe-Leete",
"given": "Louise",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Edwards",
"given": "Alexandra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bland",
"given": "Yvonne",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Dolman",
"given": "Rozzie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Foster",
"given": "Tracy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Laffey",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "McNicholas",
"given": "Bairbre",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Scully",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Casey",
"given": "Siobhan ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kernan",
"given": "Maeve",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Brennan",
"given": "Aoife",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Rangan",
"given": "Ritika",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Tully",
"given": "Riona",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Corbett",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "McCarthy",
"given": "Aine ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Duffy",
"given": "Oscar",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Burke",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Linnett",
"given": "Vanessa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Sanderson",
"given": "Amanda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ritzema",
"given": "Jenny",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Wild",
"given": "Helen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Lucas",
"given": "Rachael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Marriott",
"given": "Yvonne",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Andric",
"given": "Zdravko",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Cviljevic",
"given": "Sabina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Đimoti",
"given": "Renata",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Zapalac",
"given": "Marija",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Mirković",
"given": "Gordan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Khare",
"given": "Divya",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Pinder",
"given": "Meredith",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Gopinath",
"given": "Amitha ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kannan",
"given": "Thogulava",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Dean",
"given": "Steven",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Vanmali",
"given": "Piyush",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Depuydt",
"given": "Pieter",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "De Waele",
"given": "Jan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "De Bus",
"given": "Liesbet",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Fierens",
"given": "Jan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bracke",
"given": "Stephanie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Vermassen",
"given": "Joris",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Vermeiren",
"given": "Daisy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Pugh",
"given": "Richard",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Lean",
"given": "Richard",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Qiu",
"given": "Xinyi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Scanlan",
"given": "Jeremy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Evans",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Davies",
"given": "Gwyneth",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Lewis",
"given": "Joanne",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Plesnikova",
"given": "Yvonna",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Khoud",
"given": "Ahmed",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Coetzee",
"given": "Samantha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Puxty",
"given": "Kathryn",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Cathcart",
"given": "Susanne",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Rimmer",
"given": "Dominic",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bagot",
"given": "Catherine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Scott",
"given": "Kathryn",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Martin",
"given": "Laila",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Yusuff",
"given": "Hakeem",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Isgro",
"given": "Graziella",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Brightling",
"given": "Chris",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bourne",
"given": "Michelle",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Craner",
"given": "Michelle",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Boyles",
"given": "Rebecca",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Alexander",
"given": "Brian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Roberts",
"given": "Tracey",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Nelli",
"given": "Apoorva",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Rosenstein-Sisson",
"given": "Rachel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Speyer",
"given": "Rebecca",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Pech",
"given": "Yadira",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "McCullough",
"given": "James",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Tallott",
"given": "Mandy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Vazquez-Grande",
"given": "Gloria",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Marten",
"given": "Nicole",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Liu",
"given": "Theresa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Siddiqui",
"given": "Atif",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Khanal",
"given": "Sushil",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Amatya ",
"given": "Sameena",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Szakmany",
"given": "Tamas ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Cherian",
"given": "Shiney",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Williams",
"given": "Gemma",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "James",
"given": "Christie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Waters",
"given": "Abby",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Prout",
"given": "Rachel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Stedman",
"given": "Roger",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Davies",
"given": "Louisa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Pegler",
"given": "Suzannah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kyeremeh",
"given": "Lynsey",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Moorhouse",
"given": "Louise",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Arbane",
"given": "Gill",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Marotti",
"given": "Marina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bociek",
"given": "Aneta",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Campos",
"given": "Sara",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "van Nieuwkoop",
"given": "Kees",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ottens",
"given": "Thomas",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Visser",
"given": "Yorik",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "van den Berg",
"given": "Lettie ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "van der Kraan-Donker ",
"given": "Annemarie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Brett",
"given": "Stephen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Arias",
"given": "Sonia ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hall",
"given": "Rebecca",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Paneru",
"given": "Hem",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Koirala",
"given": "Sabin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Paudel",
"given": "Pratibha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Wilson",
"given": "Maggie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Vaara",
"given": "Suvi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Pettilä",
"given": "Leena",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Heinonen",
"given": "Jonna",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Pettilä",
"given": "Ville",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Jain",
"given": "Susan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Gupta",
"given": "Abhinav",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Holbrook",
"given": "Catherine ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Antoine",
"given": "Pierre",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Meziani",
"given": "Ferhat",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Allam",
"given": "Hayat",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Cattelan",
"given": "Jessy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Clere-Jehl",
"given": "Raphael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Helms",
"given": "Julie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kummerlen",
"given": "Christine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Merdji",
"given": "Hamid",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Monnier",
"given": "Alexandra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Rahmani",
"given": "Hassene",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Antoine",
"given": "Studer",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Schneider",
"given": "Francis",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Castelain",
"given": "Vincent",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Morel",
"given": "Guillaume",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "L’Hotellier",
"given": "Sylvie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ochin",
"given": "Evelina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Vanjak",
"given": "Christian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Rouge",
"given": "Patrick",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bendjemar",
"given": "Lynda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Albert",
"given": "Martin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Serri",
"given": "Karim",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Cavayas",
"given": "Alexandros",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Duplaix",
"given": "Mathilde",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Williams",
"given": "Virginie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Catorze",
"given": "Nuno José Teodoro Amaro dos Santos",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Pereira",
"given": "Tiago Nuno Alfaro Lima",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ferreira",
"given": "Ricardo Manuel Castro",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bastos",
"given": "Joana Margarida Pereira Sousa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Batista ",
"given": "Teresa Margarida Oliveira",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Badie",
"given": "Julio",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Berdaguer",
"given": "Fernando",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Malfroy",
"given": "Sylvain",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Mezher",
"given": "Chaouki",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bourgoin",
"given": "Charlotte",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Moneger",
"given": "Guy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bouvier",
"given": "Elodie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Muñoz-Bermúdez",
"given": "Rosana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Marin-Corral",
"given": "Judith",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Degracia",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Gómez",
"given": "Francisco",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "López ",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Aceto",
"given": "Romina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Aghemo",
"given": "Alessio",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Badalamenti",
"given": "Salvatore",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Brunetta",
"given": "Enrico",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Cecconi",
"given": "Maurizio",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ciccarelli",
"given": "Michele",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Constantini",
"given": "Elena",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Greco",
"given": "Massimiliano",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Folci",
"given": "Marco",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Selmi",
"given": "Carlo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Voza",
"given": "Antonio",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Henning",
"given": "Jeremy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bonner",
"given": "Stephen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hugill",
"given": "Keith",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Cirstea",
"given": "Emanuel ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Wilkinson",
"given": "Dean",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Jones",
"given": "Jessica",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Altomy",
"given": "Mohammed",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Karlikowski",
"given": "Michal",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Sutherland",
"given": "Helen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Wilhelmsen",
"given": "Elva",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Woods",
"given": "Jane",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "North",
"given": "Julie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Pletz",
"given": "Mathias",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hagel",
"given": "Stefan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ankert",
"given": "Juliane",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kolanos",
"given": "Steffi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bloos",
"given": "Frank",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Simons",
"given": "Koen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "van Zuylen",
"given": "Tamara",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bouman",
"given": "Angela",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kumar",
"given": "Nikhil",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Panwar",
"given": "Rakshit",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Brinkerhoff",
"given": "Gail",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Koppen",
"given": "Cassandra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Cazzola",
"given": "Federica",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Szigligeti",
"given": "Gábor",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Leszkoven",
"given": "János",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Rochwerg",
"given": "Bram",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Karachi",
"given": "Tim",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Oczkowski",
"given": "Simon",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Centofanti",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Millen",
"given": "Tina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Sundaran",
"given": "Dhinesh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hollos",
"given": "Laszlo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Williams",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Turns",
"given": "Margaret ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Walsh",
"given": "Joanne ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Al Qasim",
"given": "Eman",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Alswaidan",
"given": "Lolowa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hegazy",
"given": "Mohamed",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Arishi",
"given": "Hatim",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Al Amri",
"given": "Ali",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "AlQahtani",
"given": "Samah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Naidu",
"given": "Brintha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Tlayjeh",
"given": "Haytham",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hussain",
"given": "Sajid",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Al Enezi",
"given": "Farhan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Abdukahil ",
"given": "Sheryl Ann",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hopkins",
"given": "Phil",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Smith",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Noble",
"given": "Harriet ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "O’Reilly",
"given": "Kevin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Mehta",
"given": "Reena",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Wong",
"given": "Onyee ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Makanju",
"given": "Esther",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Rao",
"given": "Deepak",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Sikondari",
"given": "Nyma ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Saha",
"given": "Sian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Corcoran",
"given": "Ele",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Pappa",
"given": "Evita",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Cockrell",
"given": "Maeve",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Donegan",
"given": "Clare",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Balaie",
"given": "Morteza",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Nickoleit-Bitzenberger",
"given": "Daniela",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Schaaf",
"given": "Bernhard",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Meermeier",
"given": "Werner",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Prebeg",
"given": "Katharina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Azzaui",
"given": "Harun",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hower",
"given": "Martin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Brieger",
"given": "Klaus-Gerd",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Elender",
"given": "Corinna",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Sabelhaus",
"given": "Timo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Riepe",
"given": "Ansgar",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Akamp",
"given": "Ceren",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kremling",
"given": "Julius",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Klein",
"given": "Daniela",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Landsiedel-Mechenbier",
"given": "Elke",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Laha",
"given": "Shondipon",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Verlander",
"given": "Mark",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Williams",
"given": "Alexandra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Jha",
"given": "Avinash",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Megarbane",
"given": "Bruno",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Voicu",
"given": "Sebastian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Deye",
"given": "Nicolas",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Malissin",
"given": "Isabelle",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Sutterlin",
"given": "Laetitia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Mrad",
"given": "Aymen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Lehalleur",
"given": "Adrien",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Naim",
"given": "Giulia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Nguyen",
"given": "Philippe",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ekhérian",
"given": "Jean-Michel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Boué",
"given": "Yvonnick",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Sidéris",
"given": "Georgios",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Vodovar",
"given": "Dominique",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Guérin",
"given": "Emmanuelle",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Grant",
"given": "Caroline",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Brain",
"given": "Matthew",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Mineall",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Paramasivam",
"given": "Elankumaran",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Wilby",
"given": "Elizabeth",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ogg",
"given": "Bethan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Howcroft",
"given": "Clare",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Aspinwall",
"given": "Angelique",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Charlton",
"given": "Sam",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Gould",
"given": "Richard",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Mistry",
"given": "Deena",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Awan",
"given": "Sidra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bedford",
"given": "Caroline",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Carr-Wilkinson",
"given": "Joanne ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hall",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Cooke",
"given": "Jill",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Gardiner-Hill",
"given": "Caroline",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Maloney",
"given": "Carolyn",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Brunskill",
"given": "Nigel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Watchorn",
"given": "Olivia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hardy",
"given": "Chloe",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Qureshi",
"given": "Hafiz",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Flint",
"given": "Neil",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Nicholson",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Southin",
"given": "Sara",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Nicholson",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ghattaoraya",
"given": "Amardeep",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Harding",
"given": "Daniel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "O’Halloran",
"given": "Sinead",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Collins",
"given": "Amy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Smith",
"given": "Emma",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Trues",
"given": "Estefania",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Borgatta",
"given": "Barbara",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Turner-Bone",
"given": "Ian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Reddy",
"given": "Amie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Wilding",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Wilson",
"given": "Craig",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Surti",
"given": "Zuhra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "White",
"given": "Hayden",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Estensen",
"given": "Kristen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Morrison",
"given": "Lynette",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Sutton",
"given": "Joanne",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Cooper",
"given": "Melanie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Warnapura",
"given": "Loku",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Agno",
"given": "Ronan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Sathianathan",
"given": "Prasannakumari ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Shaw",
"given": "Deborah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ijaz",
"given": "Nazia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Spong",
"given": "Adam",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Sabaretnam",
"given": "Suganya",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Burns",
"given": "Dean",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Lang",
"given": "Eva",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Tate",
"given": "Margaret",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Fischer",
"given": "Roy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Biradar",
"given": "Vishwanath",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Soar",
"given": "Natalie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Golden",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Davey",
"given": "Miriam",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Seaman",
"given": "Rebecca",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Osborne",
"given": "Alexander",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bannard-Smith",
"given": "Jonathan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Clark",
"given": "Richard",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Birchall",
"given": "Kathrine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Henry",
"given": "Joanne",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Pomeroy",
"given": "Fiona",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Quayle",
"given": "Rachael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Wylie",
"given": "Katharine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Sukuraman",
"given": "Anila",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "John",
"given": "Maya",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Sibin",
"given": "Sindhu",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Khwaja",
"given": "Kosar",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Campisi",
"given": "Josie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "van Vonderen",
"given": "Marit",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Pietersma",
"given": "Mario",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Vrolijk",
"given": "Loes",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kampschreur ",
"given": "Linda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "van Gulik",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Makowski",
"given": "Arystarch",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Misztal",
"given": "Beata",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Haider",
"given": "Syeda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Liao",
"given": "Angela",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Squires",
"given": "Rebecca",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Oborska",
"given": "Aneta",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kayani",
"given": "Abdul",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kalchko-Veyssal",
"given": "Selver",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Prabakaran",
"given": "Rajalakshmi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hadebe",
"given": "Bernard",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "KalchkoVeyssal",
"given": "Selver",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Williams",
"given": "Tony",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Song",
"given": "Rima",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Lai",
"given": "Vivian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": " Habraken ",
"given": "Hannah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Stewart",
"given": "Richard",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Mwaura",
"given": "Esther",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Mew",
"given": "Louise",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Wren",
"given": "Lynn",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Willams",
"given": "Felicity",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Sutherland",
"given": "Sara-Beth",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Rebello",
"given": "Rashmi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Shehabi",
"given": "Yahya",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Al-Bassam",
"given": "Wisam",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hulley",
"given": "Amanda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kadam",
"given": "Umesh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Sathianathan",
"given": "Kushaharan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Innes",
"given": "Richard",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Doble",
"given": "Patricia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Graham",
"given": "Libby",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Shovelton",
"given": "Charmaine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Dean",
"given": "Tessa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Salahuddin",
"given": "Nawal",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Aryal",
"given": "Diptesh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Koirala",
"given": "Kanchan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Rai",
"given": "Namrata",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Luitel",
"given": "Subekshya",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Seppelt",
"given": "Ian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Whitehead",
"given": "Christina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Lowrey",
"given": "Julie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Gresham",
"given": "Rebecca",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Masters",
"given": "Kristy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hamlyn",
"given": "Vincent",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hawkins",
"given": "Nancy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Roynon-Reed",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Cutler",
"given": "Sean",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Lewis",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Lazaro",
"given": "Juan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Newman",
"given": "Tabitha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Duan",
"given": "Erick",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Tsang",
"given": "Jennifer",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Patterson",
"given": "Lisa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Austin",
"given": "Pauline",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Chapman",
"given": "Susan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Cabrelli",
"given": "Louise",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Fletcher",
"given": "Simon",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Nortje",
"given": "Jurgens",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Fottrell-Gould",
"given": "Deirdre",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Randell",
"given": "Georgina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Stammers",
"given": "Katie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Healey",
"given": "Gail",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Pinto",
"given": "Marta",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Borrill",
"given": "Zoe",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Duncan",
"given": "Tracy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ustianowski",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Uriel",
"given": "Alison",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Eltayeb",
"given": "Ayaa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Alfonso",
"given": "Jordan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hey",
"given": "Samuel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Shaw",
"given": "Joanne",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Fox",
"given": "Claire",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Lindergard",
"given": "Gabriella",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Charles",
"given": "Bethan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Blackledge",
"given": "Bethany",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Connolly",
"given": "Karen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Harris",
"given": "Jade",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Cuesta",
"given": "Jeronimo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Xavier",
"given": "Kugan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Purohit",
"given": "Dharam",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Elhassan",
"given": "Munzir",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Haldeos",
"given": "Anne",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Vincent",
"given": "Rachel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Abdelrazik",
"given": "Marwa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Jenkins",
"given": "Samuel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ganesan",
"given": "Arunkumar",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kumar",
"given": "Rohit",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Carter",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bakthavatsalam",
"given": "Dhanalakshmi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Frater",
"given": "Alasdair",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Saleem",
"given": "Malik",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Everitt",
"given": "Robert",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hacking",
"given": "Danielle",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Zaman",
"given": "Mohsin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Elmahi",
"given": "Einas",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Jones",
"given": "Andrea",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hall",
"given": "Kathryn",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Mills",
"given": "Gary",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Raithatha",
"given": "Ajay",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bauchmuller",
"given": "Kris",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ryalls",
"given": "Kim",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Harrington",
"given": "Kate",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bowler",
"given": "Helen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Sall",
"given": "Jas",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bourne",
"given": "Richard",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Gross",
"given": "Jamie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Massey",
"given": "Natalie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Adebambo",
"given": "Olumide",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Long",
"given": "Matilda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Tony",
"given": "Kiran",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Juffermans",
"given": "Nicole",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Koopmans",
"given": "Matty",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Dujardin",
"given": "Romein",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Alderink ",
"given": "Bashar",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Rowland",
"given": "Matthew",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hutton",
"given": "Paula",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bashyal",
"given": "Archana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Davidson",
"given": "Neil",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hird",
"given": "Clare",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Chhablani",
"given": "Manish",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Phalod",
"given": "Gunjan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kirkby",
"given": "Amy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Archer",
"given": "Simon",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Netherton",
"given": "Kimberley ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Reschreiter",
"given": "Henrik",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Camsooksai",
"given": "Julie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Patch",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Jenkins",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Humphrey",
"given": "Charlotte",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kruger",
"given": "Peter",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Walsham",
"given": "James",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Meyer",
"given": "Jason",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Harward",
"given": "Meg",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Venz",
"given": "Ellen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Sathe",
"given": "Sonia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Roche",
"given": "Lisa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Davies",
"given": "Ellie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Skinner",
"given": "Denise",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Gaylard",
"given": "Jane",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Newman",
"given": "Julie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Pogson",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Rose",
"given": "Steve",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Daly",
"given": "Zoe",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Brimfield",
"given": "Lutece",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Nown",
"given": "Angie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Parekh",
"given": "Dhruv",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bergin",
"given": "Colin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bates",
"given": "Michelle",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "McGhee",
"given": "Christopher",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Lynch",
"given": "Daniella",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bhandal",
"given": "Khushpreet",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Tsakiridou",
"given": "Kyriaki",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bamford",
"given": "Amy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Cooper",
"given": "Lauren",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Whitehouse",
"given": "Tony",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Veenith",
"given": "Tonny",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Forster",
"given": "Elliot",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "O'Connell",
"given": "Martin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Sim",
"given": "Malcolm",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hay",
"given": "Sophie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Henderson",
"given": "Steven",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Nygren",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Valentine",
"given": "Eliza",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Katary",
"given": "Amro",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bell",
"given": "Gillian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Wilcox",
"given": "Louise",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Mataliotakis",
"given": "Michail",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Smith",
"given": "Paul",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ali",
"given": "Murtaza",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Isguzar",
"given": "Agah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Phull",
"given": "Mandeep-Kaur",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Zaidi",
"given": "Abbas",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Pogreban",
"given": "Tatiana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Rosaroso",
"given": "Lace",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Harvey",
"given": "Daniel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Lowe",
"given": "Benjamin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Meredith",
"given": "Megan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ryan",
"given": "Lucy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Schouten",
"given": "Jeroen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Pickkers",
"given": "Peter",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Roovers",
"given": "Noortje",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Klop-Riehl",
"given": "Margreet",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "van der Eng",
"given": "Hetty",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Sloots-Cuppen",
"given": "Sonja",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Preijers",
"given": "Lieke",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "van Oosten",
"given": "Nienke",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Moine",
"given": "Pierre",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Heming",
"given": "Nicholas",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Maxime",
"given": "Virginie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bossard",
"given": "Isabelle",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Nicholier",
"given": "Tiphaine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Clair",
"given": "Bernard",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Orlikowski",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bounab",
"given": "Rania",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Abdeladim",
"given": "Lilia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Sy",
"given": "Eric",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Mailman",
"given": "Jonathan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Lee",
"given": "Stephen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Gupta",
"given": "Chiraag",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kassir",
"given": "Sandy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "López",
"given": "Rafael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Rodríguez-Gómez",
"given": "Jorge",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Cárcel",
"given": "Sheila",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Carmona",
"given": "Rosario",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "de la Fuente",
"given": "Carmen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Rodriguez",
"given": "Marina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Jan Hassing",
"given": " Robert ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Greven",
"given": "Frances",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Huijbens",
"given": "Danique",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Roebers",
"given": "Harald",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Miles",
"given": "Helen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Attokaran",
"given": "Antony",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Buehner",
"given": "Ulrike",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Williams",
"given": "Erin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Gluck",
"given": "Samuel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "O’Connor",
"given": "Stephanie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Chapman",
"given": "Marianne",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Glasby",
"given": "Kathleen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kutsogiannis",
"given": "Demetrios",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Thompson",
"given": "Patricia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Rooney",
"given": "Kevin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Rodden",
"given": "Natalie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Thomson",
"given": "Nicola",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "McGlynn",
"given": "Deborah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Abel",
"given": "Lynn",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Gemmell",
"given": "Lisa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Sundaram",
"given": "Radha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hornsby",
"given": "James",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Walden",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Keating",
"given": "Liza",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Frise",
"given": "Matthew",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Rai",
"given": "Sabi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bartley",
"given": "Shauna",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Schuster-Bruce",
"given": "Martin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Pitts",
"given": "Sally",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Miln",
"given": "Rebecca",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Purandare",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Vamplew",
"given": "Luke",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Patel",
"given": "Brijesh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Dempster",
"given": "Debra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Gummadi",
"given": "Mahitha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Dormand",
"given": "Natalie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Wang",
"given": "Shu",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Spivey",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bean",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Burt",
"given": "Karen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Moore",
"given": "Lorraine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hammonds",
"given": "Fiona",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Richards",
"given": "Carol",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Campbell",
"given": "Lewis",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Smyth",
"given": "Kirsty",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Phillips",
"given": "Margaret",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Day",
"given": "Christopher",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Zitter",
"given": "Letizia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Benyon",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Singh",
"given": "Jayaprakash",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Lynch",
"given": "Ceri ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Mikusek",
"given": "Justyna",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Deacon",
"given": "Bethan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Turner",
"given": "Keri",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Baker",
"given": "Evelyn",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hickey",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Champanerkar",
"given": "Shreekant",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Aitken",
"given": "Lindianne ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "LewisProsser",
"given": "Lorraine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ahmad",
"given": "Norfaizan ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Wiles",
"given": "Matt",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Willson",
"given": "Jayne",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Grecu",
"given": "Irina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Martin",
"given": "Jane",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Wrey Brown",
"given": "Caroline",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Arias",
"given": "Ana-Marie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bevan",
"given": "Emily",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Westlake",
"given": "Samantha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Craven",
"given": "Thomas",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hope",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Singleton",
"given": "Jo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Clark",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "McCulloch",
"given": "Corrienne",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Biddie",
"given": "Simon",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Welters",
"given": "Ingeborg",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hamilton",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Williams",
"given": "Karen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Waugh",
"given": "Victoria",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Shaw",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Mulla",
"given": "Suleman",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Waite",
"given": "Alicia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Roman",
"given": "Jaime",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Martinez",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Johnston",
"given": "Brian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Puthucheary",
"given": "Zudin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Martin",
"given": "Timothy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Santos",
"given": "Filipa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Uddin",
"given": "Ruzena ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Fernandez",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Seidu",
"given": "Fatima",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Somerville",
"given": "Alastair",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Pakats",
"given": "Mari-Liis",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Begum",
"given": "Salma",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Shahid",
"given": "Tasnin ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Presneill",
"given": "Jeffrey",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Barge",
"given": "Deborah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Byrne",
"given": "Kathleen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Driscoll",
"given": "Alana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Fortune",
"given": "Louise",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Janin",
"given": "Pierre",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Yarad",
"given": "Elizabeth",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bass",
"given": "Frances",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hammond",
"given": "Naomi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "O’Connor",
"given": "Anne",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Vuylsteke",
"given": "Alain",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Chan",
"given": "Charles",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Victor",
"given": "Saji",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Waterson",
"given": "Sharon",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "McNamara",
"given": "Robert",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Gattas",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Buhr",
"given": "Heidi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Coles",
"given": "Jennifer",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Matsa",
"given": "Ramprasad",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Gellamucho",
"given": "Minerva",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Creagh-Brown",
"given": "Ben",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Marriot",
"given": "Cheryl",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Salberg",
"given": "Armorel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Zouita",
"given": "Louisa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Stone",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Michalak",
"given": "Natalia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Donlon",
"given": "Sinead",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Mtuwa",
"given": "Shelia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Mayangao",
"given": "Irving",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Verula",
"given": "Jerik",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Burda",
"given": "Dorota",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Harris",
"given": "Celia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Jones",
"given": "Emily",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bradley",
"given": "Paul",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Tarr",
"given": "Esther",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Harden",
"given": "Lesley",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Piercy",
"given": "Charlie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Nolan",
"given": "Jerry",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kerslake",
"given": "Ian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Cook",
"given": "Tim",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Simpson",
"given": "Tom",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Dalton",
"given": "James",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Demetriou",
"given": "Carrie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Mitchard",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ramos",
"given": "Lidia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "White",
"given": "Katie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Johnson",
"given": "Toby",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Headdon",
"given": "William",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Spencer",
"given": "Stephen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "White",
"given": "Alison",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Howie",
"given": "Lucy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Reay",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Jenkins",
"given": "Steve",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Watts",
"given": "Angela",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Traverse",
"given": "Eleanor",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Jennings",
"given": "Stacey",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Anumakonda",
"given": "Vikram",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Tuckwell",
"given": "Caroline",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Pearson",
"given": "Karen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Harrow",
"given": "Kath",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Matthews",
"given": "Julie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "McGarry",
"given": "Karen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Moore",
"given": "Vanessa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Smith",
"given": "Lucie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Summerfield",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Dark",
"given": "Paul",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Harvey",
"given": "Alice",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Doonan",
"given": "Reece",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "McMorrow",
"given": "Liam",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Knowles",
"given": "Karen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Pendlebury",
"given": "Jessica",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Lee",
"given": "Stephanie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Perez",
"given": "Jane",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Marsden",
"given": "Tracy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Taylor",
"given": "Melanie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Michael",
"given": "Angiy ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Collis",
"given": "Matthew",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Claxton",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Habeichi",
"given": "Wadih",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Horner",
"given": "Dan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Slaughter",
"given": "Melanie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Thomas",
"given": "Vicky",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Proudfoot",
"given": "Nicola",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Keatley",
"given": "Claire",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Donnison",
"given": "Phil",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Casey",
"given": "Ruth",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Irving",
"given": "Ben",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Matimba-Mupaya",
"given": "Wadzanai",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Reed",
"given": "Catherine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Anthony",
"given": "Alpha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Trim",
"given": "Fiona",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Cambalova",
"given": "Lenka",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Robertson",
"given": "Debra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Wilson",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hulme",
"given": "Jonathan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kannan",
"given": "Santhana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kinney",
"given": "Fiona",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Senya",
"given": "Ho",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hayes",
"given": "Anne",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ratnam",
"given": "Valli",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Gill",
"given": "Mandy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kirk",
"given": "Jill",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Shelton",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Schweikert",
"given": "Sacha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Wibrow",
"given": "Bradley",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Anstey",
"given": "Matthew",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Rauniyar",
"given": "Rashmi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Khoso",
"given": "Nasir",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Asif",
"given": "Namra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Taqdees",
"given": "Huda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Frey",
"given": "Christian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Scano",
"given": "Riccardo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "McKee",
"given": "Madeleine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Murphy",
"given": "Peter",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Thomas",
"given": "Matt",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Worner",
"given": "Ruth",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Faulkner",
"given": "Beverley",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Gendall",
"given": "Emma",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hayes",
"given": "Kati",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Blakemore",
"given": "Hayley",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Borislavova",
"given": "Borislava",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Deshpande",
"given": "Kush",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Konecny",
"given": "Pam",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Miller",
"given": "Jennene",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kintono",
"given": "Adeline",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Tung",
"given": "Raymond",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hayes",
"given": "Leanne",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Murphy",
"given": "Lorna",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Neill",
"given": "Andy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Reidy",
"given": "Bryan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "O’Dwyer",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ryan",
"given": "Donal",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ainscough",
"given": "Kate",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hamilton-Davies",
"given": "Colin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Chan",
"given": "Carmen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Mfuko",
"given": "Celina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Abbass",
"given": "Hakam",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Mandadapu",
"given": "Vineela",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Leaver",
"given": "Susannah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Patel",
"given": "Kamal",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Farnell-Ward",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Saluzzio",
"given": "Romina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Rawlins",
"given": "Sam",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Sicat",
"given": "Christine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "De Keulenaer",
"given": "Adrian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ferrier",
"given": "Janet",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Fysh",
"given": "Ed",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Dawda",
"given": "Ashlish",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Mevavala",
"given": "Bhaumik",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Cook",
"given": "Deborah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Clarke",
"given": "Frances",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Banach",
"given": "Dorota",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Fernández de Pinedo Artaraz",
"given": "Ziortza ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Cabreros",
"given": "Leilani",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Latham",
"given": "Victoria",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kruisselbrink",
"given": "Rebecca",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Brochard",
"given": "Laurent",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Burns",
"given": "Karen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Sandhu",
"given": "Gyan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Khalid ",
"given": "Imrana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "White",
"given": "Ian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Croft",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Holland",
"given": "Nicky",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Pereira",
"given": "Rita",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Nair",
"given": "Priya",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Buscher",
"given": "Hergen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Reynolds",
"given": "Claire",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Newman",
"given": "Sally",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Santamaria",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Barbazza",
"given": "Leanne",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Homes",
"given": "Jennifer",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Smith",
"given": "Roger",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Zaki",
"given": "Ahmed",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Johnson",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Garrard",
"given": "Hywel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Juhaz",
"given": "Vera",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Brown",
"given": "Louise",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Pemberton",
"given": "Abigail",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Roy",
"given": "Alistair",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Rostron",
"given": "Anthony",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Woods",
"given": "Lindsey",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Cornell",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Fowler",
"given": "Rob",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Adhikari",
"given": "Neill",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kamra",
"given": "Maneesha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Marinoff ",
"given": "Nicole",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Garrett",
"given": "Peter",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Murray",
"given": "Lauren",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Brailsford",
"given": "Jane",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Forbes",
"given": "Loretta",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Maguire",
"given": "Teena",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Fennessy",
"given": "Gerard",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Mulder",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Morgan",
"given": "Rebecca",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "McEldrew",
"given": "Rebecca",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Pillai",
"given": "Suresh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Harford",
"given": "Rachel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ivatt",
"given": "Helen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Evans",
"given": "Debra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Richards",
"given": "Suzanne",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Roberts",
"given": "Eilir",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bowen",
"given": "James",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ainsworth",
"given": "James",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kuitunen",
"given": "Anne",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Karlsson",
"given": "Sari",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Vahtera",
"given": "Annukka",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kiiski",
"given": "Heikki",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ristimäki",
"given": "Sanna",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Albrett",
"given": "Jonathan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Jackson",
"given": "Carolyn",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kirkham",
"given": "Simon",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Tamme",
"given": "Kadri",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Reinhard",
"given": "Veronika",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ellervee",
"given": "Anneli",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Põldots",
"given": "Liisi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Rennit",
"given": "Pille",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Svitškar",
"given": "Nikolai",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Browne",
"given": "Troy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Grimwade",
"given": "Kate",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Goodson",
"given": "Jennifer",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Keet",
"given": "Owen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Callender",
"given": "Owen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Udy",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "McCracken",
"given": "Phoebe",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Young",
"given": "Meredith",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Board",
"given": "Jasmin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Martin",
"given": "Emma",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kasipandian",
"given": "Vidya",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Patel",
"given": "Amit",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Allibone",
"given": "Suzanne",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Mary-Genetu",
"given": "Roman",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "English",
"given": "Shane",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Watpool",
"given": "Irene",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Porteous",
"given": "Rebecca",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Miezitis",
"given": "Sydney",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "McIntyre",
"given": "Lauralyn",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Brady",
"given": "Kara",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Vale",
"given": "Cassandra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Shekar",
"given": "Kiran",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Lavana",
"given": "Jayshree",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Parmar",
"given": "Dinesh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Peake",
"given": "Sandra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kurenda",
"given": "Catherine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hormis",
"given": "Anil",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Walker",
"given": "Rachel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Collier",
"given": "Dawn",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kimpton",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Oakley",
"given": "Susan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bhagani",
"given": "Sanjay",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "De Neef",
"given": "Mark",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Garcia",
"given": "Sara",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Maharajh",
"given": "Amitaa ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Nandani",
"given": "Aarti",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Dobson",
"given": "Jade",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Fernando",
"given": "Gloria",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Eastgate",
"given": "Christine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Gomez",
"given": "Keith",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Abdi",
"given": "Zakee",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Tatham",
"given": "Kate",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Jhanji",
"given": "Shaman",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Black",
"given": "Ethel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Dela Rosa",
"given": "Arnold",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Howle",
"given": "Ryan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Baikady",
"given": "Ravishankar",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Tully",
"given": "Redmond",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Drummond",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Dearden",
"given": "Joy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Philbin",
"given": "Jennifer",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Munt",
"given": "Sheila",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Gopal",
"given": "Shameer",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Pooni",
"given": "Jagtar- Singh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ganguly",
"given": "Saibal",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Smallwood",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Metherell",
"given": "Stella",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Naeem",
"given": "Anas",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Fagan",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ryan",
"given": "Emily",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Mariappa",
"given": "Vasanth",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Smith",
"given": "Judith",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Foulds",
"given": "Angela",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Revill",
"given": "Adam",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bhattarai",
"given": "Binita",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "de Jonge",
"given": "Evert",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Wigbers",
"given": "Jeanette",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "del Prado",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Cremer",
"given": "Olaf",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Mulier",
"given": "Jelle",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Peters",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Romberg",
"given": "Birgit",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Schutgens",
"given": "Roger",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Troeman",
"given": "Darren",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "van Opdorp",
"given": "Marjolein",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Besten",
"given": "Henny",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Brakké",
"given": "Karen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Barber",
"given": "Russell",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hilldrith",
"given": "Anette",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kluge",
"given": "Stefan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Nierhaus",
"given": "Axel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Jarczak",
"given": "Dominik",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Roedl ",
"given": "Kevin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kochanek",
"given": "Matthias",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Rueß-Paterno",
"given": "Giusi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Mc-Kenzie",
"given": "Josette",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Eichenauer",
"given": "Dennis",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Shimabukuro-Vornhagen",
"given": "Alexander",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Wilcox",
"given": "Elizabeth",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "del Sorbo",
"given": "Lorenzo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Abdelhady",
"given": "Hesham",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Romagnuolo ",
"given": "Tina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Simpson",
"given": "Scott",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Maiden",
"given": "Matthew",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bone",
"given": "Allison",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Horton",
"given": "Michelle",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Salerno",
"given": "Tania",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Krajinovic",
"given": "Vladimir",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kutleša",
"given": "Marko",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kotarski",
"given": "Viktor",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Brohi",
"given": "Farooq",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Jagannathan",
"given": "Vijay",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Clark",
"given": "Michele",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Purvis",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Wetherill",
"given": "Bill",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Brajković",
"given": "Ana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Babel",
"given": "Jakša",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Sever",
"given": "Helena",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Dragija",
"given": "Lidija",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kušan",
"given": "Ira",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Dushianthan",
"given": "Ahilanandan ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Cusack",
"given": "Rebecca",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "de Courcy-Golder",
"given": "Kim",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Salmon",
"given": "Karen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Burnish",
"given": "Rachel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Smith",
"given": "Simon",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Jackson",
"given": "Susan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ruiz",
"given": "Winningtom ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Duke",
"given": "Zoe",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Johns",
"given": "Magaret ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Male",
"given": "Michelle",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Gladas",
"given": "Kirsty",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Virdee",
"given": "Satwinder ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Swabe",
"given": "Jacqueline",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Tomlinson",
"given": "Helen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Rohde",
"given": "Gernot",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Grünewaldt",
"given": "Achim",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bojunga",
"given": "Jörg",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Petros",
"given": "Sirak",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kunz",
"given": "Kevin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Schütze",
"given": "Bianka",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Weismann",
"given": "Dirk",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Frey",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Drayss",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Goebeler",
"given": "M.E.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Flor",
"given": "Thomas",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Fragner",
"given": "Gertrud",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Wahl",
"given": "Nadine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Totzke",
"given": "Juliane",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Sayehli",
"given": "Cyrus",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bewley",
"given": "Jeremy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Sweet",
"given": "Katie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Grimmer",
"given": "Lisa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Johnson",
"given": "Rebekah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Wyatt",
"given": "Rachel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Morgan",
"given": "Karen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Varghese",
"given": "Siby",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Willis",
"given": "Joanna",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Stratton",
"given": "Emma",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kyle",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Putensen",
"given": "Daniel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Drury",
"given": "Kay",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Skorko",
"given": "Agnieszka",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bremmer",
"given": "Pamela",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ward",
"given": "Geraldine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bassford",
"given": "Christopher",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Sligl",
"given": "Wendy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Baig",
"given": "Nadia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Rewa",
"given": "Oleksa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bagshaw",
"given": "Sean",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Basile",
"given": "Kim",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Stavor",
"given": "Dara",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Burbee",
"given": "Dylan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "McNamara",
"given": "Amanda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Wunderley",
"given": "Renee",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bensen",
"given": "Nicole",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Richardson",
"given": "Aaron",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Adams",
"given": "Peter",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Vita",
"given": "Tina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Buhay",
"given": "Megan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Scholl",
"given": "Denise",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Gilliam",
"given": "Matthew",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Winters",
"given": "James",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Doherty",
"given": "Kaleigh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Berryman",
"given": "Emily",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ghaffari",
"given": "Mehrdad",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Marroquin",
"given": "Oscar",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Quinn",
"given": "Kevin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Garrard",
"given": "William",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kalchthaler",
"given": "Kyle",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Beard",
"given": "Gregory",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Skrtich",
"given": "Aimee",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bagavathy",
"given": "Kavitha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Drapola",
"given": "Debra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bryan-Morris",
"given": "Kayla",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Arnold",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Reynolds",
"given": "Bob",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hussain",
"given": "Mahwish",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Dunsavage",
"given": "Janice",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Saiyed",
"given": "Salim",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hernandez",
"given": "Erik",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Goldman",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Brown",
"given": "Cynthia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Comp",
"given": "Susan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Raczek",
"given": "James",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Morris",
"given": "Jenny",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Vargas Jr.",
"given": "Jesus",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Weiss",
"given": "Daniel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hensley",
"given": "Joseph",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kochert",
"given": "Erik",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Wnuk",
"given": "Chris",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Nemeth",
"given": "Christopher",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Mowery",
"given": "Brent",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hutchinson",
"given": "Christina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Winters",
"given": "Lauren",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "McAdams",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Walker",
"given": "Gena",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Minnier",
"given": "Tami",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Wisniewski",
"given": "Mary",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Mayak",
"given": "Katelyn",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "McCreary",
"given": "Erin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Martin",
"given": "Elise",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bariola",
"given": "Ryan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Viehman",
"given": "Alex",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Daley",
"given": "Jessica",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Lopus",
"given": "Alyssa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Schmidhofer",
"given": "Mark",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ambrosino",
"given": "Richard",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Keen",
"given": "Sherbrina ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Toffalo",
"given": "Sue",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Stambaugh",
"given": "Martha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Trimmer",
"given": "Ken",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Perri",
"given": "Reno",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Casali",
"given": "Sherry",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Medva",
"given": "Rebecca",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Massar",
"given": "Brent",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Beyerl",
"given": "Ashley",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Burkey",
"given": "Jason",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Keeler",
"given": "Sheryl",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Lowery",
"given": "Maryalyce",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Oncea",
"given": "Lynne",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Daugherty",
"given": "Jason",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Sevilla",
"given": "Chanthou",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Woelke",
"given": "Amy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Dice",
"given": "Julie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Weber",
"given": "Lisa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Roth",
"given": "Jason",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ferringer",
"given": "Cindy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Beer",
"given": "Deborah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Fesz",
"given": "Jessica",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Carpio",
"given": "Lillian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Colin",
"given": "Gwenhael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Zinzoni",
"given": "Vanessa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Maquigneau",
"given": "Natacha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Henri-Lagarrigue",
"given": "Matthieu",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Pouplet",
"given": "Caroline",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Reill",
"given": "Lorenz",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Distler",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Maselli",
"given": "Astrid",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Martynoga",
"given": "Robert",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Trask",
"given": "Kara",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Butler",
"given": "Amelia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Attwood",
"given": "Ben",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Parsons",
"given": "Penny",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Campbell",
"given": "Bridget",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Smith",
"given": "Alex",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Page",
"given": "Valerie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Zhao",
"given": "Xiao",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Oza",
"given": "Deepali",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Abrahamson",
"given": "Gail",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Sheath",
"given": "Ben",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ellis",
"given": "Chiara",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Young",
"given": "Paul",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Young",
"given": "Chelsea",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Lesona",
"given": "Eden",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Navarra",
"given": "Leanlove",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Cruz",
"given": "Raulle",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Delaney",
"given": "Kirsha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Cruz",
"given": "Rhoze",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Aguilar-Dano",
"given": "April",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Rhodes",
"given": "Jonathan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Anderson",
"given": "Thomas",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Morris",
"given": "Sheila",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Fuchs",
"given": "Ralph",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Lambert",
"given": "Bridget",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Tai",
"given": "Charlotte",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Thomas",
"given": "Amy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Keen",
"given": "Alexandra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Tierney",
"given": "Carey",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Omer",
"given": "Nimca",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bacon",
"given": "Gina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Tridente",
"given": "Ascanio",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Shuker",
"given": "Karen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Anders",
"given": "Jeanette",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Greer",
"given": "Sandra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Scott",
"given": "Paula",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Millington",
"given": "Amy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Buchanan",
"given": "Philip",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kirk",
"given": "Jodie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Binnie",
"given": "Alexandra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Powell",
"given": "Elizabeth",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "McMillan",
"given": "Alexandra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Luk",
"given": "Tracy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Aref",
"given": "Noah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Denmade",
"given": "Craig",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Sadera",
"given": "Girendra ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Jacob",
"given": "Reni",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Jones",
"given": "Cathy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hughes",
"given": "Debbie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Sterba",
"given": "Martin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Geng",
"given": "Wenli",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Digby",
"given": "Stephen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Southern",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Reddy",
"given": "Harsha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hulse",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Campbell",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Garton",
"given": "Mark",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Watkins",
"given": "Claire",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Smuts",
"given": "Sara",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Quinn",
"given": "Alison",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Simpson",
"given": "Benjamin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "McMillan",
"given": "Catherine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Finch",
"given": "Cheryl",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hill",
"given": "Claire",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Cooper",
"given": "Josh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Budd",
"given": "Joanna",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Small",
"given": "Charlotte",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "O’Leary",
"given": "Ryan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Birch",
"given": "Janine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Collins",
"given": "Emma",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Holland",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Alexander",
"given": "Peter",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Felton",
"given": "Tim",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ferguson",
"given": "Susan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Sellers",
"given": "Katharine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ward",
"given": "Luke",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Yates",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Birkinshaw",
"given": "Isobel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kell",
"given": "Kay",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Scott",
"given": "Zoe",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Pearson",
"given": "Harriet",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hashmi",
"given": "Madiha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ali",
"given": "Maryam",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hassan",
"given": "Noor",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Panjwani",
"given": "Ashok",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Umrani",
"given": "Zulfiqar",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Shaikh",
"given": "Mohiuddin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Siddiqui",
"given": "Ayesha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ain",
"given": "Quratul",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kanwal",
"given": "Darakhshan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "van Bree",
"given": "Sjoerd",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bouw-Ruiter",
"given": "Marianne",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Osinga",
"given": "Margreet",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": " van Zanten ",
"given": "Arthur",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Zammit ",
"given": "Claire",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Rashan",
"given": "Sumayyah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Azergui",
"given": "Nora",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Bari",
"given": "Sara",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Beltran",
"given": "Mercedes",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Brugman",
"given": "Curt",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Groeneveld",
"given": "Erika",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Jafarzadeh",
"given": "Mina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Keijzer-Timmers",
"given": "Nicole",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kester",
"given": "Esmee",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Koelink",
"given": "Maaike",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kwakkenbos-Craanen",
"given": "Marion",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Okundaye",
"given": "Clementina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Parker",
"given": "Lorraine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Peters",
"given": "Svenja",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Post",
"given": "Sophie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Rietveld",
"given": "Ilse",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Scheepstra-Beukers",
"given": "Irma",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Schreuder",
"given": "Gerwin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Smit ",
"given": "Albertine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Brillinger",
"given": "Nicole",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Markgraf",
"given": "René",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Eichinger",
"given": "Fred",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Doran",
"given": "Peter",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Anjum",
"given": "Aisha ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Best-Lane",
"given": "Janis",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Fagbodun",
"given": "Elizabeth",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Barton",
"given": "Frances",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Charles",
"given": "Walton",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Miller",
"given": "Lorna",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Parry-Billings",
"given": "Karen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Peters",
"given": "Sam",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Richards-Belle",
"given": "Alvin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Saull",
"given": "Michelle",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Sprinckmoller",
"given": "Stefan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Wiley",
"given": "Daisy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Darnell",
"given": "Robert",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Au",
"given": "Carly",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Stronach",
"given": "Lucy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Burrell",
"given": "Aidan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Forbes",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Heritier",
"given": "Stephane",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Saxena",
"given": "Manoj",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Trapani",
"given": "Tony",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Cuthbertson",
"given": "Brian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Manoharan",
"given": "Venika",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Dondrop",
"given": "Arjen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Jayakumar",
"given": "Deva",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ehrmann",
"given": "Stephan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hullegie",
"given": "Sebastiaan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Povoa",
"given": "Pedro",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Beasley",
"given": "Richard",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Daneman",
"given": "Nick",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Fowler",
"given": "Robert",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "McGloughlin",
"given": "Steve",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Paterson",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Venkatesh",
"given": "Bala",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "de Jong",
"given": "Menno",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Uyeki",
"given": "Tim",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Baillie",
"given": "Kenneth",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hashmi",
"given": "Madhia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Netea",
"given": "Mihai",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Orr",
"given": "Katrina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Patanwala",
"given": "Asad",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Tong",
"given": "Steve",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Cooper",
"given": "Nichola",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Galea",
"given": "James",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ogungbenro",
"given": "Kayode",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Patawala",
"given": "Asad",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Rademaker",
"given": "Emma",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Tong",
"given": "Steven",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Youngstein",
"given": "Taryn",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Fergusson",
"given": "Dean",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kumar",
"given": "Anand",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Laffan",
"given": "Mike",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Lother",
"given": "Sylvain",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "de Man",
"given": "Angelique",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Masse",
"given": "Marie-Helene",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Abraham",
"given": "Jacinta",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Arnold",
"given": "Donald",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Begin",
"given": "Phillipe",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Charlewood",
"given": "Richard",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Chasse",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Cooper",
"given": "Jamie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Coyne",
"given": "Mark",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Daly",
"given": "James",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Gosbell",
"given": "Iain",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Harvala-Simmonds",
"given": "Heli",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "MacLennan",
"given": "Sheila",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "McDyer",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Menon",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Pridee",
"given": "Nicole",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Roberts",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Thomas",
"given": "Helen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Tinmouth",
"given": "Alan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Triulzi",
"given": "Darrell",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Walsh",
"given": "Tim",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Wood",
"given": "Erica",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Calfee",
"given": "Carolyn",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "O’Kane",
"given": "Cecilia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Shyamsundar",
"given": "Murali",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Sinha",
"given": "Pratik",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Thompson",
"given": "Taylor",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Young",
"given": "Ian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ferguson",
"given": "Niall",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Hodgson",
"given": "Carol",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Orford",
"given": "Neil",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Phua",
"given": "Jason",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Baron",
"given": "Rebecca",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Epelman",
"given": "Slava ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Frankfurter",
"given": "Claudia ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Gommans",
"given": "Frank ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Kim",
"given": "Edy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Leaf",
"given": "David ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Vaduganathan",
"given": "Muthiah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "van Kimmenade",
"given": "Roland",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Sanil",
"given": "Ashish",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "van Beurden",
"given": "Marloes",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Effelaar",
"given": "Evelien",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Schotsman",
"given": "Joost",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Boyd",
"given": "Craig",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Harland",
"given": "Cain",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Shearer",
"given": "Audrey",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Wren",
"given": "Jess",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Clermont",
"given": "Giles",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ricketts",
"given": "Daniel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Attanayaka",
"given": "Udara",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Darshana",
"given": "Sri",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Ishani",
"given": "Pramodya",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "REMAP-CAP Writing Committee for the REMAP-CAP Investigators"
}
],
"family": "Udayanga",
"given": "Ishara ",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Bristol, Bristol, England"
}
],
"family": "Bradbury",
"given": "Charlotte A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Peter Munk Cardiac Centre at University Health Network, Toronto, Ontario, Canada"
},
{
"name": "University of Toronto, Toronto, Ontario, Canada"
}
],
"family": "Lawler",
"given": "Patrick R.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Oxford, Oxford, England"
},
{
"name": "NHS Blood and Transplant, Oxford, England"
}
],
"family": "Stanworth",
"given": "Simon J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Pittsburgh, Pittsburgh, Pennsylvania"
}
],
"family": "McVerry",
"given": "Bryan J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Monash University, Melbourne, Victoria, Australia"
},
{
"name": "Monash Health, Melbourne, Victoria, Australia"
}
],
"family": "McQuilten",
"given": "Zoe",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Monash University, Melbourne, Victoria, Australia"
}
],
"family": "Higgins",
"given": "Alisa M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Intensive Care National Audit and Research Centre (ICNARC), London, England"
}
],
"family": "Mouncey",
"given": "Paul R.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Imperial College London, London, England"
}
],
"family": "Al-Beidh",
"given": "Farah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Intensive Care National Audit and Research Centre (ICNARC), London, England"
}
],
"family": "Rowan",
"given": "Kathryn M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Berry Consultants, Austin, Texas"
}
],
"family": "Berry",
"given": "Lindsay R.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Berry Consultants, Austin, Texas"
}
],
"family": "Lorenzi",
"given": "Elizabeth",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Manitoba, Winnipeg, Canada"
}
],
"family": "Zarychanski",
"given": "Ryan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "King Saud bin Abdulaziz University for Health Sciences and King Abdullah International Medical Research Center, Riyadh, Saudi Arabia"
}
],
"family": "Arabi",
"given": "Yaseen M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Hospital Raymond Poincaré (Assistance Publique Hôpitaux de Paris), Garches, France"
},
{
"name": "Université Versailles SQY–Université Paris Saclay, Montigny-le-Bretonneux, France"
}
],
"family": "Annane",
"given": "Djillali",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Oxford, Oxford, England"
}
],
"family": "Beane",
"given": "Abi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University Medical Center Utrecht, Utrecht, the Netherlands"
}
],
"family": "van Bentum-Puijk",
"given": "Wilma",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "St Michael’s Hospital Unity Health, Toronto, Ontario, Canada"
}
],
"family": "Bhimani",
"given": "Zahra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Flinders University, Bedford Park, South Australia, Australia"
}
],
"family": "Bihari",
"given": "Shailesh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University Medical Center Utrecht, Utrecht, the Netherlands"
}
],
"family": "Bonten",
"given": "Marc J. M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Jena University Hospital, Jena, Germany"
}
],
"family": "Brunkhorst",
"given": "Frank M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Monash University, Melbourne, Victoria, Australia"
}
],
"family": "Buzgau",
"given": "Adrian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Global Coalition for Adaptive Research, Los Angeles, California"
}
],
"family": "Buxton",
"given": "Meredith",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Ottawa Hospital Research Institute, Ottawa, Ontario, Canada"
},
{
"name": "Institut du Savoir Montfort, Ottawa, Ontario, Canada"
}
],
"family": "Carrier",
"given": "Marc",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Monash University, Melbourne, Victoria, Australia"
},
{
"name": "Alfred Health, Melbourne, Victoria, Australia"
}
],
"family": "Cheng",
"given": "Allen C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Yong Loo Lin School of Medicine, National University of Singapore, Singapore"
}
],
"family": "Cove",
"given": "Matthew",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Berry Consultants, Austin, Texas"
}
],
"family": "Detry",
"given": "Michelle A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "NHS Blood and Transplant, Oxford, England"
}
],
"family": "Estcourt",
"given": "Lise J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Berry Consultants, Austin, Texas"
}
],
"family": "Fitzgerald",
"given": "Mark",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Pittsburgh, Pittsburgh, Pennsylvania"
}
],
"family": "Girard",
"given": "Timothy D.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Peter Munk Cardiac Centre at University Health Network, Toronto, Ontario, Canada"
},
{
"name": "University of Toronto, Toronto, Ontario, Canada"
}
],
"family": "Goligher",
"given": "Ewan C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Antwerp, Wilrijk, Belgium"
}
],
"family": "Goossens",
"given": "Herman",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Oxford, Bangkok, Thailand"
},
{
"name": "National Intensive Care Surveillance (NICST), Colombo, Sri Lanka"
}
],
"family": "Haniffa",
"given": "Rashan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Medical Research Institute of New Zealand (MRINZ), Wellington, New Zealand"
}
],
"family": "Hills",
"given": "Thomas",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Pittsburgh, Pittsburgh, Pennsylvania"
}
],
"family": "Huang",
"given": "David T.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "UPMC Children’s Hospital of Pittsburgh, Pittsburgh, Pennsylvania"
}
],
"family": "Horvat",
"given": "Christopher M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Kings Healthcare Partners, London, England"
}
],
"family": "Hunt",
"given": "Beverley J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "The University of Tokyo, Tokyo, Japan"
}
],
"family": "Ichihara",
"given": "Nao",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Université de Sherbrooke, Sherbrooke, Québec, Canada"
}
],
"family": "Lamontagne",
"given": "Francois",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University Medical Center Utrecht, Utrecht, the Netherlands"
}
],
"family": "Leavis",
"given": "Helen L.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Pittsburgh, Pittsburgh, Pennsylvania"
}
],
"family": "Linstrum",
"given": "Kelsey M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Fiona Stanley Hospital, Perth, Western Australia, Australia"
},
{
"name": "University of Western Australia, Perth, Australia"
}
],
"family": "Litton",
"given": "Edward",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "St Michael’s Hospital Unity Health, Toronto, Ontario, Canada"
}
],
"family": "Marshall",
"given": "John C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Queen’s University Belfast, Belfast, Northern Ireland"
},
{
"name": "Royal Victoria Hospital, Belfast, Northern Ireland"
}
],
"family": "McAuley",
"given": "Daniel F.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Berry Consultants, Austin, Texas"
}
],
"family": "McGlothlin",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Monash University, Melbourne, Victoria, Australia"
},
{
"name": "Auckland City Hospital, Auckland, New Zealand"
}
],
"family": "McGuinness",
"given": "Shay P.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Radboud University Medical Center, Nijmegen, the Netherlands"
}
],
"family": "Middeldorp",
"given": "Saskia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Pittsburgh, Pittsburgh, Pennsylvania"
}
],
"family": "Montgomery",
"given": "Stephanie K.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Middlemore Hospital, Auckland, New Zealand"
}
],
"family": "Morpeth",
"given": "Susan C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of British Columbia, Vancouver, Canada"
}
],
"family": "Murthy",
"given": "Srinivas",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Pittsburgh, Pittsburgh, Pennsylvania"
}
],
"family": "Neal",
"given": "Matthew D.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Monash University, Melbourne, Victoria, Australia"
},
{
"name": "University College Dublin, Dublin, Ireland"
}
],
"family": "Nichol",
"given": "Alistair D.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Auckland City Hospital, Auckland, New Zealand"
},
{
"name": "University of Auckland, Auckland, New Zealand"
}
],
"family": "Parke",
"given": "Rachael L.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Monash University, Melbourne, Victoria, Australia"
}
],
"family": "Parker",
"given": "Jane C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Universidad de La Sabana, Chia, Colombia"
},
{
"name": "Clinica Universidad de La Sabana, Chia, Colombia"
}
],
"family": "Reyes",
"given": "Luis F.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "St Marianna University School of Medicine, Yokohama City Seibu Hospital, Yokohama, Japan"
}
],
"family": "Saito",
"given": "Hiroki",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "St Michael’s Hospital Unity Health, Toronto, Ontario, Canada"
}
],
"family": "Santos",
"given": "Marlene S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Berry Consultants, Austin, Texas"
}
],
"family": "Saunders",
"given": "Christina T.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Monash University, Melbourne, Victoria, Australia"
},
{
"name": "Hospital Israelita Albert Einstein, Sao Paulo, Brazil"
}
],
"family": "Serpa-Neto",
"given": "Ary",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Pittsburgh, Pittsburgh, Pennsylvania"
}
],
"family": "Seymour",
"given": "Christopher W.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "King’s College London, London, England"
},
{
"name": "Guy’s and St Thomas’ NHS Foundation Trust, London, England"
}
],
"family": "Shankar-Hari",
"given": "Manu",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Monash University, Melbourne, Victoria, Australia"
}
],
"family": "Singh",
"given": "Vanessa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "National Intensive Care Surveillance (NICST), Colombo, Sri Lanka"
}
],
"family": "Tolppa",
"given": "Timo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Université Laval, Québec City, Québec, Canada"
},
{
"name": "CHU de Québec–Université Laval Research Center, Québec City, Québec, Canada"
}
],
"family": "Turgeon",
"given": "Alexis F.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Medical Research Institute of New Zealand (MRINZ), Wellington, New Zealand"
}
],
"family": "Turner",
"given": "Anne M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Radboud University Medical Center, Nijmegen, the Netherlands"
}
],
"family": "van de Veerdonk",
"given": "Frank L.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Monash University, Melbourne, Victoria, Australia"
}
],
"family": "Green",
"given": "Cameron",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Berry Consultants, Austin, Texas"
},
{
"name": "Harbor-UCLA Medical Center, Torrance, California"
}
],
"family": "Lewis",
"given": "Roger J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Pittsburgh, Pittsburgh, Pennsylvania"
}
],
"family": "Angus",
"given": "Derek C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Auckland City Hospital, Auckland, New Zealand"
}
],
"family": "McArthur",
"given": "Colin J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Berry Consultants, Austin, Texas"
}
],
"family": "Berry",
"given": "Scott",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University Medical Center Utrecht, Utrecht, the Netherlands"
}
],
"family": "Derde",
"given": "Lennie P. G.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Monash University, Melbourne, Victoria, Australia"
},
{
"name": "St John of God Hospital, Subiaco, Western Australia, Australia"
}
],
"family": "Webb",
"given": "Steve A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Imperial College London, London, England"
},
{
"name": "Imperial College Healthcare NHS Trust, St Mary’s Hospital, London, England"
}
],
"family": "Gordon",
"given": "Anthony C.",
"sequence": "additional"
}
],
"container-title": "JAMA",
"container-title-short": "JAMA",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
3,
22
]
],
"date-time": "2022-03-22T09:31:02Z",
"timestamp": 1647941462000
},
"deposited": {
"date-parts": [
[
2022,
4,
5
]
],
"date-time": "2022-04-05T15:02:39Z",
"timestamp": 1649170959000
},
"indexed": {
"date-parts": [
[
2024,
3,
29
]
],
"date-time": "2024-03-29T10:33:04Z",
"timestamp": 1711708384809
},
"is-referenced-by-count": 79,
"issue": "13",
"issued": {
"date-parts": [
[
2022,
4,
5
]
]
},
"journal-issue": {
"issue": "13",
"published-print": {
"date-parts": [
[
2022,
4,
5
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://jamanetwork.com/journals/jama/articlepdf/2790488/jama_bradbury_2022_oi_220025_1648650455.00328.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "10",
"original-title": [],
"page": "1247",
"prefix": "10.1001",
"published": {
"date-parts": [
[
2022,
4,
5
]
]
},
"published-print": {
"date-parts": [
[
2022,
4,
5
]
]
},
"publisher": "American Medical Association (AMA)",
"reference": [
{
"DOI": "10.1016/j.jacc.2020.04.031",
"article-title": "COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review.",
"author": "Bikdeli",
"doi-asserted-by": "publisher",
"first-page": "2950",
"issue": "23",
"journal-title": "J Am Coll Cardiol",
"key": "joi220025r1",
"volume": "75",
"year": "2020"
},
{
"DOI": "10.1007/s00134-020-06062-x",
"article-title": "High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study.",
"author": "Helms",
"doi-asserted-by": "publisher",
"first-page": "1089",
"issue": "6",
"journal-title": "Intensive Care Med",
"key": "joi220025r2",
"volume": "46",
"year": "2020"
},
{
"DOI": "10.1016/j.thromres.2020.04.013",
"article-title": "Incidence of thrombotic complications in critically ill ICU patients with COVID-19.",
"author": "Klok",
"doi-asserted-by": "publisher",
"first-page": "145",
"journal-title": "Thromb Res",
"key": "joi220025r3",
"volume": "191",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.13372",
"article-title": "Thrombosis in hospitalized patients with COVID-19 in a New York City health system.",
"author": "Bilaloglu",
"doi-asserted-by": "publisher",
"first-page": "799",
"issue": "8",
"journal-title": "JAMA",
"key": "joi220025r4",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1182/blood.2020006520",
"article-title": "COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection.",
"author": "Al-Samkari",
"doi-asserted-by": "publisher",
"first-page": "489",
"issue": "4",
"journal-title": "Blood",
"key": "joi220025r5",
"volume": "136",
"year": "2020"
},
{
"DOI": "10.1186/s13054-020-03260-3",
"article-title": "Thrombotic and haemorrhagic complications in critically ill patients with COVID-19: a multicentre observational study.",
"author": "Shah",
"doi-asserted-by": "publisher",
"first-page": "561",
"issue": "1",
"journal-title": "Crit Care",
"key": "joi220025r6",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1111/jth.v18.8",
"article-title": "Incidence of venous thromboembolism in hospitalized patients with COVID-19.",
"author": "Middeldorp",
"doi-asserted-by": "publisher",
"first-page": "1995",
"issue": "8",
"journal-title": "J Thromb Haemost",
"key": "joi220025r7",
"volume": "18",
"year": "2020"
},
{
"DOI": "10.1111/bjh.v194.3",
"article-title": "COVID-19 and immunothrombosis: emerging understanding and clinical management.",
"author": "Shaw",
"doi-asserted-by": "publisher",
"first-page": "518",
"issue": "3",
"journal-title": "Br J Haematol",
"key": "joi220025r8",
"volume": "194",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2105911",
"article-title": "Therapeutic anticoagulation with heparin in noncritically ill patients with Covid-19.",
"author": "Lawler",
"doi-asserted-by": "crossref",
"first-page": "790",
"issue": "9",
"journal-title": "N Engl J Med",
"key": "joi220025r9",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2103417",
"article-title": "Therapeutic anticoagulation with heparin in critically ill patients with Covid-19.",
"author": "Goligher",
"doi-asserted-by": "crossref",
"first-page": "777",
"issue": "9",
"journal-title": "N Engl J Med",
"key": "joi220025r10",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1182/blood.2020007214",
"article-title": "Platelet gene expression and function in patients with COVID-19.",
"author": "Manne",
"doi-asserted-by": "publisher",
"first-page": "1317",
"issue": "11",
"journal-title": "Blood",
"key": "joi220025r11",
"volume": "136",
"year": "2020"
},
{
"DOI": "10.1161/CIRCRESAHA.120.317703",
"article-title": "Platelets can associate with SARS-CoV-2 RNA and are hyperactivated in COVID-19.",
"author": "Zaid",
"doi-asserted-by": "publisher",
"first-page": "1404",
"issue": "11",
"journal-title": "Circ Res",
"key": "joi220025r12",
"volume": "127",
"year": "2020"
},
{
"DOI": "10.7326/M20-2003",
"article-title": "Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study.",
"author": "Wichmann",
"doi-asserted-by": "publisher",
"first-page": "268",
"issue": "4",
"journal-title": "Ann Intern Med",
"key": "joi220025r13",
"volume": "173",
"year": "2020"
},
{
"DOI": "10.7326/M20-2566",
"article-title": "Pulmonary arterial thrombosis in COVID-19 with fatal outcome: results from a prospective, single-center, clinicopathologic case series.",
"author": "Lax",
"doi-asserted-by": "publisher",
"first-page": "350",
"issue": "5",
"journal-title": "Ann Intern Med",
"key": "joi220025r14",
"volume": "173",
"year": "2020"
},
{
"DOI": "10.1016/j.eclinm.2020.100434",
"article-title": "Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: a case series.",
"author": "Rapkiewicz",
"doi-asserted-by": "crossref",
"journal-title": "EClinicalMedicine",
"key": "joi220025r15",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.17022",
"article-title": "Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial.",
"author": "Angus",
"doi-asserted-by": "crossref",
"first-page": "1317",
"issue": "13",
"journal-title": "JAMA",
"key": "joi220025r16",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1007/s00134-021-06448-5",
"article-title": "Lopinavir-ritonavir and hydroxychloroquine for critically ill patients with COVID-19: REMAP-CAP randomized controlled trial.",
"author": "Arabi",
"doi-asserted-by": "publisher",
"first-page": "867",
"issue": "8",
"journal-title": "Intensive Care Med",
"key": "joi220025r17",
"volume": "47",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2100433",
"article-title": "Interleukin-6 receptor antagonists in critically ill patients with Covid-19.",
"author": "Gordon",
"doi-asserted-by": "crossref",
"first-page": "1491",
"issue": "16",
"journal-title": "N Engl J Med",
"key": "joi220025r18",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1001/jama.2021.18178",
"article-title": "Effect of convalescent plasma on organ support–free days in critically ill patients with COVID-19: a randomized clinical trial.",
"author": "Estcourt",
"doi-asserted-by": "crossref",
"first-page": "1690",
"issue": "17",
"journal-title": "JAMA",
"key": "joi220025r19",
"volume": "326",
"year": "2021"
},
{
"DOI": "10.1513/AnnalsATS.202003-192SD",
"article-title": "The REMAP-CAP (Randomized Embedded Multifactorial Adaptive Platform for Community-Acquired Pneumonia) study: rationale and design.",
"author": "Angus",
"doi-asserted-by": "publisher",
"first-page": "879",
"issue": "7",
"journal-title": "Ann Am Thorac Soc",
"key": "joi220025r20",
"volume": "17",
"year": "2020"
},
{
"DOI": "10.1001/jama.2013.281053",
"article-title": "World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects.",
"author": "World Medical Association",
"doi-asserted-by": "publisher",
"first-page": "2191",
"issue": "20",
"journal-title": "JAMA",
"key": "joi220025r21",
"volume": "310",
"year": "2013"
},
{
"DOI": "10.1016/S0140-6736(21)01825-0",
"article-title": "Aspirin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial.",
"author": "Group",
"doi-asserted-by": "crossref",
"first-page": "143",
"issue": "10320",
"journal-title": "Lancet",
"key": "joi220025r22",
"volume": "399",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(20)30937-5",
"article-title": "Endothelial cell infection and endotheliitis in COVID-19.",
"author": "Varga",
"doi-asserted-by": "publisher",
"first-page": "1417",
"issue": "10234",
"journal-title": "Lancet",
"key": "joi220025r23",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/j.thromres.2020.04.014",
"article-title": "Severe COVID-19 infection associated with endothelial activation.",
"author": "Escher",
"doi-asserted-by": "publisher",
"first-page": "62",
"journal-title": "Thromb Res",
"key": "joi220025r24",
"volume": "190",
"year": "2020"
},
{
"DOI": "10.1016/S2352-3026(20)30216-7",
"article-title": "Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study.",
"author": "Goshua",
"doi-asserted-by": "publisher",
"first-page": "e575",
"issue": "8",
"journal-title": "Lancet Haematol",
"key": "joi220025r25",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1182/blood.2020007008",
"article-title": "Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome.",
"author": "Middleton",
"doi-asserted-by": "publisher",
"first-page": "1169",
"issue": "10",
"journal-title": "Blood",
"key": "joi220025r26",
"volume": "136",
"year": "2020"
},
{
"DOI": "10.1016/S2352-3026(20)30215-5",
"article-title": "Endothelial cells orchestrate COVID-19 coagulopathy.",
"author": "O’Sullivan",
"doi-asserted-by": "publisher",
"first-page": "e553",
"issue": "8",
"journal-title": "Lancet Haematol",
"key": "joi220025r27",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1503/cmaj.201240",
"article-title": "Anticipating and managing coagulopathy and thrombotic manifestations of severe COVID-19.",
"author": "Godoy",
"doi-asserted-by": "publisher",
"first-page": "E1156",
"issue": "40",
"journal-title": "CMAJ",
"key": "joi220025r28",
"volume": "192",
"year": "2020"
},
{
"DOI": "10.1111/jth.v18.7",
"article-title": "Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis.",
"author": "Panigada",
"doi-asserted-by": "publisher",
"first-page": "1738",
"issue": "7",
"journal-title": "J Thromb Haemost",
"key": "joi220025r29",
"volume": "18",
"year": "2020"
},
{
"DOI": "10.1111/jth.v18.7",
"article-title": "The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome.",
"author": "Ranucci",
"doi-asserted-by": "publisher",
"first-page": "1747",
"issue": "7",
"journal-title": "J Thromb Haemost",
"key": "joi220025r30",
"volume": "18",
"year": "2020"
},
{
"DOI": "10.1111/jth.v18.4",
"article-title": "Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia.",
"author": "Tang",
"doi-asserted-by": "publisher",
"first-page": "844",
"issue": "4",
"journal-title": "J Thromb Haemost",
"key": "joi220025r31",
"volume": "18",
"year": "2020"
},
{
"DOI": "10.1016/j.lfs.2020.118617",
"article-title": "Preventing the development of severe COVID-19 by modifying immunothrombosis.",
"author": "Morris",
"doi-asserted-by": "crossref",
"journal-title": "Life Sci",
"key": "joi220025r32",
"volume": "264",
"year": "2021"
},
{
"DOI": "10.1159/000453002",
"article-title": "Immunothrombosis in acute respiratory distress syndrome: cross talks between inflammation and coagulation.",
"author": "Frantzeskaki",
"doi-asserted-by": "publisher",
"first-page": "212",
"issue": "3",
"journal-title": "Respiration",
"key": "joi220025r33",
"volume": "93",
"year": "2017"
},
{
"DOI": "10.1111/bjh.v192.3",
"article-title": "Debate: should the dose or duration of anticoagulants for the prevention of venous thrombosis be increased in patients with COVID-19 while we are awaiting the results of clinical trials?",
"author": "Gomez",
"doi-asserted-by": "publisher",
"first-page": "459",
"issue": "3",
"journal-title": "Br J Haematol",
"key": "joi220025r34",
"volume": "192",
"year": "2021"
},
{
"DOI": "10.1136/bmj.n2400",
"article-title": "Effectiveness of therapeutic heparin vs prophylactic heparin on death, mechanical ventilation, or intensive care unit admission in moderately ill patients with COVID-19 admitted to hospital: RAPID randomised clinical trial.",
"author": "Sholzberg",
"doi-asserted-by": "crossref",
"first-page": "n2400",
"issue": "2400",
"journal-title": "BMJ",
"key": "joi220025r35",
"volume": "375",
"year": "2021"
},
{
"DOI": "10.1001/jamainternmed.2021.6203",
"article-title": "Efficacy and safety of therapeutic-dose heparin vs standard prophylactic or intermediate-dose heparins for thromboprophylaxis in high-risk hospitalized patients with COVID-19: the HEP-COVID randomized clinical trial.",
"author": "Spyropoulos",
"doi-asserted-by": "publisher",
"first-page": "1612",
"issue": "12",
"journal-title": "JAMA Intern Med",
"key": "joi220025r36",
"volume": "181",
"year": "2021"
},
{
"DOI": "10.1001/jama.2021.4152",
"article-title": "Effect of intermediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit: the INSPIRATION randomized clinical trial.",
"author": "Sadeghipour",
"doi-asserted-by": "crossref",
"first-page": "1620",
"issue": "16",
"journal-title": "JAMA",
"key": "joi220025r37",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(21)01203-4",
"article-title": "Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial.",
"author": "Lopes",
"doi-asserted-by": "publisher",
"first-page": "2253",
"issue": "10291",
"journal-title": "Lancet",
"key": "joi220025r38",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1016/j.thromres.2013.07.002",
"article-title": "Use of antiplatelet agents in sepsis: a glimpse into the future.",
"author": "Akinosoglou",
"doi-asserted-by": "publisher",
"first-page": "131",
"issue": "2",
"journal-title": "Thromb Res",
"key": "joi220025r39",
"volume": "133",
"year": "2014"
},
{
"DOI": "10.1213/ANE.0000000000005292",
"article-title": "Aspirin use is associated with decreased mechanical ventilation, intensive care unit admission, and in-hospital mortality in hospitalized patients with coronavirus disease 2019.",
"author": "Chow",
"doi-asserted-by": "publisher",
"first-page": "930",
"issue": "4",
"journal-title": "Anesth Analg",
"key": "joi220025r40",
"volume": "132",
"year": "2021"
},
{
"DOI": "10.1111/his.v77.2",
"article-title": "Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction.",
"author": "Menter",
"doi-asserted-by": "publisher",
"first-page": "198",
"issue": "2",
"journal-title": "Histopathology",
"key": "joi220025r41",
"volume": "77",
"year": "2020"
},
{
"DOI": "10.1001/jama.2021.23605",
"article-title": "Effect of P2Y12 inhibitors on survival free of organ support among non–critically ill hospitalized patients with COVID-19: a randomized clinical trial.",
"author": "Berger",
"doi-asserted-by": "publisher",
"first-page": "227",
"issue": "3",
"journal-title": "JAMA",
"key": "joi220025r42",
"volume": "327",
"year": "2022"
},
{
"DOI": "10.1001/jama.2021.23866",
"article-title": "Antiplatelet therapy in patients with COVID-19—more is less?",
"author": "Spaetgens",
"doi-asserted-by": "publisher",
"first-page": "223",
"issue": "3",
"journal-title": "JAMA",
"key": "joi220025r43",
"volume": "327",
"year": "2022"
}
],
"reference-count": 43,
"references-count": 43,
"relation": {},
"resource": {
"primary": {
"URL": "https://jamanetwork.com/journals/jama/fullarticle/2790488"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [
"A Randomized Clinical Trial"
],
"title": "Effect of Antiplatelet Therapy on Survival and Organ Support–Free Days in Critically Ill Patients With COVID-19",
"type": "journal-article",
"volume": "327"
}