Astodrimer Sodium Nasal Spray versus Placebo in Non-Hospitalised Patients with COVID-19: A Randomised, Double-Blinded, Placebo-Controlled Trial
et al., Pharmaceutics, doi:10.3390/pharmaceutics16091173, ISRCTN70449927, Sep 2024
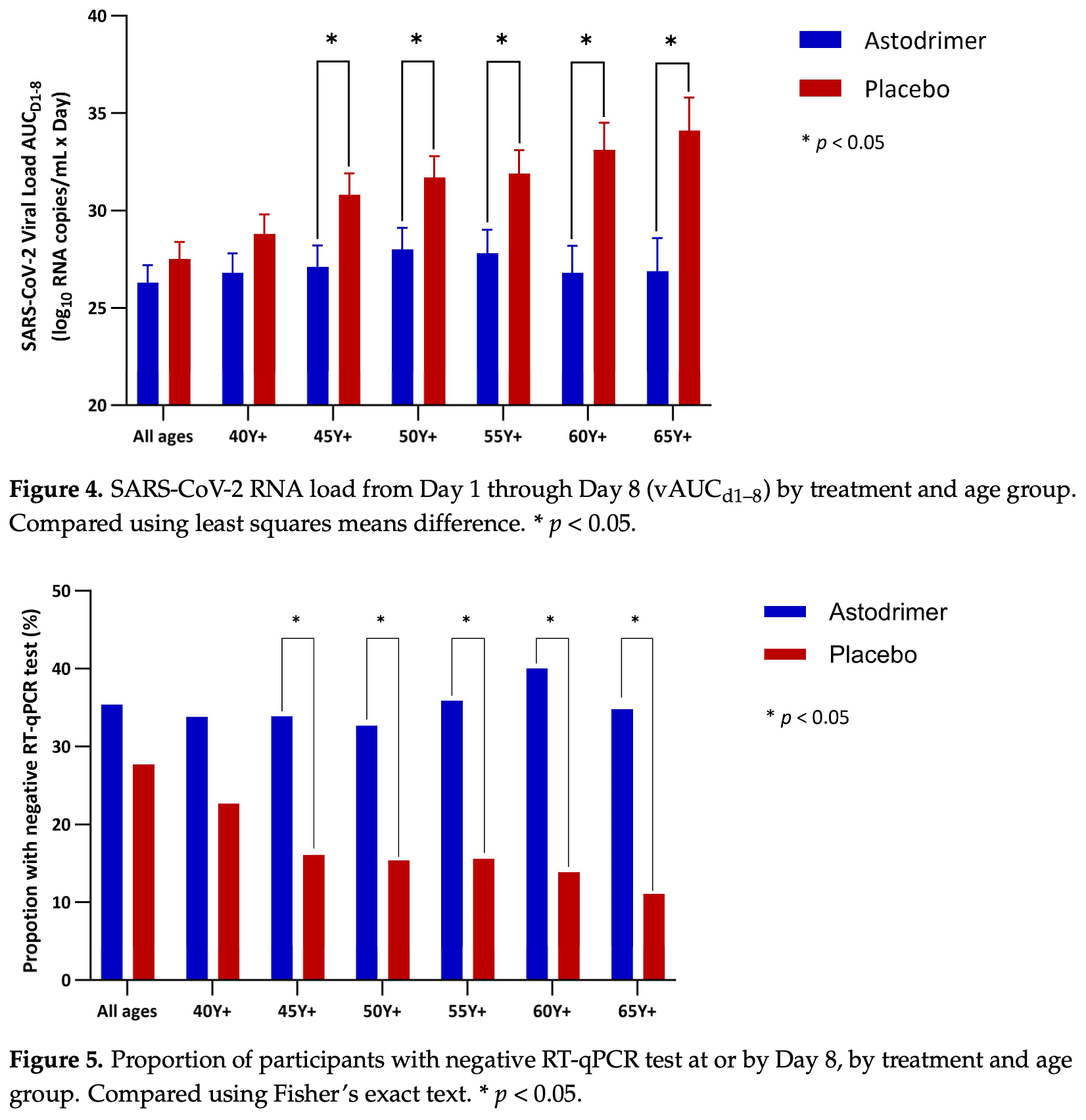
RCT 222 non-hospitalized low risk COVID-19 patients showing lower SARS-CoV-2 viral load, faster viral clearance, and improvements in symptoms, particularly anosmia, with astodrimer sodium nasal spray compared to placebo. The reduction in viral load and benefits were statistically significant in patients aged 45 years and older, with greater effects in older age groups. There was only one hospitalization event. Treatment delay is not specified and may be relatively late - authors suggest that patients were "already at or past the time of peak viral load, and the infection was already in its decline phase".
Standard of Care (SOC) for COVID-19 in the study country,
the United Kingdom, is very poor with very low average efficacy for approved treatments1.
The United Kingdom focused on expensive high-profit treatments, approving only one low-cost early treatment, which required a prescription and had limited adoption. The high-cost prescription treatment strategy reduces the probability of early treatment due to access and cost barriers, and eliminates complementary and synergistic benefits seen with many low-cost treatments.
|
risk of hospitalization, 205.2% higher, RR 3.05, p = 0.49, treatment 1 of 96 (1.0%), control 0 of 101 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm).
|
|
risk of no recovery, 19.2% lower, RR 0.81, p = 0.41, treatment 23 of 79 (29.1%), control 31 of 86 (36.0%), NNT 14, symptomatic patients, day 8, Table S8.
|
|
risk of no viral clearance, 23.7% lower, HR 0.76, p = 0.30, treatment 62 of 96 (64.6%), control 73 of 101 (72.3%), inverted to make HR<1 favor treatment, Cox proportional hazards, day 8.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Winchester et al., 6 Sep 2024, Double Blind Randomized Controlled Trial, placebo-controlled, United Kingdom, peer-reviewed, median age 50.0, 9 authors, study period 9 January, 2023 - 20 September, 2023, trial ISRCTN70449927.
Contact: jeremy.paull@starpharma.com (corresponding author), s.winchester@nhs.net, alex.castellarnau@starpharma.com, kashif.jabbar@nhs.net, meera.nadir@nhs.net, kapila.ranasinghe@nhs.net, isaac.john@nhs.net, xavier.masramon@sail-biometria.com, g.r.kinghorn@sheffield.ac.uk.
Astodrimer Sodium Nasal Spray versus Placebo in Non-Hospitalised Patients with COVID-19: A Randomised, Double-Blinded, Placebo-Controlled Trial
Pharmaceutics, doi:10.3390/pharmaceutics16091173
Background/Objectives: Dendrimer-based astodrimer sodium nasal spray was assessed for its ability to reduce SARS-CoV-2 load in outpatients with COVID-19, which remains a severe illness for vulnerable groups. Methods: This was a randomised, double-blind, placebo-controlled clinical investigation evaluating the efficacy of astodrimer nasal spray in reducing SARS-CoV-2 viral burden in the nasopharynx of outpatients with COVID-19. Non-hospitalised adults with SARS-CoV-2 infection were randomised 1:1 to astodrimer or placebo four times daily from Day 1 to Day 7. Nasopharyngeal swabs for SARS-CoV-2 load determination were self-obtained daily from Day 1 to Day 8. The primary endpoint was an area under the curve of SARS-CoV-2 RNA copies/mL through Day 8 (vAUC d1-8 ). The primary analysis population was the modified intent-totreat population (mITT: all randomised participants exposed to the study treatment who had at least one post-baseline viral load determination). Safety analyses included all randomised participants exposed to the study treatment. Study registration: ISRCTN70449927; Results: 231 participants were recruited between 9 January and 20 September 2023. The safety population comprised 109 and 113 participants randomised to astodrimer and placebo, respectively, with 96 and 101 participants in the mITT. Astodrimer sodium nasal spray reduced the SARS-CoV-2 burden (vAUC d1-8 ) vs. placebo in non-hospitalised COVID-19 patients aged 16 years and over (-1.2 log 10 copies/mL × Day). The reduction in SARS-CoV-2 load was statistically significant in those aged 45 years and older (-3.7, p = 0.017) and the effect increased in older age groups, including in those aged 65 years and older (-7.3, p = 0.005). Astodrimer sodium nasal spray increased the rate of viral clearance and helped alleviate some COVID-19 symptoms, especially loss of sense of smell. Overall, 31 participants (14%) had ≥1 adverse event (AE). Four AEs were deemed possibly related to treatment. Most AEs were of mild severity and occurred at similar rates in both treatment arms. Conclusions: Astodrimer nasal spray reduces viral burden and accelerates viral clearance, especially in older populations, and is well tolerated.
Informed Consent Statement: Informed consent was obtained from all subjects involved in the study.
Data Availability Statement: The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author. Acknowledgments: First and foremost, we sincerely thank all of the study participants for their participation. We also thank Ana Glennon for her contribution to implementing the study, following up with participants and collecting and cleaning data. Thanks to Samiya Hussain for monitoring and supporting the clinical site and contributing to data cleaning activities. We are grateful to Amelia Spiers for her support with data entry and cleaning. We also thank Freda Gomes for her assistance in communicating with the ethics committee. Our gratitude extends to Mario Garcia for his support with data cleaning, statistical programming and analysis. We acknowledge Mireia Riera for coding adverse events, medical history conditions and concomitant medications. We thank Adrian Wildfire and Martin Schutten of CHIMunomics Ltd. for their help interpreting the study results. Additionally, we thank Graham Heery, Aynaz Seta and Arnulf Company for their support in making the study product available, and Renée Brown and Sivananthan Baalakumaran for their quality assurance oversight. We are also grateful to all site and laboratory staff who contributed to this study. Finally, we sincerely thank the..
References
Anwar, Badawi, Eltablawy, Can the Coronavirus Infection Penetrates the Brain Resulting in Sudden Anosmia Followed by Severe Neurological Disorders?, eNeurologicalSci, doi:10.1016/j.ensci.2020.100290
Aydin, Benk, Geckil, May Viral Load Detected in Saliva in the Early Stages of Infection Be a Prognostic Indicator in COVID-19 Patients?, J. Virol. Methods, doi:10.1016/j.jviromet.2021.114198
Boldrini, Canoll, Klein, How COVID-19 Affects the Brain, JAMA Psychiatry, doi:10.1001/jamapsychiatry.2021.0500
Castellarnau, Heery, Seta, Luscombe, Kinghorn et al., Astodrimer Sodium Antiviral Nasal Spray for Reducing Respiratory Infections Is Safe and Well Tolerated in a Randomized Controlled Trial, Sci. Rep, doi:10.1038/s41598-022-14601-3
Da Silva, De Lima, Da Silva, Kohl, Pena, Viral Load in COVID-19 Patients: Implications for Prognosis and Vaccine Efficacy in the Context of Emerging SARS-CoV-2 Variants, Front. Med, doi:10.3389/fmed.2021.836826
Dabisch, Biryukov, Beck, Boydston, Sanjak et al., Seroconversion and Fever Are Dose-Dependent in a Nonhuman Primate Model of Inhalational COVID-19, PLoS Pathog, doi:10.1371/journal.ppat.1009865
Dadras, Afsahi, Pashaei, Mojdeganlou, Karimi et al., The Relationship between COVID-19 Viral Load and Disease Severity: A Systematic Review, Immun. Inflamm. Dis, doi:10.1002/iid3.580
Fajnzylber, Regan, Coxen, Corry, Wong et al., SARS-CoV-2 Viral Load Is Associated with Increased Disease Severity and Mortality, Nat. Commun, doi:10.1038/s41467-020-19057-5
Gallay, Luscombe, Stauffer, Bobardt, Heery et al., Effects of Astodrimer Sodium against SARS-CoV-2 Variants (α, β, γ, δ, κ) in Vitro, Top. Antivir. Med
Imai, Iwatsuki-Horimoto, Hatta, Loeber, Halfmann et al., Syrian Hamsters as a Small Animal Model for SARS-CoV-2 Infection and Countermeasure Development, Proc. Natl. Acad. Sci, doi:10.1073/pnas.2009799117
Kawasuji, Takegoshi, Kaneda, Ueno, Miyajima et al., Transmissibility of COVID-19 Depends on the Viral Load around Onset in Adult and Symptomatic Patients, PLoS ONE, doi:10.1371/journal.pone.0243597
Lewis, Is the Coronavirus Airborne? Experts Can't Agree, Nature, doi:10.1038/d41586-020-00974-w
Little, Read, Amlôt, Chadborn, Rice et al., Reducing Risks from Coronavirus Transmission in the Home-the Role of Viral Load, BMJ, doi:10.1136/bmj.m1728
Liu, Yan, Wan, Xiang, Le et al., Viral Dynamics in Mild and Severe Cases of COVID-19, Lancet Infect. Dis, doi:10.1016/S1473-3099(20)30232-2
Maltezou, Raftopoulos, Vorou, Papadima, Mellou et al., Association Between Upper Respiratory Tract Viral Load, Comorbidities, Disease Severity, and Outcome of Patients with SARS-CoV-2 Infection, J. Infect. Dis, doi:10.1093/infdis/jiaa804
Meinhardt, Radke, Dittmayer, Franz, Thomas et al., Olfactory Transmucosal SARS-CoV-2 Invasion as a Port of Central Nervous System Entry in Individuals with COVID-19, Nat. Neurosci, doi:10.1038/s41593-020-00758-5
Memoli, Czajkowski, Reed, Athota, Bristol et al., Validation of the Wild-Type Influenza A Human Challenge Model H1N1pdMIST: An A(H1N1)Pdm09 Dose-Finding Investigational New Drug Study, Clin. Infect. Dis, doi:10.1093/cid/ciu924
Meyerowitz, Scott, Richterman, Male, Cevik, Clinical Course and Management of COVID-19 in the Era of Widespread Population Immunity, Nat. Rev. Microbiol, doi:10.1038/s41579-023-01001-1
Najjar-Debbiny, Gronich, Weber, Khoury, Amar et al., Effectiveness of Paxlovid in Reducing Severe Coronavirus Disease 2019 and Mortality in High-Risk Patients, Clin Infect Dis, doi:10.1093/cid/ciac443
Paull, Heery, Bobardt, Castellarnau, Luscombe et al., Virucidal and Antiviral Activity of Astodrimer Sodium against SARS-CoV-2 in Vitro, Antiviral Res, doi:10.1016/j.antiviral.2021.105089
Paull, Luscombe, Castellarnau, Heery, Bobardt et al., Protective Effects of Astodrimer Sodium 1% Nasal Spray Formulation against SARS-CoV-2 Nasal Challenge in K18-hACE2 Mice, Viruses, doi:10.3390/v13081656
Powers, Bacci, Guerrero, Leidy, Stringer et al., Reliability, Validity, and Responsiveness of InFLUenza Patient-Reported Outcome (FLU-PRO©) Scores in Influenza-Positive Patients, Value Health, doi:10.1016/j.jval.2017.04.014
Powers, Guerrero, Leidy, Fairchok, Rosenberg et al., Development of the Flu-PRO: A Patient-Reported Outcome (PRO) Instrument to Evaluate Symptoms of Influenza, BMC Infect. Dis, doi:10.1186/s12879-015-1330-0
Ruiz, Andrew, COVID-19 Vaccination and Hybrid Immunity in Older Adults, Lancet Healthy Longev, doi:10.1016/S2666-7568(23)00112-5
Tang, Mao, Jones, Tan, Ji et al., Aerosol Transmission of SARS-CoV-2? Evidence, Prevention and Control, Environ. Int, doi:10.1016/j.envint.2020.106039
Vegvari, Hadjichrysanthou, Cauët, Lawrence, Cori et al., How Can Viral Dynamics Models Inform Endpoint Measures in Clinical Trials of Therapies for Acute Viral Infections?, PLoS ONE, doi:10.1371/journal.pone.0158237
Vilarello, Jacobson, Tervo, Waring, Gudis et al., Olfaction and Neurocognition after COVID-19: A Scoping Review, Front. Neurosci
Winchester, John, Jabbar, John, Clinical Efficacy of Nitric Oxide Nasal Spray (NONS) for the Treatment of Mild COVID-19 Infection, J. Infect, doi:10.1016/j.jinf.2021.05.009
Yan, Grantham, Pantelic, Bueno De Mesquita, Albert et al., EMIT Consortium Infectious Virus in Exhaled Breath of Symptomatic Seasonal Influenza Cases from a College Community, Proc. Natl. Acad. Sci, doi:10.1073/pnas.1716561115
DOI record:
{
"DOI": "10.3390/pharmaceutics16091173",
"ISSN": [
"1999-4923"
],
"URL": "http://dx.doi.org/10.3390/pharmaceutics16091173",
"abstract": "<jats:p>Background/Objectives: Dendrimer-based astodrimer sodium nasal spray was assessed for its ability to reduce SARS-CoV-2 load in outpatients with COVID-19, which remains a severe illness for vulnerable groups. Methods: This was a randomised, double-blind, placebo-controlled clinical investigation evaluating the efficacy of astodrimer nasal spray in reducing SARS-CoV-2 viral burden in the nasopharynx of outpatients with COVID-19. Non-hospitalised adults with SARS-CoV-2 infection were randomised 1:1 to astodrimer or placebo four times daily from Day 1 to Day 7. Nasopharyngeal swabs for SARS-CoV-2 load determination were self-obtained daily from Day 1 to Day 8. The primary endpoint was an area under the curve of SARS-CoV-2 RNA copies/mL through Day 8 (vAUCd1–8). The primary analysis population was the modified intent-to-treat population (mITT: all randomised participants exposed to the study treatment who had at least one post-baseline viral load determination). Safety analyses included all randomised participants exposed to the study treatment. Study registration: ISRCTN70449927; Results: 231 participants were recruited between 9 January and 20 September 2023. The safety population comprised 109 and 113 participants randomised to astodrimer and placebo, respectively, with 96 and 101 participants in the mITT. Astodrimer sodium nasal spray reduced the SARS-CoV-2 burden (vAUCd1–8) vs. placebo in non-hospitalised COVID-19 patients aged 16 years and over (−1.2 log10 copies/mL × Day). The reduction in SARS-CoV-2 load was statistically significant in those aged 45 years and older (−3.7, p = 0.017) and the effect increased in older age groups, including in those aged 65 years and older (−7.3, p = 0.005). Astodrimer sodium nasal spray increased the rate of viral clearance and helped alleviate some COVID-19 symptoms, especially loss of sense of smell. Overall, 31 participants (14%) had ≥1 adverse event (AE). Four AEs were deemed possibly related to treatment. Most AEs were of mild severity and occurred at similar rates in both treatment arms. Conclusions: Astodrimer nasal spray reduces viral burden and accelerates viral clearance, especially in older populations, and is well tolerated.</jats:p>",
"alternative-id": [
"pharmaceutics16091173"
],
"author": [
{
"affiliation": [
{
"name": "Frimley Health National Health Service Foundation Trust, Camberley GU16 7UJ, UK"
}
],
"family": "Winchester",
"given": "Stephen",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Starpharma Pty Ltd., Abbotsford, VIC 3067, Australia"
}
],
"family": "Castellarnau",
"given": "Alex",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Ashford and St. Peter’s Hospitals National Health Service Foundation Trust, Chertsey KT16 0PZ, UK"
}
],
"family": "Jabbar",
"given": "Kashif",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Ashford and St. Peter’s Hospitals National Health Service Foundation Trust, Chertsey KT16 0PZ, UK"
}
],
"family": "Nadir",
"given": "Meera",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Ashford and St. Peter’s Hospitals National Health Service Foundation Trust, Chertsey KT16 0PZ, UK"
}
],
"family": "Ranasinghe",
"given": "Kapila",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Servicio de Asesoría a la Investigación y Logística (SAIL), 08027 Barcelona, Spain"
}
],
"family": "Masramon",
"given": "Xavier",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Royal Hallamshire Hospital, University of Sheffield, Western Bank, Sheffield S10 2TN, UK"
}
],
"family": "Kinghorn",
"given": "George R.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2856-6180",
"affiliation": [
{
"name": "Ashford and St. Peter’s Hospitals National Health Service Foundation Trust, Chertsey KT16 0PZ, UK"
},
{
"name": "Royal Holloway, University of London, Egham TW20 0EX, UK"
}
],
"authenticated-orcid": false,
"family": "John",
"given": "Isaac",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9981-421X",
"affiliation": [
{
"name": "Starpharma Pty Ltd., Abbotsford, VIC 3067, Australia"
}
],
"authenticated-orcid": false,
"family": "Paull",
"given": "Jeremy R. A.",
"sequence": "additional"
}
],
"container-title": "Pharmaceutics",
"container-title-short": "Pharmaceutics",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
9,
6
]
],
"date-time": "2024-09-06T09:02:28Z",
"timestamp": 1725613348000
},
"deposited": {
"date-parts": [
[
2024,
9,
6
]
],
"date-time": "2024-09-06T09:12:06Z",
"timestamp": 1725613926000
},
"funder": [
{
"name": "Starpharma Pty Ltd."
}
],
"indexed": {
"date-parts": [
[
2024,
9,
7
]
],
"date-time": "2024-09-07T00:30:30Z",
"timestamp": 1725669030258
},
"is-referenced-by-count": 0,
"issue": "9",
"issued": {
"date-parts": [
[
2024,
9,
6
]
]
},
"journal-issue": {
"issue": "9",
"published-online": {
"date-parts": [
[
2024,
9
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
9,
6
]
],
"date-time": "2024-09-06T00:00:00Z",
"timestamp": 1725580800000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/1999-4923/16/9/1173/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "1173",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2024,
9,
6
]
]
},
"published-online": {
"date-parts": [
[
2024,
9,
6
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1136/bmj.m1728",
"article-title": "Reducing Risks from Coronavirus Transmission in the Home-the Role of Viral Load",
"author": "Little",
"doi-asserted-by": "crossref",
"first-page": "m1728",
"journal-title": "BMJ",
"key": "ref_1",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.1073/pnas.2009799117",
"article-title": "Syrian Hamsters as a Small Animal Model for SARS-CoV-2 Infection and Countermeasure Development",
"author": "Imai",
"doi-asserted-by": "crossref",
"first-page": "16587",
"journal-title": "Proc. Natl. Acad. Sci. USA",
"key": "ref_2",
"volume": "117",
"year": "2020"
},
{
"DOI": "10.1371/journal.ppat.1009865",
"doi-asserted-by": "crossref",
"key": "ref_3",
"unstructured": "Dabisch, P.A., Biryukov, J., Beck, K., Boydston, J.A., Sanjak, J.S., Herzog, A., Green, B., Williams, G., Yeager, J., and Bohannon, J.K. (2021). Seroconversion and Fever Are Dose-Dependent in a Nonhuman Primate Model of Inhalational COVID-19. PLoS Pathog., 17."
},
{
"DOI": "10.1093/cid/ciu924",
"article-title": "Validation of the Wild-Type Influenza A Human Challenge Model H1N1pdMIST: An A(H1N1)Pdm09 Dose-Finding Investigational New Drug Study",
"author": "Memoli",
"doi-asserted-by": "crossref",
"first-page": "693",
"journal-title": "Clin. Infect. Dis.",
"key": "ref_4",
"volume": "60",
"year": "2015"
},
{
"DOI": "10.1016/S1473-3099(20)30232-2",
"article-title": "Viral Dynamics in Mild and Severe Cases of COVID-19",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "656",
"journal-title": "Lancet Infect. Dis.",
"key": "ref_5",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1038/s41467-020-19057-5",
"article-title": "SARS-CoV-2 Viral Load Is Associated with Increased Disease Severity and Mortality",
"author": "Fajnzylber",
"doi-asserted-by": "crossref",
"first-page": "5493",
"journal-title": "Nat. Commun.",
"key": "ref_6",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1093/infdis/jiaa804",
"article-title": "Association Between Upper Respiratory Tract Viral Load, Comorbidities, Disease Severity, and Outcome of Patients with SARS-CoV-2 Infection",
"author": "Maltezou",
"doi-asserted-by": "crossref",
"first-page": "1132",
"journal-title": "J. Infect. Dis.",
"key": "ref_7",
"volume": "223",
"year": "2021"
},
{
"DOI": "10.3389/fmed.2021.836826",
"doi-asserted-by": "crossref",
"key": "ref_8",
"unstructured": "da Silva, S.J.R., de Lima, S.C., da Silva, R.C., Kohl, A., and Pena, L. (2021). Viral Load in COVID-19 Patients: Implications for Prognosis and Vaccine Efficacy in the Context of Emerging SARS-CoV-2 Variants. Front. Med., 8."
},
{
"DOI": "10.1371/journal.pone.0243597",
"doi-asserted-by": "crossref",
"key": "ref_9",
"unstructured": "Kawasuji, H., Takegoshi, Y., Kaneda, M., Ueno, A., Miyajima, Y., Kawago, K., Fukui, Y., Yoshida, Y., Kimura, M., and Yamada, H. (2020). Transmissibility of COVID-19 Depends on the Viral Load around Onset in Adult and Symptomatic Patients. PLoS ONE, 15."
},
{
"DOI": "10.1038/s41579-023-01001-1",
"article-title": "Clinical Course and Management of COVID-19 in the Era of Widespread Population Immunity",
"author": "Meyerowitz",
"doi-asserted-by": "crossref",
"first-page": "75",
"journal-title": "Nat. Rev. Microbiol.",
"key": "ref_10",
"volume": "22",
"year": "2024"
},
{
"DOI": "10.1038/d41586-020-00974-w",
"article-title": "Is the Coronavirus Airborne? Experts Can’t Agree",
"author": "Lewis",
"doi-asserted-by": "crossref",
"first-page": "175",
"journal-title": "Nature",
"key": "ref_11",
"volume": "580",
"year": "2020"
},
{
"DOI": "10.1016/j.envint.2020.106039",
"article-title": "Aerosol Transmission of SARS-CoV-2? Evidence, Prevention and Control",
"author": "Tang",
"doi-asserted-by": "crossref",
"first-page": "106039",
"journal-title": "Environ. Int.",
"key": "ref_12",
"volume": "144",
"year": "2020"
},
{
"DOI": "10.1073/pnas.1716561115",
"article-title": "EMIT Consortium Infectious Virus in Exhaled Breath of Symptomatic Seasonal Influenza Cases from a College Community",
"author": "Yan",
"doi-asserted-by": "crossref",
"first-page": "1081",
"journal-title": "Proc. Natl. Acad. Sci. USA",
"key": "ref_13",
"volume": "115",
"year": "2018"
},
{
"DOI": "10.1016/j.antiviral.2021.105089",
"article-title": "Virucidal and Antiviral Activity of Astodrimer Sodium against SARS-CoV-2 in Vitro",
"author": "Paull",
"doi-asserted-by": "crossref",
"first-page": "105089",
"journal-title": "Antiviral Res.",
"key": "ref_14",
"volume": "191",
"year": "2021"
},
{
"article-title": "Effects of Astodrimer Sodium against SARS-CoV-2 Variants (α, β, γ, δ, κ) in Vitro [CROI Abstract 478]. Special Issue: Abstracts from the Virtual 2022 Conference on Retroviruses and Opportunistic Infections",
"author": "Gallay",
"first-page": "182",
"journal-title": "Top. Antivir. Med.",
"key": "ref_15",
"volume": "30",
"year": "2022"
},
{
"DOI": "10.3390/v13081656",
"doi-asserted-by": "crossref",
"key": "ref_16",
"unstructured": "Paull, J.R.A., Luscombe, C.A., Castellarnau, A., Heery, G.P., Bobardt, M.D., and Gallay, P.A. (2021). Protective Effects of Astodrimer Sodium 1% Nasal Spray Formulation against SARS-CoV-2 Nasal Challenge in K18-hACE2 Mice. Viruses, 13."
},
{
"DOI": "10.1038/s41598-022-14601-3",
"doi-asserted-by": "crossref",
"key": "ref_17",
"unstructured": "Castellarnau, A., Heery, G.P., Seta, A., Luscombe, C.A., Kinghorn, G.R., Button, P., McCloud, P., and Paull, J.R.A. (2022). Astodrimer Sodium Antiviral Nasal Spray for Reducing Respiratory Infections Is Safe and Well Tolerated in a Randomized Controlled Trial. Sci. Rep., 12."
},
{
"key": "ref_18",
"unstructured": "WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection (2020). A Minimal Common Outcome Measure Set for COVID-19 Clinical Research. Lancet Infect. Dis., 20, e192–e197."
},
{
"DOI": "10.1186/s12879-015-1330-0",
"doi-asserted-by": "crossref",
"key": "ref_19",
"unstructured": "Powers, J.H., Guerrero, M.L., Leidy, N.K., Fairchok, M.P., Rosenberg, A., Hernández, A., Stringer, S., Schofield, C., Rodríguez-Zulueta, P., and Kim, K. (2016). Development of the Flu-PRO: A Patient-Reported Outcome (PRO) Instrument to Evaluate Symptoms of Influenza. BMC Infect. Dis., 16."
},
{
"DOI": "10.1016/j.jval.2017.04.014",
"article-title": "Reliability, Validity, and Responsiveness of InFLUenza Patient-Reported Outcome (FLU-PRO©) Scores in Influenza-Positive Patients",
"author": "Powers",
"doi-asserted-by": "crossref",
"first-page": "210",
"journal-title": "Value Health",
"key": "ref_20",
"volume": "21",
"year": "2018"
},
{
"DOI": "10.1002/iid3.580",
"article-title": "The Relationship between COVID-19 Viral Load and Disease Severity: A Systematic Review",
"author": "Dadras",
"doi-asserted-by": "crossref",
"first-page": "e580",
"journal-title": "Immun. Inflamm. Dis.",
"key": "ref_21",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1016/j.jinf.2021.05.009",
"article-title": "Clinical Efficacy of Nitric Oxide Nasal Spray (NONS) for the Treatment of Mild COVID-19 Infection",
"author": "Winchester",
"doi-asserted-by": "crossref",
"first-page": "237",
"journal-title": "J. Infect.",
"key": "ref_22",
"volume": "83",
"year": "2021"
},
{
"DOI": "10.1016/j.jviromet.2021.114198",
"article-title": "May Viral Load Detected in Saliva in the Early Stages of Infection Be a Prognostic Indicator in COVID-19 Patients?",
"author": "Aydin",
"doi-asserted-by": "crossref",
"first-page": "114198",
"journal-title": "J. Virol. Methods",
"key": "ref_23",
"volume": "294",
"year": "2021"
},
{
"DOI": "10.1371/journal.pone.0158237",
"doi-asserted-by": "crossref",
"key": "ref_24",
"unstructured": "Vegvari, C., Hadjichrysanthou, C., Cauët, E., Lawrence, E., Cori, A., de Wolf, F., and Anderson, R.M. (2016). How Can Viral Dynamics Models Inform Endpoint Measures in Clinical Trials of Therapies for Acute Viral Infections?. PLoS ONE, 11."
},
{
"DOI": "10.1016/S2666-7568(23)00112-5",
"article-title": "COVID-19 Vaccination and Hybrid Immunity in Older Adults",
"author": "Ruiz",
"doi-asserted-by": "crossref",
"first-page": "e364",
"journal-title": "Lancet Healthy Longev.",
"key": "ref_25",
"volume": "4",
"year": "2023"
},
{
"DOI": "10.1093/cid/ciac443",
"article-title": "Effectiveness of Paxlovid in Reducing Severe Coronavirus Disease 2019 and Mortality in High-Risk Patients",
"author": "Gronich",
"doi-asserted-by": "crossref",
"first-page": "e342",
"journal-title": "Clin Infect Dis",
"key": "ref_26",
"volume": "76",
"year": "2023"
},
{
"DOI": "10.1038/s41593-020-00758-5",
"article-title": "Olfactory Transmucosal SARS-CoV-2 Invasion as a Port of Central Nervous System Entry in Individuals with COVID-19",
"author": "Meinhardt",
"doi-asserted-by": "crossref",
"first-page": "168",
"journal-title": "Nat. Neurosci.",
"key": "ref_27",
"volume": "24",
"year": "2021"
},
{
"DOI": "10.1001/jamapsychiatry.2021.0500",
"article-title": "How COVID-19 Affects the Brain",
"author": "Boldrini",
"doi-asserted-by": "crossref",
"first-page": "682",
"journal-title": "JAMA Psychiatry",
"key": "ref_28",
"volume": "78",
"year": "2021"
},
{
"DOI": "10.1016/j.ensci.2020.100290",
"article-title": "Can the Coronavirus Infection Penetrates the Brain Resulting in Sudden Anosmia Followed by Severe Neurological Disorders?",
"author": "Anwar",
"doi-asserted-by": "crossref",
"first-page": "100290",
"journal-title": "eNeurologicalSci",
"key": "ref_29",
"volume": "21",
"year": "2020"
},
{
"DOI": "10.3389/fnins.2023.1198267",
"doi-asserted-by": "crossref",
"key": "ref_30",
"unstructured": "Vilarello, B.J., Jacobson, P.T., Tervo, J.P., Waring, N.A., Gudis, D.A., Goldberg, T.E., Devanand, D.P., and Overdevest, J.B. (2023). Olfaction and Neurocognition after COVID-19: A Scoping Review. Front. Neurosci., 17."
}
],
"reference-count": 30,
"references-count": 30,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/1999-4923/16/9/1173"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Astodrimer Sodium Nasal Spray versus Placebo in Non-Hospitalised Patients with COVID-19: A Randomised, Double-Blinded, Placebo-Controlled Trial",
"type": "journal-article",
"volume": "16"
}