Astodrimer sodium nasal spray forms a barrier to SARS-CoV-2 in vitro and preserves normal mucociliary function in human nasal epithelium
et al., Scientific Reports, doi:10.1038/s41598-024-72262-w, Sep 2024
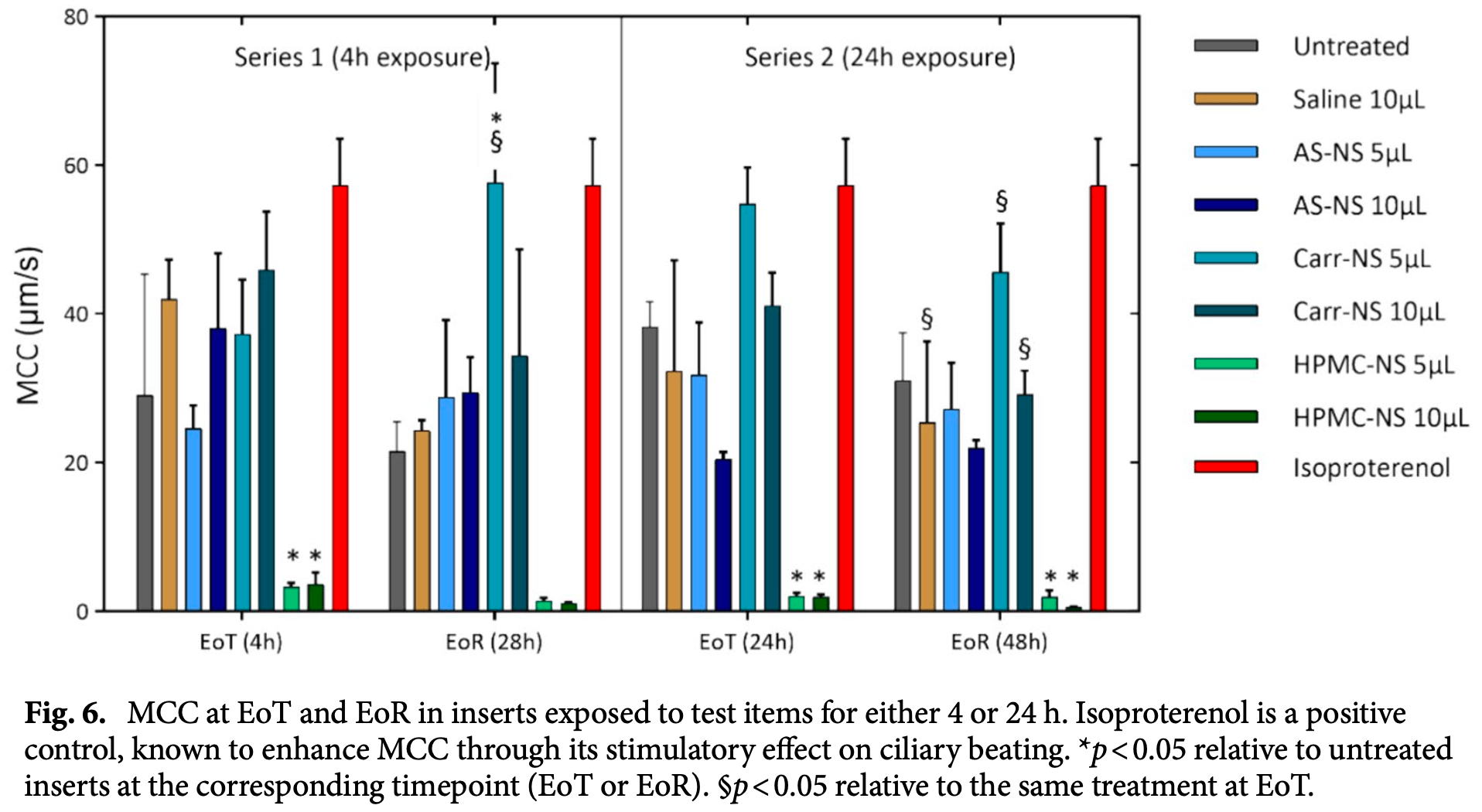
In vitro study showing that astodrimer sodium nasal spray forms an effective barrier against SARS-CoV-2 infection while preserving normal mucociliary function in human nasal epithelium. Authors demonstrated a 96.6% reduction in infectious SARS-CoV-2 delta variant particles in Calu-3 lung epithelial cells treated with astodrimer sodium nasal spray compared to controls. The spray showed minimal cytotoxicity in Vero E6 cells and maintained normal tissue integrity, ciliary beating frequency, and mucociliary clearance in a MucilAir human nasal epithelium model. In contrast, a low pH hydroxypropyl methylcellulose nasal spray disrupted epithelial integrity and mucociliary function. The study suggests astodrimer sodium nasal spray could potentially reduce exposure to infectious respiratory viruses by forming a protective barrier in the nasal cavity without compromising normal nasal functions. The antiviral mechanism involves astodrimer binding to positively charged regions of the SARS-CoV-2 spike protein, blocking its interaction with cell-surface heparan sulfate proteoglycans.
3 preclinical studies support the efficacy of astodrimer sodium for COVID-19:
1 in vivo animal study3
1.
Paull et al., Astodrimer sodium nasal spray forms a barrier to SARS-CoV-2 in vitro and preserves normal mucociliary function in human nasal epithelium, Scientific Reports, doi:10.1038/s41598-024-72262-w.
Paull et al., 11 Sep 2024, peer-reviewed, 8 authors.
Contact: jeremy.paull@starpharma.com.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Astodrimer sodium nasal spray forms a barrier to SARS-CoV-2 in vitro and preserves normal mucociliary function in human nasal epithelium
Scientific Reports, doi:10.1038/s41598-024-72262-w
COVID-19 remains a severe condition for many including immunocompromised individuals. There remains a need for effective measures against this and other respiratory infections, which transmit via virus-laden droplets that reach the nasal or oral mucosae. Nasal sprays offer potential protection against viruses. Such formulations should preserve normal nasal mucociliary function. The antiviral barrier efficacy and effects on mucociliary function of astodrimer sodium nasal spray (AS-NS) were evaluated and compared with other available nasal sprays-low pH hydroxypropyl methylcellulose (HPMC-NS), iota-carrageenan (Carr-NS), nitric oxide (NO-NS), and povidone iodine (PI-NS). Assays simulated clinical conditions. Antiviral barrier function and cell viability were assessed in airway cell monolayers, while a model of fully differentiated human nasal epithelium (MucilAir™) was utilized to evaluate tissue integrity, cytotoxicity, cilia beating frequency, and mucociliary clearance. AS-NS reduced infectious virus in cell monolayers and demonstrated a benign cytotoxicity profile. In human nasal epithelium ex vivo, AS-NS had no impact on mucociliary function (cilia beating nor mucociliary clearance). Carr-NS, HPMC-NS, NO-NS and PI-NS demonstrated limited antiviral effects, while HPMC-NS caused inhibition of mucociliary function. Astodrimer sodium nasal spray demonstrates an acceptable nonclinical efficacy and safety profile as a barrier nasal spray against respiratory viral infection in the nasal cavity. Since the height of the COVID-19 pandemic, the severity of the condition has lessened, indicated by a decline in mortality rates as well as a reduction in the proportion of patients hospitalized due to COVID-19 and that require intensive medical intervention. This shift in COVID-19 morbidity and mortality patterns can be attributed to the widespread access to highly effective COVID-19 vaccines, a high level of immunity induced by prior infection within the population, availability of outpatient treatment options and changes in the SARS-CoV-2 virus itself 1 . Despite these improvements in managing the spread and impact of COVID-19, it remains a severe condition for many, particularly the elderly, unvaccinated or immunocompromised individuals, and those with respiratory comorbidities. Therefore, the search for effective strategies to complement other preventive and therapeutic options and lessen the incidence and clinical severity of the disease as well as other respiratory infections, including those that may emerge as potential pandemic viruses in future, is ongoing. Respiratory viruses primarily transmit when an infected individual expels droplets laden with the virus a short distance through the air. These droplets can either directly contact the nasal or oral mucous membranes of a susceptible individual or be indirectly transferred through contact with contaminated surfaces 2-4 . The first line of defence against respiratory viral infections includes..
Author contributions
Funding The authors declare that this study was funded by Starpharma Pty Ltd. The funder had the following involvement with the study: conceptualisation, supervision and project administration, aspects of study design and methodology, analysis, and interpretation of data, writing and reviewing this article, and made the decision to submit the article for publication.
Competing interests The authors declare the following financial interests/personal relationships which may be considered as potential conflicts of interest: J.R.A.P., A.S. and G.P.H. are paid employees of Starpharma Pty Ltd. A.C. and C.A.L. are paid consultants to Starpharma Pty Ltd. S.C. is a paid employee of Epithelix Sarl. The remaining authors M.B. and P.A.G. declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Bar-On, Flamholz, Phillips, Milo, SARS-CoV-2 (COVID-19) by the numbers, eLife
Bernstein, Evaluations of unformulated and formulated dendrimer-based microbicide candidates in mouse and guinea pig models of genital herpes, Antimicrob. Agents Chemother
Castellarnau, Astodrimer sodium antiviral nasal spray for reducing respiratory infections is safe and well tolerated in a randomized controlled trial, Sci. Rep
Connell, Lortat-Jacob, Human immunodeficiency virus and heparan sulfate: From attachment to entry inhibition, Front. Immunol
Eccles, Efficacy and safety of iota-carrageenan nasal spray versus placebo in early treatment of the common cold in adults: The ICICC trial, Respir. Res
Fais, Drug-free nasal spray as a barrier against SARS-CoV-2 and its delta variant: In vitro study of safety and efficacy in human nasal airway epithelia, Int. J. Mol. Sci
Gallay, Effects of astodrimer sodium against SARS-CoV-2 variants (α, β, γ, δ, κ) in vitro, Top. Antivir. Med
Gizurarson, Anatomical and histological factors affecting intranasal drug and vaccine delivery, Curr. Drug Deliv
Gong, Evaluation of dendrimer SPL7013, a lead microbicide candidate against herpes simplex viruses, Antivir. Res
Gudis, Cohen, Cilia Dysfunction, None, Otolaryngol. Clin. N. Am
Jiang, SPL7013 gel as a topical microbicide for prevention of vaginal transmission of SHIV89.6P in macaques, AIDS Res. Hum. Retrovir
Jiao, Zhang, Influence of intranasal drugs on human nasal mucociliary clearance and ciliary beat frequency, Allergy Asthma Immunol. Res
Lewis, Is the coronavirus airborne? Experts can't agree, Nature
Mccarthy, Dendrimers as drugs: Discovery and preclinical and clinical development of dendrimer-based microbicides for HIV and STI prevention, Mol. Pharm
Meyerowitz, Scott, Richterman, Male, Cevik, Clinical course and management of COVID-19 in the era of widespread population immunity, Nat. Rev. Microbiol
Paull, Protective effects of astodrimer sodium 1% nasal spray formulation against SARS-CoV-2 nasal challenge in K18-hACE2 mice, Viruses
Paull, Virucidal and antiviral activity of astodrimer sodium against SARS-CoV-2 in vitro, Antivir. Res
Sarrazin, Lamanna, Esko, Heparan sulfate proteoglycans, Cold Spring Harb Perspect Biol
Shukla, Spear, Herpesviruses and heparan sulfate: An intimate relationship in aid of viral entry, J. Clin. Investig
Suman, Current understanding of nasal morphology and physiology as a drug delivery target, Drug Deliv. Transl. Res
Sungnak, SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes, Nat. Med
Tang, Aerosol transmission of SARS-CoV-2? Evidence, prevention and control, Environ. Int
Telwatte, Virucidal activity of the dendrimer microbicide SPL7013 against HIV-1, Antivir. Res
Tyssen, Structure activity relationship of dendrimer microbicides with dual action antiviral activity, PLoS ONE
Yan, Infectious virus in exhaled breath of symptomatic seasonal influenza cases from a college community, Proc. Natl. Acad. Sci. USA
Zhang, Heparan sulfate assists SARS-CoV-2 in cell entry and can be targeted by approved drugs in vitro, Cell Discov
Zhang, Zhang, Wang, Surface charge changes in spike RBD mutations of SARS-CoV-2 and its variant strains alter the virus evasiveness via HSPGs: A review and mechanistic hypothesis, Front. Public Health
DOI record:
{
"DOI": "10.1038/s41598-024-72262-w",
"ISSN": [
"2045-2322"
],
"URL": "http://dx.doi.org/10.1038/s41598-024-72262-w",
"abstract": "<jats:title>Abstract</jats:title><jats:p>COVID-19 remains a severe condition for many including immunocompromised individuals. There remains a need for effective measures against this and other respiratory infections, which transmit via virus-laden droplets that reach the nasal or oral mucosae. Nasal sprays offer potential protection against viruses. Such formulations should preserve normal nasal mucociliary function. The antiviral barrier efficacy and effects on mucociliary function of astodrimer sodium nasal spray (AS-NS) were evaluated and compared with other available nasal sprays—low pH hydroxypropyl methylcellulose (HPMC-NS), iota-carrageenan (Carr-NS), nitric oxide (NO-NS), and povidone iodine (PI-NS). Assays simulated clinical conditions. Antiviral barrier function and cell viability were assessed in airway cell monolayers, while a model of fully differentiated human nasal epithelium (MucilAir™) was utilized to evaluate tissue integrity, cytotoxicity, cilia beating frequency, and mucociliary clearance. AS-NS reduced infectious virus in cell monolayers and demonstrated a benign cytotoxicity profile. In human nasal epithelium ex vivo, AS-NS had no impact on mucociliary function (cilia beating nor mucociliary clearance). Carr-NS, HPMC-NS, NO-NS and PI-NS demonstrated limited antiviral effects, while HPMC-NS caused inhibition of mucociliary function. Astodrimer sodium nasal spray demonstrates an acceptable nonclinical efficacy and safety profile as a barrier nasal spray against respiratory viral infection in the nasal cavity.</jats:p>",
"alternative-id": [
"72262"
],
"article-number": "21259",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "22 March 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "5 September 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "11 September 2024"
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1,
"value": "The authors declare the following financial interests/personal relationships which may be considered as potential conflicts of interest: J.R.A.P., A.S. and G.P.H. are paid employees of Starpharma Pty Ltd. A.C. and C.A.L. are paid consultants to Starpharma Pty Ltd. S.C. is a paid employee of Epithelix Sarl. The remaining authors M.B. and P.A.G. declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest."
}
],
"author": [
{
"affiliation": [],
"family": "Paull",
"given": "Jeremy R. A.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Luscombe",
"given": "Carolyn A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Seta",
"given": "Aynaz",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Heery",
"given": "Graham P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bobardt",
"given": "Michael D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gallay",
"given": "Philippe A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Constant",
"given": "Samuel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Castellarnau",
"given": "Alex",
"sequence": "additional"
}
],
"container-title": "Scientific Reports",
"container-title-short": "Sci Rep",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2024,
9,
11
]
],
"date-time": "2024-09-11T16:05:23Z",
"timestamp": 1726070723000
},
"deposited": {
"date-parts": [
[
2024,
9,
11
]
],
"date-time": "2024-09-11T16:11:55Z",
"timestamp": 1726071115000
},
"funder": [
{
"name": "Starpharma Pty Ltd"
}
],
"indexed": {
"date-parts": [
[
2024,
9,
12
]
],
"date-time": "2024-09-12T00:31:49Z",
"timestamp": 1726101109161
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2024,
9,
11
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2024,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
9,
11
]
],
"date-time": "2024-09-11T00:00:00Z",
"timestamp": 1726012800000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
9,
11
]
],
"date-time": "2024-09-11T00:00:00Z",
"timestamp": 1726012800000
}
}
],
"link": [
{
"URL": "https://www.nature.com/articles/s41598-024-72262-w.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-024-72262-w",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-024-72262-w.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1038",
"published": {
"date-parts": [
[
2024,
9,
11
]
]
},
"published-online": {
"date-parts": [
[
2024,
9,
11
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1038/s41579-023-01001-1",
"author": "EA Meyerowitz",
"doi-asserted-by": "publisher",
"first-page": "75",
"journal-title": "Nat. Rev. Microbiol.",
"key": "72262_CR1",
"unstructured": "Meyerowitz, E. A., Scott, J., Richterman, A., Male, V. & Cevik, M. Clinical course and management of COVID-19 in the era of widespread population immunity. Nat. Rev. Microbiol. 22, 75–88 (2024).",
"volume": "22",
"year": "2024"
},
{
"DOI": "10.1038/d41586-020-00974-w",
"author": "D Lewis",
"doi-asserted-by": "publisher",
"first-page": "175",
"journal-title": "Nature",
"key": "72262_CR2",
"unstructured": "Lewis, D. Is the coronavirus airborne? Experts can’t agree. Nature 580, 175 (2020).",
"volume": "580",
"year": "2020"
},
{
"DOI": "10.1016/j.envint.2020.106039",
"author": "S Tang",
"doi-asserted-by": "publisher",
"first-page": "106039",
"journal-title": "Environ. Int.",
"key": "72262_CR3",
"unstructured": "Tang, S. et al. Aerosol transmission of SARS-CoV-2? Evidence, prevention and control. Environ. Int. 144, 106039 (2020).",
"volume": "144",
"year": "2020"
},
{
"DOI": "10.1073/pnas.1716561115",
"author": "J Yan",
"doi-asserted-by": "publisher",
"first-page": "1081",
"journal-title": "Proc. Natl. Acad. Sci. USA",
"key": "72262_CR4",
"unstructured": "Yan, J. et al. Infectious virus in exhaled breath of symptomatic seasonal influenza cases from a college community. Proc. Natl. Acad. Sci. USA 115, 1081–1086 (2018).",
"volume": "115",
"year": "2018"
},
{
"DOI": "10.1021/mp050023q",
"author": "TD McCarthy",
"doi-asserted-by": "publisher",
"first-page": "312",
"journal-title": "Mol. Pharm.",
"key": "72262_CR5",
"unstructured": "McCarthy, T. D. et al. Dendrimers as drugs: Discovery and preclinical and clinical development of dendrimer-based microbicides for HIV and STI prevention. Mol. Pharm. 2, 312–318 (2005).",
"volume": "2",
"year": "2005"
},
{
"DOI": "10.1016/j.antiviral.2005.08.004",
"author": "E Gong",
"doi-asserted-by": "publisher",
"first-page": "139",
"journal-title": "Antivir. Res.",
"key": "72262_CR6",
"unstructured": "Gong, E. et al. Evaluation of dendrimer SPL7013, a lead microbicide candidate against herpes simplex viruses. Antivir. Res. 68, 139–146 (2005).",
"volume": "68",
"year": "2005"
},
{
"DOI": "10.1371/journal.pone.0012309",
"author": "D Tyssen",
"doi-asserted-by": "publisher",
"first-page": "e12309",
"journal-title": "PLoS ONE",
"key": "72262_CR7",
"unstructured": "Tyssen, D. et al. Structure activity relationship of dendrimer microbicides with dual action antiviral activity. PLoS ONE 5, e12309 (2010).",
"volume": "5",
"year": "2010"
},
{
"DOI": "10.1016/j.antiviral.2011.03.186",
"author": "S Telwatte",
"doi-asserted-by": "publisher",
"first-page": "195",
"journal-title": "Antivir. Res.",
"key": "72262_CR8",
"unstructured": "Telwatte, S. et al. Virucidal activity of the dendrimer microbicide SPL7013 against HIV-1. Antivir. Res. 90, 195–199 (2011).",
"volume": "90",
"year": "2011"
},
{
"DOI": "10.1089/aid.2005.21.207",
"author": "Y-H Jiang",
"doi-asserted-by": "publisher",
"first-page": "207",
"journal-title": "AIDS Res. Hum. Retrovir.",
"key": "72262_CR9",
"unstructured": "Jiang, Y.-H. et al. SPL7013 gel as a topical microbicide for prevention of vaginal transmission of SHIV89.6P in macaques. AIDS Res. Hum. Retrovir. 21, 207–213 (2005).",
"volume": "21",
"year": "2005"
},
{
"DOI": "10.1128/AAC.47.12.3784-3788.2003",
"author": "DI Bernstein",
"doi-asserted-by": "publisher",
"first-page": "3784",
"journal-title": "Antimicrob. Agents Chemother.",
"key": "72262_CR10",
"unstructured": "Bernstein, D. I. et al. Evaluations of unformulated and formulated dendrimer-based microbicide candidates in mouse and guinea pig models of genital herpes. Antimicrob. Agents Chemother. 47, 3784–3788 (2003).",
"volume": "47",
"year": "2003"
},
{
"DOI": "10.1016/j.antiviral.2021.105089",
"author": "JRA Paull",
"doi-asserted-by": "publisher",
"first-page": "105089",
"journal-title": "Antivir. Res.",
"key": "72262_CR11",
"unstructured": "Paull, J. R. A. et al. Virucidal and antiviral activity of astodrimer sodium against SARS-CoV-2 in vitro. Antivir. Res. 191, 105089 (2021).",
"volume": "191",
"year": "2021"
},
{
"key": "72262_CR12",
"unstructured": "Gallay, P. A. et al. Effects of astodrimer sodium against SARS-CoV-2 variants (α, β, γ, δ, κ) in vitro [CROI Abstract 478]. Special Issue: Abstracts from the virtual 2022 Conference on Retroviruses and Opportunistic Infections. Top. Antivir. Med. 30 (2022)."
},
{
"DOI": "10.3390/v13081656",
"author": "JRA Paull",
"doi-asserted-by": "publisher",
"first-page": "1656",
"journal-title": "Viruses",
"key": "72262_CR13",
"unstructured": "Paull, J. R. A. et al. Protective effects of astodrimer sodium 1% nasal spray formulation against SARS-CoV-2 nasal challenge in K18-hACE2 mice. Viruses 13, 1656 (2021).",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1101/cshperspect.a004952",
"doi-asserted-by": "crossref",
"key": "72262_CR14",
"unstructured": "Sarrazin, S., Lamanna, W. C. & Esko, J. D. Heparan sulfate proteoglycans. Cold Spring Harb Perspect Biol 3, (2011)."
},
{
"DOI": "10.1038/s41421-020-00222-5",
"author": "Q Zhang",
"doi-asserted-by": "publisher",
"first-page": "80",
"journal-title": "Cell Discov.",
"key": "72262_CR15",
"unstructured": "Zhang, Q. et al. Heparan sulfate assists SARS-CoV-2 in cell entry and can be targeted by approved drugs in vitro. Cell Discov. 6, 80 (2020).",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.3389/fpubh.2022.952916",
"author": "Z Zhang",
"doi-asserted-by": "publisher",
"first-page": "952916",
"journal-title": "Front. Public Health",
"key": "72262_CR16",
"unstructured": "Zhang, Z., Zhang, J. & Wang, J. Surface charge changes in spike RBD mutations of SARS-CoV-2 and its variant strains alter the virus evasiveness via HSPGs: A review and mechanistic hypothesis. Front. Public Health 10, 952916 (2022).",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1172/JCI200113799",
"author": "D Shukla",
"doi-asserted-by": "publisher",
"first-page": "503",
"journal-title": "J. Clin. Investig.",
"key": "72262_CR17",
"unstructured": "Shukla, D. & Spear, P. G. Herpesviruses and heparan sulfate: An intimate relationship in aid of viral entry. J. Clin. Investig. 108, 503–510 (2001).",
"volume": "108",
"year": "2001"
},
{
"DOI": "10.3389/fimmu.2013.00385",
"author": "BJ Connell",
"doi-asserted-by": "publisher",
"first-page": "385",
"journal-title": "Front. Immunol.",
"key": "72262_CR18",
"unstructured": "Connell, B. J. & Lortat-Jacob, H. Human immunodeficiency virus and heparan sulfate: From attachment to entry inhibition. Front. Immunol. 4, 385 (2013).",
"volume": "4",
"year": "2013"
},
{
"DOI": "10.1038/s41591-020-0868-6",
"author": "W Sungnak",
"doi-asserted-by": "publisher",
"first-page": "681",
"journal-title": "Nat. Med.",
"key": "72262_CR19",
"unstructured": "Sungnak, W. et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 26, 681–687 (2020).",
"volume": "26",
"year": "2020"
},
{
"author": "DA Gudis",
"first-page": "vii",
"issue": "461–472",
"journal-title": "Otolaryngol. Clin. N. Am.",
"key": "72262_CR20",
"unstructured": "Gudis, D. A. & Cohen, N. A. Cilia dysfunction. Otolaryngol. Clin. N. Am. 43(461–472), vii (2010).",
"volume": "43",
"year": "2010"
},
{
"DOI": "10.4168/aair.2019.11.3.306",
"author": "J Jiao",
"doi-asserted-by": "publisher",
"first-page": "306",
"journal-title": "Allergy Asthma Immunol. Res.",
"key": "72262_CR21",
"unstructured": "Jiao, J. & Zhang, L. Influence of intranasal drugs on human nasal mucociliary clearance and ciliary beat frequency. Allergy Asthma Immunol. Res. 11, 306–319 (2019).",
"volume": "11",
"year": "2019"
},
{
"DOI": "10.7554/eLife.57309",
"author": "YM Bar-On",
"doi-asserted-by": "publisher",
"first-page": "e57309",
"journal-title": "eLife",
"key": "72262_CR22",
"unstructured": "Bar-On, Y. M., Flamholz, A., Phillips, R. & Milo, R. SARS-CoV-2 (COVID-19) by the numbers. eLife 9, e57309 (2020).",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.3390/ijms23074062",
"author": "F Fais",
"doi-asserted-by": "publisher",
"first-page": "4062",
"journal-title": "Int. J. Mol. Sci.",
"key": "72262_CR23",
"unstructured": "Fais, F. et al. Drug-free nasal spray as a barrier against SARS-CoV-2 and its delta variant: In vitro study of safety and efficacy in human nasal airway epithelia. Int. J. Mol. Sci. 23, 4062 (2022).",
"volume": "23",
"year": "2022"
},
{
"DOI": "10.2174/156720112803529828",
"author": "S Gizurarson",
"doi-asserted-by": "publisher",
"first-page": "566",
"journal-title": "Curr. Drug Deliv.",
"key": "72262_CR24",
"unstructured": "Gizurarson, S. Anatomical and histological factors affecting intranasal drug and vaccine delivery. Curr. Drug Deliv. 9, 566–582 (2012).",
"volume": "9",
"year": "2012"
},
{
"DOI": "10.1007/s13346-012-0121-z",
"author": "JD Suman",
"doi-asserted-by": "publisher",
"first-page": "4",
"journal-title": "Drug Deliv. Transl. Res.",
"key": "72262_CR25",
"unstructured": "Suman, J. D. Current understanding of nasal morphology and physiology as a drug delivery target. Drug Deliv. Transl. Res. 3, 4–15 (2013).",
"volume": "3",
"year": "2013"
},
{
"DOI": "10.1038/s41598-022-14601-3",
"author": "A Castellarnau",
"doi-asserted-by": "publisher",
"first-page": "10210",
"journal-title": "Sci. Rep.",
"key": "72262_CR26",
"unstructured": "Castellarnau, A. et al. Astodrimer sodium antiviral nasal spray for reducing respiratory infections is safe and well tolerated in a randomized controlled trial. Sci. Rep. 12, 10210 (2022).",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1186/s12931-015-0281-8",
"author": "R Eccles",
"doi-asserted-by": "publisher",
"first-page": "121",
"journal-title": "Respir. Res.",
"key": "72262_CR27",
"unstructured": "Eccles, R. et al. Efficacy and safety of iota-carrageenan nasal spray versus placebo in early treatment of the common cold in adults: The ICICC trial. Respir. Res. 16, 121 (2015).",
"volume": "16",
"year": "2015"
},
{
"DOI": "10.1080/14015450510042116",
"doi-asserted-by": "crossref",
"key": "72262_CR28",
"unstructured": "Airway diseases. A shared approach to management. In: Conjunction with the 6th International Symposium on Human Pepsin (ISHP). Logopedics Phoniatrics Vocology, Vol. 30, 41–47 (2005)."
}
],
"reference-count": 28,
"references-count": 28,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.nature.com/articles/s41598-024-72262-w"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Astodrimer sodium nasal spray forms a barrier to SARS-CoV-2 in vitro and preserves normal mucociliary function in human nasal epithelium",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "14"
}