Virucidal and antiviral activity of astodrimer sodium against SARS-CoV-2 in vitro
et al., Antiviral Research, doi:10.1016/j.antiviral.2021.105089, Jul 2021
In vitro study showing potent antiviral and virucidal activity of astodrimer sodium against SARS-CoV-2.
3 preclinical studies support the efficacy of astodrimer sodium for COVID-19:
1 in vivo animal study3
1.
Paull et al., Astodrimer sodium nasal spray forms a barrier to SARS-CoV-2 in vitro and preserves normal mucociliary function in human nasal epithelium, Scientific Reports, doi:10.1038/s41598-024-72262-w.
Paull et al., 31 Jul 2021, USA, peer-reviewed, 7 authors.
Contact: jeremy.paull@starpharma.com.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Virucidal and antiviral activity of astodrimer sodium against SARS-CoV-2 in vitro
Antiviral Research, doi:10.1016/j.antiviral.2021.105089
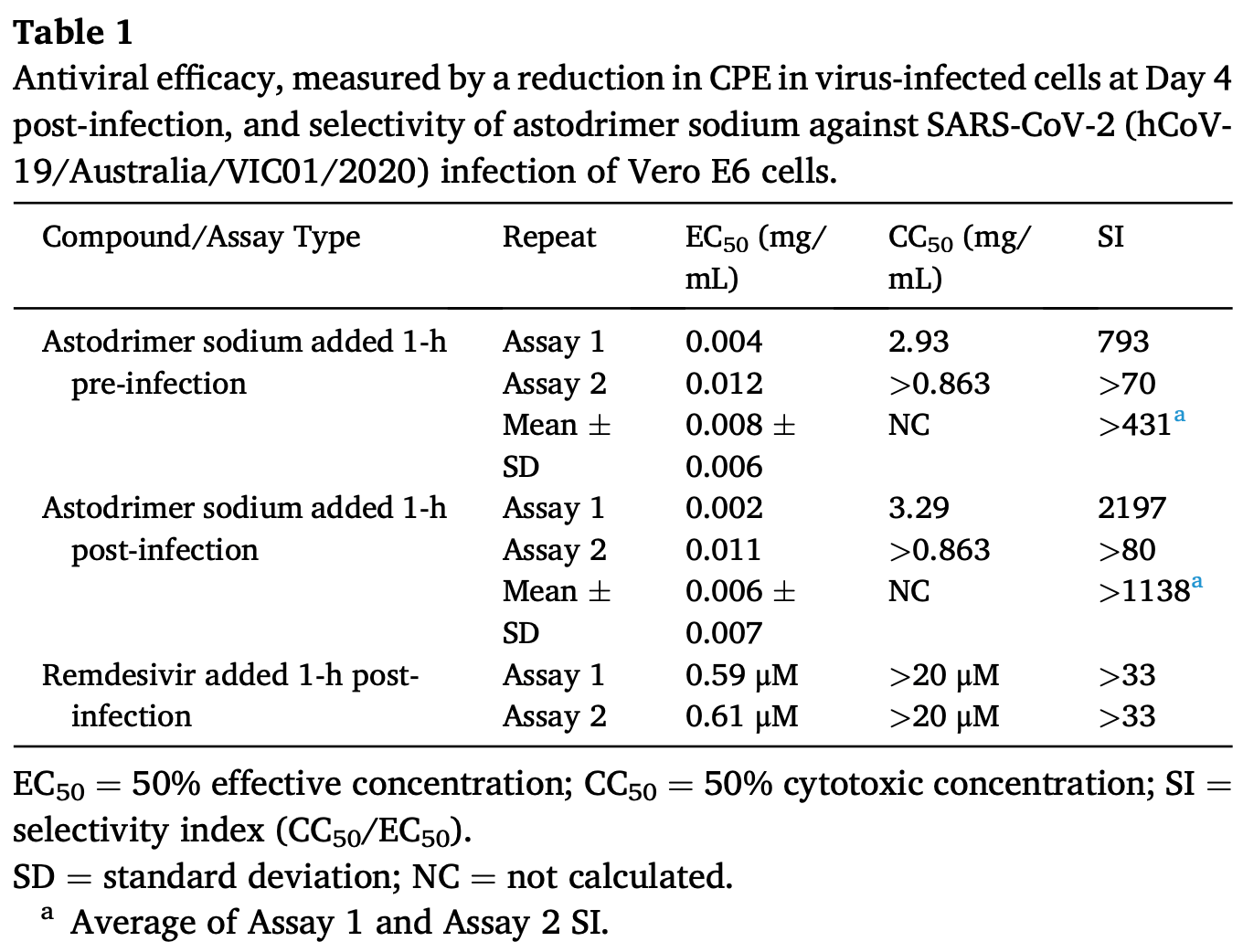
An effective response to the ongoing coronavirus disease (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) will involve a range of complementary preventive modalities. The current studies were conducted to evaluate the in vitro SARS-CoV-2 antiviral and virucidal (irreversible) activity of astodrimer sodium, a dendrimer with broad spectrum antimicrobial activity, including against enveloped viruses in in vitro and in vivo models, that is marketed for antiviral and antibacterial applications. We report that astodrimer sodium inhibits replication of SARS-CoV-2 in Vero E6 and Calu-3 cells, with 50% effective concentrations (EC 50 ) for i) reducing virus-induced cytopathic effect of 0.002-0.012 mg/mL in Vero E6 cells, and ii) infectious virus release by plaque assay of 0.019-0.032 mg/mL in Vero E6 cells and 0.030-0.037 mg/mL in Calu-3 cells. The selectivity index (SI) in these assays was as high as 2197. Astodrimer sodium was also virucidal, irreversibly reducing SARS-CoV-2 infectivity by >99.9% (>3 log 10 ) within 1 min of exposure, and up to >99.999% (>5 log 10 ) shown at astodrimer sodium concentrations of 10-30 mg/mL in Vero E6 and Calu-3 cell lines. Astodrimer sodium also inhibited infection in a primary human airway epithelial cell line. The data were similar for all investigations and were consistent with the potent antiviral and virucidal activity of astodrimer sodium being due to irreversible inhibition of virus-host cell interactions, as previously demonstrated for other viruses. Further studies will confirm if astodrimer sodium binds to SARS-CoV-2 spike protein and physically blocks initial attachment of the virus to the host cell. Given the in vitro effectiveness and significantly high SI, astodrimer sodium warrants further investigation for potential as a topically administered agent for SARS-CoV-2 therapeutic applications.
Glossary
References
Bansal, Jonsson, Taylor, Figueroa, Vanesa et al., Iota-carrageenan and xylitol inhibit SARS-CoV-2 in cell culture, bioRxiv, doi:10.1101/2020.08.19.225854
Bernstein, Stanberry, Sacks, Ayisi, Gong et al., Evaluations of unformulated and formulated dendrimer-based microbicide candidates in mouse and Guinea pig models of genital herpes, Antimicrob. Agents Chemother, doi:10.1128/aac.47.12.3784-3788.2003
Chavoustie, Carter, Waldbaum, Donders, Peters et al., Two phase 3, double-blinded, placebo-controlled studies of the efficacy and safety of Astodrimer 1% Gel for the treatment of bacterial vaginosis, Eur. J. Obstet. Gynecol. Reprod. Biol, doi:10.1016/j.ejogrb.2019.11.032
Chen, Millwood, Wand, Poynten, Law et al., A randomized controlled trial of the safety of candidate microbicide SPL7013 gel when applied to the penis, J. Acquir. Immune Defic. Syndr, doi:10.1097/QAI.0b013e318198a7e6
Connell, Lortat-Jacob, Human immunodeficiency virus and heparan sulfate: from attachment to entry inhibition, Front. Immunol, doi:10.3389/fimmu.2013.00385
Ghosh, Chattopadhyay, Marschall, Karmakar, Mandal et al., Focus on antivirally active sulfated polysaccharides: from structure-activity analysis to clinical evaluation, Glycobiology, doi:10.1093/glycob/cwn092
Gong, Matthews, Mccarthy, Chu, Holan et al., Evaluation of dendrimer SPL7013, a lead microbicide candidate against herpes simplex virus, Antivir. Res, doi:10.1016/j.antiviral.2005.08.004
Hoffmann, Kleine-Weber, Pöhlmann, A multibasic cleavage site in the spike protein of SARS-CoV-2 is essential for infection of human lung cells, Mol. Cell, doi:10.1016/j.molcel.2020.04.022
Hoffmann, Kleine-Weber, Schroeder, Krüger, Herrler et al., SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor, Cell, doi:10.1016/j.cell.2020.02.052
Hou, Okuda, Edwards, SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract, Cell, doi:10.1016/j.cell.2020.05.042
Jiang, Emau, Cairns, Flanary, Morton et al., SPL7013 gel as a topical microbicide for prevention of vaginal transmission of SHIV 89.6P in macaques, AIDS Res. Hum. Retrovir, doi:10.1089/aid.2005.21.207
Korber, Fischer, Gnanakaran, Yoon, Theiler et al., Spike mutation pipeline reveals the emergence of a more transmissible form of SARS-CoV-2, bioRxiv, doi:10.1101/2020.04.29.069054
Lackman-Smith, Osterling, Luckenbaugh, Mankowski, Snyder et al., Development of a comprehensive human immunodeficiency virus type-1 screening algorithm for discovery and preclinical testing of topical microbicides, Antimicrob. Agents Chemother, doi:10.1128/AAC.01328-07
Lang, Yang, Deng, Liu, Yang et al., Inhibition of SARS pseudovirus cell entry by lactoferrin binding to heparan sulfate proteoglycans, PloS One, doi:10.1371/journal.pone.0023710
Liu, Chopra, Li, Wolfert, Tompkins et al., SARS-CoV-2 spike protein binds heparan sulfate in a length-and sequence-dependent manner, bioRxiv, doi:10.1101/2020.05.10.087288
Mccarthy, Karellas, Henderson, Giannis, O'keefe et al., Dendrimers as drugs: discovery and preclinical and clinical development of dendrimer microbicides for HIV and STI prevention, Mol. Pharm, doi:10.1021/mp050023q
Mcgowan, Gomez, Bruder, Febo, Chen et al., Phase 1 randomized trial of the vaginal safety and acceptability of SPL7013 gel (VivaGel®) in sexually active young women (MTN-004), AIDS, doi:10.1097/QAD.0b013e328346bd3e
Milewska, Zarebski, Nowak, Stozek, Potempa et al., Human coronavirus NL63 utilizes heparan sulfate proteoglycans for attachment to target cells, J. Virol, doi:10.1128/JVI.02078-14
Mycroft-West, Su, Elli, Li, Guimond et al., The 2019 coronavirus (SARS-CoV-2) surface protein (spike) S1 receptor binding domain undergoes conformational change upon heparin binding, bioRxiv, doi:10.1101/2020.02.29.971093
Mycroft-West, Su, Li, Guimond, Rudd et al., SARS CoV-2 spike S1 receptor binding domain undergoes conformational change upon interaction with low molecular weight heparins, bioRxiv, doi:10.1101/2020.04.29.068486
Mycroft-West, Su, Pagani, Rudd, Elli et al., Heparin inhibits cellular invasion of SARS-CoV-2: structural dependence on the interaction of the surface protein (spike) S1 receptor binding domain with heparin, doi:10.1101/2020.04.28.066761
O'loughlin, Millwood, Mcdonald, Price, Kaldor et al., Safety, tolerability, and pharmacokinetics of SPL7013 gel (VivaGel®): a dose ranging phase 1 study, Sex. Transm. Dis, doi:10.1097/OLQ.0b013e3181bc0aac
Ogando, Dalebout, Zevenhoven-Dobbe, Limpens, Van Der Meer et al., SARS-coronavirus-2 replication in Vero E6 cells: replication kinetics, rapid adaption and cytopathology, J. General Virol, doi:10.1099/jgv.0.001453
Pauwels, Balzarini, Baba, Snoeck, Schols et al., Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds, J. Virol. Methods, doi:10.1016/0166-0934(88)90134-6
Pizzorno, Padey, Dubois, Julien, Traversier et al., Vitro Evaluation of Antiviral Activity of Single and Combined Repurposable Drugs against SARS-CoV-2, doi:10.1016/j.antiviral.2020.104878
Qiao, Olvera De La Cruz, The distal polybasic cleavage sites of SARS-CoV-2 spike protein enhance spike protein-ACE2 binding, bioRxiv, doi:10.1101/2020.06.09.142877
Sarrazin, Lamanna, Esko, Heparan sulfate proteoglycans, Cold Spring Harb. Perspect. Biol, doi:10.1101/cshperspect.a004952
Schwebke, Carter, Waldbaum, Price, Castellarnau et al., A phase 3, randomized, controlled trial of Astodrimer 1% Gel for preventing recurrent bacterial vaginosis, Eur. J. Obstet. Gynecol. Reprod. Biol. X, doi:10.1016/j.eurox.2021.100121
Sungnak, Huang, Bécavin, Berg, Queen et al., SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes, Nat. Med, doi:10.1038/s41591-020-0868-6
Telwatte, Moore, Johnson, Tyssen, Sterjovski et al., Virucidal activity of the dendrimer microbicide SPL7013 against HIV-1, Antivir. Res, doi:10.1016/j.antiviral.2011.03.186
Tyssen, Henderson, Johnson, Sterjovski, Moore et al., Structure activity relationship of dendrimer microbicides with dual action antiviral activity, PloS One, doi:10.1371/journal.pone.0012309
Van Den Worm, Eriksson, Zevenhoven, Weber, Zust et al., Reverse genetics of SARS-related coronavirus using vaccinia virus-based recombination, PloS One, doi:10.1371/journal.pone.0032857
Waldbaum, Schwebke, Paull, Price, Edmondson et al., A phase 2, double-blind, multicenter, randomized, placebo-controlled, dose-ranging study of the efficacy and safety of Astodrimer Gel for the treatment of bacterial vaginosis, PloS One, doi:10.1371/journal.pone.0232394
Walls, Park, Tortorici, Wall, Mcguire et al., Structure, function and antigenicity of the SARS-CoV-2 spike glycoprotein, Cell, doi:10.1016/j.cell.2020.02.058
Wang, Cao, Zhang, Yang, Liu et al., Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res, doi:10.1038/s41422-020-0282-0
Wrapp, Wang, Corbett, Goldsmith, Hsieh et al., Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation, Science, doi:10.1126/science.abb2507
Yuan, Wu, Zhu, Lee, So et al., A highly conserved cryptic epitope in the receptor binding domains of SARS-CoV-2 and SARS-CoV, Science, doi:10.1126/science.abb7269
DOI record:
{
"DOI": "10.1016/j.antiviral.2021.105089",
"ISSN": [
"0166-3542"
],
"URL": "http://dx.doi.org/10.1016/j.antiviral.2021.105089",
"alternative-id": [
"S0166354221000796"
],
"article-number": "105089",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Virucidal and antiviral activity of astodrimer sodium against SARS-CoV-2 in vitro"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Antiviral Research"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.antiviral.2021.105089"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2021 Starpharma Pty Ltd. Published by Elsevier B.V."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-9981-421X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Paull",
"given": "Jeremy R.A.",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0003-1852-5836",
"affiliation": [],
"authenticated-orcid": false,
"family": "Heery",
"given": "Graham P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bobardt",
"given": "Michael D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Castellarnau",
"given": "Alex",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1153-2099",
"affiliation": [],
"authenticated-orcid": false,
"family": "Luscombe",
"given": "Carolyn A.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6380-8929",
"affiliation": [],
"authenticated-orcid": false,
"family": "Fairley",
"given": "Jacinth K.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gallay",
"given": "Philippe A.",
"sequence": "additional"
}
],
"container-title": "Antiviral Research",
"container-title-short": "Antiviral Research",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2021,
5,
16
]
],
"date-time": "2021-05-16T21:41:22Z",
"timestamp": 1621201282000
},
"deposited": {
"date-parts": [
[
2021,
6,
16
]
],
"date-time": "2021-06-16T01:25:22Z",
"timestamp": 1623806722000
},
"indexed": {
"date-parts": [
[
2024,
9,
6
]
],
"date-time": "2024-09-06T10:11:43Z",
"timestamp": 1725617503517
},
"is-referenced-by-count": 24,
"issued": {
"date-parts": [
[
2021,
7
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
7,
1
]
],
"date-time": "2021-07-01T00:00:00Z",
"timestamp": 1625097600000
}
},
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
5,
15
]
],
"date-time": "2021-05-15T00:00:00Z",
"timestamp": 1621036800000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S0166354221000796?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S0166354221000796?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "105089",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2021,
7
]
]
},
"published-print": {
"date-parts": [
[
2021,
7
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1128/AAC.47.12.3784-3788.2003",
"article-title": "Evaluations of unformulated and formulated dendrimer-based microbicide candidates in mouse and Guinea pig models of genital herpes",
"author": "Bernstein",
"doi-asserted-by": "crossref",
"first-page": "3784",
"issue": "12",
"journal-title": "Antimicrob. Agents Chemother.",
"key": "10.1016/j.antiviral.2021.105089_bib1",
"volume": "47",
"year": "2003"
},
{
"author": "Bansal",
"key": "10.1016/j.antiviral.2021.105089_bib2"
},
{
"DOI": "10.1016/j.ejogrb.2019.11.032",
"article-title": "Two phase 3, double-blinded, placebo-controlled studies of the efficacy and safety of Astodrimer 1% Gel for the treatment of bacterial vaginosis",
"author": "Chavoustie",
"doi-asserted-by": "crossref",
"first-page": "13",
"journal-title": "Eur. J. Obstet. Gynecol. Reprod. Biol.",
"key": "10.1016/j.antiviral.2021.105089_bib3",
"volume": "245",
"year": "2020"
},
{
"DOI": "10.1097/QAI.0b013e318198a7e6",
"article-title": "A randomized controlled trial of the safety of candidate microbicide SPL7013 gel when applied to the penis",
"author": "Chen",
"doi-asserted-by": "crossref",
"first-page": "375",
"issue": "4",
"journal-title": "J. Acquir. Immune Defic. Syndr.",
"key": "10.1016/j.antiviral.2021.105089_bib4",
"volume": "50",
"year": "2009"
},
{
"DOI": "10.3389/fimmu.2013.00385",
"article-title": "Human immunodeficiency virus and heparan sulfate: from attachment to entry inhibition",
"author": "Connell",
"doi-asserted-by": "crossref",
"first-page": "385",
"journal-title": "Front. Immunol.",
"key": "10.1016/j.antiviral.2021.105089_bib5",
"volume": "4",
"year": "2013"
},
{
"DOI": "10.1093/glycob/cwn092",
"article-title": "Focus on antivirally active sulfated polysaccharides: from structure-activity analysis to clinical evaluation",
"author": "Ghosh",
"doi-asserted-by": "crossref",
"first-page": "2",
"issue": "1",
"journal-title": "Glycobiology",
"key": "10.1016/j.antiviral.2021.105089_bib6",
"volume": "19",
"year": "2009"
},
{
"DOI": "10.1016/j.antiviral.2005.08.004",
"article-title": "Evaluation of dendrimer SPL7013, a lead microbicide candidate against herpes simplex virus",
"author": "Gong",
"doi-asserted-by": "crossref",
"first-page": "139",
"issue": "3",
"journal-title": "Antivir. Res.",
"key": "10.1016/j.antiviral.2021.105089_bib7",
"volume": "68",
"year": "2005"
},
{
"DOI": "10.1016/j.molcel.2020.04.022",
"article-title": "A multibasic cleavage site in the spike protein of SARS-CoV-2 is essential for infection of human lung cells",
"author": "Hoffmann",
"doi-asserted-by": "crossref",
"first-page": "779",
"issue": "4",
"journal-title": "Mol. Cell",
"key": "10.1016/j.antiviral.2021.105089_bib8",
"volume": "78",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"article-title": "SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor",
"author": "Hoffmann",
"doi-asserted-by": "crossref",
"first-page": "271",
"issue": "2",
"journal-title": "Cell",
"key": "10.1016/j.antiviral.2021.105089_bib9",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.05.042",
"article-title": "SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract",
"author": "Hou",
"doi-asserted-by": "crossref",
"first-page": "429",
"issue": "2",
"journal-title": "Cell",
"key": "10.1016/j.antiviral.2021.105089_bib10",
"volume": "182",
"year": "2020"
},
{
"DOI": "10.1089/aid.2005.21.207",
"article-title": "SPL7013 gel as a topical microbicide for prevention of vaginal transmission of SHIV89.6P in macaques",
"author": "Jiang",
"doi-asserted-by": "crossref",
"first-page": "207",
"issue": "3",
"journal-title": "AIDS Res. Hum. Retrovir.",
"key": "10.1016/j.antiviral.2021.105089_bib11",
"volume": "21",
"year": "2005"
},
{
"author": "Korber",
"key": "10.1016/j.antiviral.2021.105089_bib12"
},
{
"DOI": "10.1128/AAC.01328-07",
"article-title": "Development of a comprehensive human immunodeficiency virus type-1 screening algorithm for discovery and preclinical testing of topical microbicides",
"author": "Lackman-Smith",
"doi-asserted-by": "crossref",
"first-page": "1768",
"issue": "5",
"journal-title": "Antimicrob. Agents Chemother.",
"key": "10.1016/j.antiviral.2021.105089_bib13",
"volume": "52",
"year": "2008"
},
{
"DOI": "10.1371/journal.pone.0023710",
"article-title": "Inhibition of SARS pseudovirus cell entry by lactoferrin binding to heparan sulfate proteoglycans",
"author": "Lang",
"doi-asserted-by": "crossref",
"issue": "8",
"journal-title": "PloS One",
"key": "10.1016/j.antiviral.2021.105089_bib14",
"volume": "6",
"year": "2011"
},
{
"author": "Liu",
"key": "10.1016/j.antiviral.2021.105089_bib15"
},
{
"DOI": "10.1021/mp050023q",
"article-title": "Dendrimers as drugs: discovery and preclinical and clinical development of dendrimer microbicides for HIV and STI prevention",
"author": "McCarthy",
"doi-asserted-by": "crossref",
"first-page": "312",
"issue": "4",
"journal-title": "Mol. Pharm.",
"key": "10.1016/j.antiviral.2021.105089_bib16",
"volume": "2",
"year": "2005"
},
{
"DOI": "10.1097/QAD.0b013e328346bd3e",
"article-title": "Phase 1 randomized trial of the vaginal safety and acceptability of SPL7013 gel (VivaGel®) in sexually active young women (MTN-004)",
"author": "McGowan",
"doi-asserted-by": "crossref",
"first-page": "1057",
"issue": "8",
"journal-title": "AIDS",
"key": "10.1016/j.antiviral.2021.105089_bib17",
"volume": "25",
"year": "2011"
},
{
"DOI": "10.1128/JVI.02078-14",
"article-title": "Human coronavirus NL63 utilizes heparan sulfate proteoglycans for attachment to target cells",
"author": "Milewska",
"doi-asserted-by": "crossref",
"first-page": "13221",
"issue": "22",
"journal-title": "J. Virol.",
"key": "10.1016/j.antiviral.2021.105089_bib18",
"volume": "88",
"year": "2014"
},
{
"author": "Mycroft-West",
"key": "10.1016/j.antiviral.2021.105089_bib19"
},
{
"author": "Mycroft-West",
"key": "10.1016/j.antiviral.2021.105089_bib20"
},
{
"author": "Mycroft-West",
"key": "10.1016/j.antiviral.2021.105089_bib21"
},
{
"DOI": "10.1097/OLQ.0b013e3181bc0aac",
"article-title": "Safety, tolerability, and pharmacokinetics of SPL7013 gel (VivaGel®): a dose ranging phase 1 study",
"author": "O'Loughlin",
"doi-asserted-by": "crossref",
"first-page": "100",
"issue": "2",
"journal-title": "Sex. Transm. Dis.",
"key": "10.1016/j.antiviral.2021.105089_bib22",
"volume": "37",
"year": "2010"
},
{
"DOI": "10.1099/jgv.0.001453",
"article-title": "SARS-coronavirus-2 replication in Vero E6 cells: replication kinetics, rapid adaption and cytopathology",
"author": "Ogando",
"doi-asserted-by": "crossref",
"first-page": "925",
"issue": "9",
"journal-title": "J. General Virol.",
"key": "10.1016/j.antiviral.2021.105089_bib23",
"volume": "101",
"year": "2020"
},
{
"DOI": "10.1016/0166-0934(88)90134-6",
"article-title": "Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds",
"author": "Pauwels",
"doi-asserted-by": "crossref",
"first-page": "309",
"issue": "4",
"journal-title": "J. Virol. Methods",
"key": "10.1016/j.antiviral.2021.105089_bib24",
"volume": "20",
"year": "1988"
},
{
"article-title": "Antiviral Res. 104878",
"author": "Pizzorno",
"key": "10.1016/j.antiviral.2021.105089_bib25",
"series-title": "In Vitro Evaluation of Antiviral Activity of Single and Combined Repurposable Drugs against SARS-CoV-2",
"year": "2020"
},
{
"article-title": "The distal polybasic cleavage sites of SARS-CoV-2 spike protein enhance spike protein-ACE2 binding",
"author": "Qiao",
"journal-title": "bioRxiv June",
"key": "10.1016/j.antiviral.2021.105089_bib26",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1101/cshperspect.a004952",
"article-title": "Heparan sulfate proteoglycans",
"author": "Sarrazin",
"doi-asserted-by": "crossref",
"issue": "7",
"journal-title": "Cold Spring Harb. Perspect. Biol.",
"key": "10.1016/j.antiviral.2021.105089_bib27",
"volume": "3",
"year": "2011"
},
{
"DOI": "10.1016/j.eurox.2021.100121",
"article-title": "A phase 3, randomized, controlled trial of Astodrimer 1% Gel for preventing recurrent bacterial vaginosis",
"author": "Schwebke",
"doi-asserted-by": "crossref",
"journal-title": "Eur. J. Obstet. Gynecol. Reprod. Biol. X",
"key": "10.1016/j.antiviral.2021.105089_bib28",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1038/s41591-020-0868-6",
"article-title": "SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes",
"author": "Sungnak",
"doi-asserted-by": "crossref",
"first-page": "681",
"issue": "5",
"journal-title": "Nat. Med.",
"key": "10.1016/j.antiviral.2021.105089_bib29",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1016/j.antiviral.2011.03.186",
"article-title": "Virucidal activity of the dendrimer microbicide SPL7013 against HIV-1",
"author": "Telwatte",
"doi-asserted-by": "crossref",
"first-page": "195",
"issue": "3",
"journal-title": "Antivir. Res.",
"key": "10.1016/j.antiviral.2021.105089_bib30",
"volume": "90",
"year": "2011"
},
{
"DOI": "10.1371/journal.pone.0012309",
"article-title": "Structure activity relationship of dendrimer microbicides with dual action antiviral activity",
"author": "Tyssen",
"doi-asserted-by": "crossref",
"issue": "8",
"journal-title": "PloS One",
"key": "10.1016/j.antiviral.2021.105089_bib31",
"volume": "5",
"year": "2010"
},
{
"DOI": "10.1371/journal.pone.0032857",
"article-title": "Reverse genetics of SARS-related coronavirus using vaccinia virus-based recombination",
"author": "van den Worm",
"doi-asserted-by": "crossref",
"issue": "3",
"journal-title": "PloS One",
"key": "10.1016/j.antiviral.2021.105089_bib32",
"volume": "7",
"year": "2012"
},
{
"DOI": "10.1371/journal.pone.0232394",
"article-title": "A phase 2, double-blind, multicenter, randomized, placebo-controlled, dose-ranging study of the efficacy and safety of Astodrimer Gel for the treatment of bacterial vaginosis",
"author": "Waldbaum",
"doi-asserted-by": "crossref",
"issue": "5",
"journal-title": "PloS One",
"key": "10.1016/j.antiviral.2021.105089_bib33",
"volume": "15",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.02.058",
"article-title": "Structure, function and antigenicity of the SARS-CoV-2 spike glycoprotein",
"author": "Walls",
"doi-asserted-by": "crossref",
"first-page": "281",
"issue": "2",
"journal-title": "Cell",
"key": "10.1016/j.antiviral.2021.105089_bib34",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"article-title": "Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "269",
"issue": "3",
"journal-title": "Cell Res.",
"key": "10.1016/j.antiviral.2021.105089_bib35",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1126/science.abb2507",
"article-title": "Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation",
"author": "Wrapp",
"doi-asserted-by": "crossref",
"first-page": "1260",
"issue": "6483",
"journal-title": "Science",
"key": "10.1016/j.antiviral.2021.105089_bib36",
"volume": "367",
"year": "2020"
},
{
"DOI": "10.1126/science.abb7269",
"article-title": "A highly conserved cryptic epitope in the receptor binding domains of SARS-CoV-2 and SARS-CoV",
"author": "Yuan",
"doi-asserted-by": "crossref",
"first-page": "630",
"issue": "6491",
"journal-title": "Science",
"key": "10.1016/j.antiviral.2021.105089_bib37",
"volume": "368",
"year": "2020"
}
],
"reference-count": 37,
"references-count": 37,
"relation": {
"has-preprint": [
{
"asserted-by": "object",
"id": "10.21203/rs.3.rs-440941/v1",
"id-type": "doi"
},
{
"asserted-by": "object",
"id": "10.1101/2020.08.20.260190",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S0166354221000796"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"special_numbering": "C",
"subject": [],
"subtitle": [],
"title": "Virucidal and antiviral activity of astodrimer sodium against SARS-CoV-2 in vitro",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "191"
}