Sep 11 2024 |
et al., Scientific Reports, doi:10.1038/s41598-024-72262-w | Astodrimer sodium nasal spray forms a barrier to SARS-CoV-2 in vitro and preserves normal mucociliary function in human nasal epithelium |
| In vitro study showing that astodrimer sodium nasal spray forms an effective barrier against SARS-CoV-2 infection while preserving normal mucociliary function in human nasal epithelium. Authors demonstrated a 96.6% reduction in infectious.. | ||
Sep 6 2024 |
et al., Pharmaceutics, doi:10.3390/pharmaceutics16091173 | Astodrimer Sodium Nasal Spray versus Placebo in Non-Hospitalised Patients with COVID-19: A Randomised, Double-Blinded, Placebo-Controlled Trial |
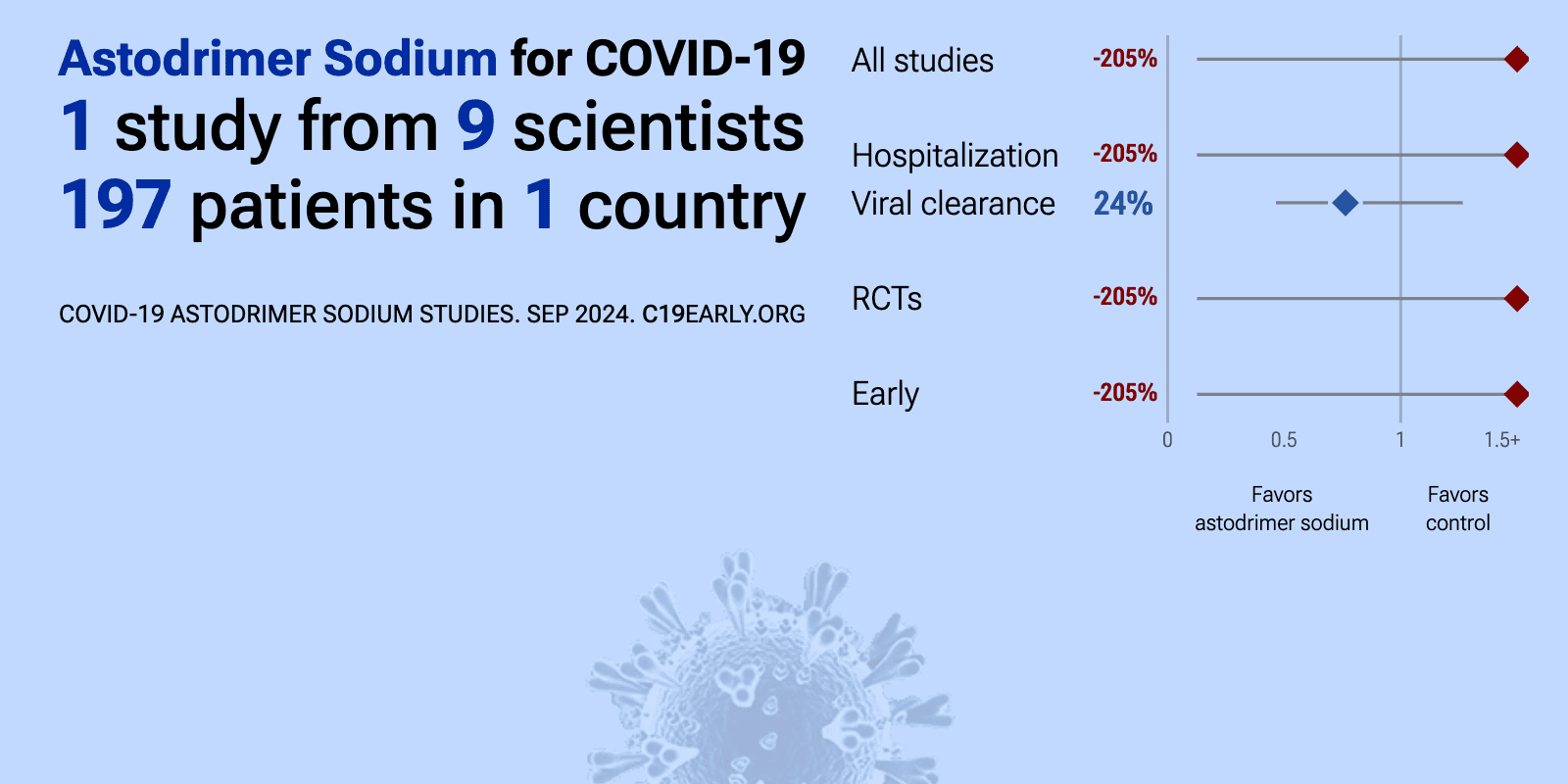
| 19% improved recovery (p=0.41) and 24% improved viral clearance (p=0.3). RCT 222 non-hospitalized low risk COVID-19 patients showing lower SARS-CoV-2 viral load, faster viral clearance, and improvements in symptoms, particularly anosmia, with astodrimer sodium nasal spray compared to placebo. The reduction in .. | ||
Nov 17 2022 |
et al., Biomedicines, doi:10.3390/biomedicines10112966 | Improving Nasal Protection for Preventing SARS-CoV-2 Infection |
| Review of strategies for improving nasal protection to prevent SARS-CoV-2 infection. Authors note the nasal epithelium is the primary entry point for SARS-CoV-2, especially for Omicron variants which replicate efficiently in the upper res.. | ||
Jun 17 2022 |
et al., Scientific Reports, doi:10.1038/s41598-022-14601-3 | Astodrimer sodium antiviral nasal spray for reducing respiratory infections is safe and well tolerated in a randomized controlled trial |
| RCT 40 healthy volunteers showing astodrimer sodium 1% nasal spray was well tolerated with no systemic absorption detected. Treatment emergent adverse events occurred in a greater proportion of participants receiving placebo than astodrim.. | ||
Aug 20 2021 |
et al., Viruses, doi:10.3390/v13081656 | Protective Effects of Astodrimer Sodium 1% Nasal Spray Formulation against SARS-CoV-2 Nasal Challenge in K18-hACE2 Mice |
| Mouse study showing astodrimer sodium 1% nasal spray significantly reduced SARS-CoV-2 replication, tissue viral loads, and proinflammatory cytokine production in K18-hACE2 mice. Astodrimer sodium reduced viral genome copies and infectious.. | ||
Jul 31 2021 |
et al., Antiviral Research, doi:10.1016/j.antiviral.2021.105089 | Virucidal and antiviral activity of astodrimer sodium against SARS-CoV-2 in vitro |
| In vitro study showing potent antiviral and virucidal activity of astodrimer sodium against SARS-CoV-2. | ||