Colchicine in Recently Hospitalized Patients with COVID-19: A Randomized Controlled Trial (COL-COVID)
et al., International Journal of General Medicine, doi:10.2147/IJGM.S329810, NCT04350320, Sep 2021
Colchicine for COVID-19
5th treatment shown to reduce risk in
September 2020, now with p = 0.0000049 from 54 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
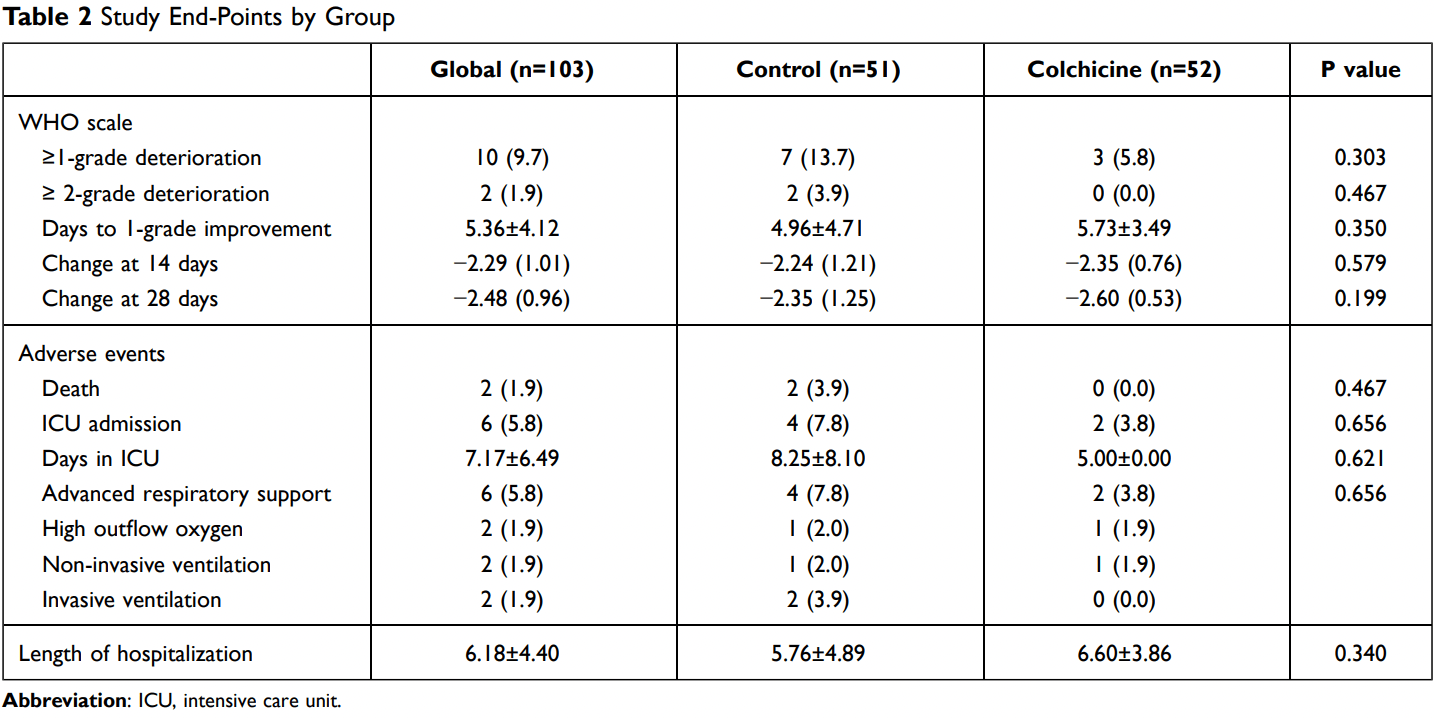
RCT with 52 colchicine patients and 51 control patients, showing lower risk of clinical deterioration with treatment. COL-COVID. NCT04350320 (history).
|
risk of death, 80.2% lower, RR 0.20, p = 0.24, treatment 0 of 52 (0.0%), control 2 of 51 (3.9%), NNT 26, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of mechanical ventilation, 80.2% lower, RR 0.20, p = 0.24, treatment 0 of 52 (0.0%), control 2 of 51 (3.9%), NNT 26, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of ICU admission, 51.0% lower, RR 0.49, p = 0.44, treatment 2 of 52 (3.8%), control 4 of 51 (7.8%), NNT 25.
|
|
risk of 7-point scale, 87.5% lower, RR 0.13, p = 0.03, treatment 3 of 52 (5.8%), control 7 of 51 (13.7%), adjusted per study, odds ratio converted to relative risk, deterioration ≥1 point, multivariable, primary outcome.
|
|
risk of 7-point scale, 80.2% lower, RR 0.20, p = 0.24, treatment 0 of 52 (0.0%), control 2 of 51 (3.9%), NNT 26, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), deterioration ≥2 points.
|
|
hospitalization time, 14.6% higher, relative time 1.15, p = 0.34, treatment 52, control 51.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Pascual-Figal et al., 11 Sep 2021, Randomized Controlled Trial, Spain, peer-reviewed, 14 authors, study period 30 April, 2020 - 4 December, 2020, dosage 1.5mg day 1, 1mg days 2-8, 0.5mg days 9-36, trial NCT04350320 (history).
Colchicine in Recently Hospitalized Patients with COVID-19: A Randomized Controlled Trial (COL-COVID)
International Journal of General Medicine, doi:10.2147/ijgm.s329810
Background: Colchicine has been proposed as a potential therapy in coronavirus disease 2019 (COVID-19) due to their anti-inflammatory actions. Methods: The COL-COVID study was a prospective, randomized, controlled and openlabel clinical trial that compared colchicine added to standard treatment vs standard treatment in hospitalized COVID-19 patients that do not need mechanical ventilatory support. Colchicine was initiated within the first 48 hours of admission at a 1.5 mg loading dose, followed by 0.5 mg b.i.d. for one week and 0.5 mg per day for 28 days. The study endpoints were clinical status (7-points WHO ordinal scale) and inflammatory biomarkers (IL-6 and CRP). Results: A total of 103 patients (51±12 years, 52% male) were randomly allocated to colchicine arm (n=52) and control arm (n=51). At day 28, all patients in the colchicine group were alive and discharged, whereas in the control group, two patients died in-hospital and one patient remained hospitalized. Clinical improvement in terms of changes on WHO scale at day 14 and 28 and time to 1-point clinical improvement did not differ between the two groups. Clinical deterioration (increase of at least 1-point in WHO scale) was observed in a higher proportion of cases in colchicine group (13.8%) vs control group (5.8%) (p=0.303); after adjustment by baseline risk factors and concomitant therapies, colchicine therapy was associated with a lower risk of clinical deterioration (p=0.030). Inflammatory biomarkers CRP and IL-6 concentrations course did not differ between the two arms.
Conclusion: In hospitalized COVID-19 patients, colchicine treatment neither improved the clinical status, nor the inflammatory response, over the standard treatment. Nevertheless, a preventive effect for further clinical deterioration might be possible. Trial Registration: NCT04350320.
finding the observed low rate of thrombosis in the COLCORONA trial. 21 Among the limitations of our study are the small sample size and the limited number of adverse events that underpowered the ability to reach conclusions. The sample size also limited the ability to control other relevant comorbidities, such as diabetes and related therapies, 28 and to investigate the effect for subgroups. Indeed, despite the controlled randomization, rates of dexamethasone, remdesivir and inhaled bronchodilators were higher in patients allocated to colchicine suggesting a higher risk from the caring physician perspective. Nevertheless, this study adds new evidence to the body of randomized clinical trials evaluating the role of colchicine in preventing the progression of COVID-19. Indeed, a recent meta-analysis showed a positive effect of colchicine in preventing the severity and mortality rate in COVID-19 patients. 29 However, only three studies were randomized trials, 20, 21, 24 which complicates reaching a conclusion regarding the effect, especially considering subgroups and populations; as the authors stated, there is a need for more randomized clinical trials. 29 The RECOVERY trial included colchicine as one therapeutic candidate to compare with usual care alone in
Ethics Statement The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the Clinical University Hospital Virgen de la Arrixaca (EUDRACT..
References
Abani, Abbas, Abbas, Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial, Lancet
Aimo, Figal, Bayes-Genis, Emdin, Georgiopoulos, Effect of low-dose colchicine in acute and chronic coronary syndromes: a systematic review and meta-analysis, Eur J Clin Invest
Anon, Dexamethasone in hospitalized patients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2021436
Brunetti, Diawara, Tsai, Colchicine to weather the cytokine storm in hospitalized patients with COVID-19, J Clin Med, doi:10.3390/jcm9092961
Burrage, Koushesh, Sofat, Immunomodulatory drugs in the management of SARS-CoV-2, Front Immunol
Channappanavar, Perlman, Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology, Semin Immunopathol, doi:10.1007/s00281-017-0629-x
Conti, Ronconi, Caraffa, Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by COVID-19: anti-inflammatory strategies, J Biol Regul Homeost Agents
Deftereos, Giannopoulos, Vrachatis, Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: the GRECCO-19 randomized clinical trial, JAMA Netw open, doi:10.1001/jamanetworkopen.2020.13136
Della-Torre, Della-Torre, Kusanovic, Treating COVID-19 with colchicine in community healthcare setting, Clin Immunol
Guan, Ni, Hu, Clinical characteristics of coronavirus disease 2019 in China, N Engl J Med, doi:10.1056/NEJMoa2002032
Ivan, Devina, Halim, Jodhinata, Colchicine treatment can improve outcomes of coronavirus 19): a systematic review and analysis, Clin Exp Pharmacol Physiol
Ivan, Kurniawan, Metformin use is associated with reduced mortality rate from coronavirus disease 2019 (COVID-19) infection, Obes Med, doi:10.1016/j.obmed.2020.100290
Landray, Colchicine in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial
Lin, Lu, Cao, Li, Hypothesis for potential pathogenesis of SARS-CoV-2 infection -a review of immune changes in patients with viral pneumonia, Emerg Microbes Infect
Lopes, Bonjorno, Giannini, Beneficial effects of colchicine for moderate to severe COVID-19: an interim analysis of a randomized, double-blinded, placebo controlled clinical trial, medRxiv
Otani, Watanabe, Shimada, Colchicine prevents NSAID-induced small intestinal injury by inhibiting activation of the NLRP3 inflammasome, Sci Rep, doi:10.1038/srep32587
Perricone, Bartoloni, Gerli, Colchicine, an anti-rheumatic agent, as a potential compound for the treatment of COVID-19, Reumatologia, doi:10.5114/reum.2020.100088
Pope, Tschopp, The role of interleukin-1 and the inflammasome in gout: implications for therapy, Arthritis Rheum, doi:10.1002/art.22938
Ravelli, Gigant, Curmi, Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain, Nature, doi:10.1038/nature02393
Rizk, Kalantar-Zadeh, Mehra, Lavie, Rizk et al., Pharmaco-immunomodulatory therapy in COVID-19, Drugs, doi:10.1007/s40265-020-01367-z
Sandhu, Tieng, Chilimuri, Franchin, A case control study to evaluate the impact of colchicine on patients admitted to the hospital with moderate to severe covid-19 infection, Can J Infect Dis Med Microbiol
Scarsi, Piantoni, Colombo, Association between treatment with colchicine and improved survival in a single-centre cohort of adult hospitalised patients with COVID-19 pneumonia and acute respiratory distress syndrome, Ann Rheum Dis, doi:10.1136/annrheumdis-2020-217712
Siddiqi, Mehra, COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal, J Hear Lung Transplant, doi:10.1016/j.healun.2020.03.012
Slobodnick, Shah, Krasnokutsky, Pillinger, Update on colchicine, Rheumatology, doi:10.1093/rheumatology/kex453
Tardif, Bouabdallaoui, Allier, Colchicine for community-treated patients with COVID-19 (COLCORONA): a Phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial, Lancet Respir Med
Vitiello, Ferrara, Ferrara, Colchicine and SARS-CoV-2: management of the hyperinflammatory state, Respir Med, doi:10.1016/j.rmed.2021.106322
Yousefi, Mashouri, Okpechi, Alahari, Alahari, Repurposing existing drugs for the treatment of COVID-19/SARS-CoV-2 infection: a review describing drug mechanisms of action, Biochem Pharmacol
Zhou, Yu, Du, Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a Retrospective Cohort Study, Lancet, doi:10.1016/S0140-6736(20)30566-3
DOI record:
{
"DOI": "10.2147/ijgm.s329810",
"ISSN": [
"1178-7074"
],
"URL": "http://dx.doi.org/10.2147/ijgm.s329810",
"author": [
{
"ORCID": "http://orcid.org/0000-0002-4993-9540",
"affiliation": [],
"authenticated-orcid": true,
"family": "Pascual-Figal",
"given": "Domingo A",
"sequence": "first"
},
{
"affiliation": [],
"family": "Roura-Piloto",
"given": "Aychel E",
"sequence": "additional",
"suffix": "Snr"
},
{
"affiliation": [],
"family": "Moral-Escudero",
"given": "Encarnación",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bernal",
"given": "Enrique",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Albendin-Iglesias",
"given": "Helena",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pérez-Martínez",
"given": "M Teresa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Noguera-Velasco",
"given": "Jose Antonio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cebreiros-López",
"given": "Iria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hernández-Vicente",
"given": "Álvaro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vázquez-Andrés",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sánchez-Pérez",
"given": "Carmen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Khan",
"given": "Amjad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sánchez-Cabo",
"given": "Fátima",
"sequence": "additional"
},
{
"affiliation": [],
"family": "García-Vázquez",
"given": "Elisa",
"sequence": "additional"
}
],
"container-title": [
"International Journal of General Medicine"
],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
9,
10
]
],
"date-time": "2021-09-10T16:30:07Z",
"timestamp": 1631291407000
},
"deposited": {
"date-parts": [
[
2021,
9,
10
]
],
"date-time": "2021-09-10T16:30:23Z",
"timestamp": 1631291423000
},
"indexed": {
"date-parts": [
[
2022,
2,
3
]
],
"date-time": "2022-02-03T12:29:55Z",
"timestamp": 1643891395675
},
"is-referenced-by-count": 4,
"issn-type": [
{
"type": "electronic",
"value": "1178-7074"
}
],
"issued": {
"date-parts": [
[
2021,
9
]
]
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc/3.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
9,
1
]
],
"date-time": "2021-09-01T00:00:00Z",
"timestamp": 1630454400000
}
}
],
"link": [
{
"URL": "https://www.dovepress.com/getfile.php?fileID=73562",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.dovepress.com/getfile.php?fileID=73562",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "301",
"original-title": [],
"page": "5517-5526",
"prefix": "10.2147",
"published": {
"date-parts": [
[
2021,
9
]
]
},
"published-online": {
"date-parts": [
[
2021,
9
]
]
},
"publisher": "Informa UK Limited",
"reference": [
{
"DOI": "10.1056/NEJMoa2002032",
"author": "Guan",
"doi-asserted-by": "publisher",
"first-page": "1708",
"journal-title": "N Engl J Med",
"key": "ref1",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"author": "Zhou",
"doi-asserted-by": "publisher",
"first-page": "1054",
"journal-title": "Lancet",
"key": "ref2",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/j.healun.2020.03.012",
"author": "Siddiqi",
"doi-asserted-by": "publisher",
"first-page": "405",
"journal-title": "J Hear Lung Transplant",
"key": "ref3",
"volume": "39",
"year": "2020"
},
{
"author": "Lin",
"first-page": "1",
"journal-title": "Emerg Microbes Infect",
"key": "ref4",
"year": "2020"
},
{
"DOI": "10.1007/s00281-017-0629-x",
"author": "Channappanavar",
"doi-asserted-by": "publisher",
"first-page": "529",
"journal-title": "Semin Immunopathol",
"key": "ref5",
"volume": "39",
"year": "2017"
},
{
"author": "Conti",
"first-page": "1",
"journal-title": "J Biol Regul Homeost Agents",
"key": "ref6",
"volume": "34",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2020.01844",
"author": "Burrage",
"doi-asserted-by": "crossref",
"first-page": "1844",
"journal-title": "Front Immunol",
"key": "ref7",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1007/s40265-020-01367-z",
"author": "Rizk",
"doi-asserted-by": "publisher",
"first-page": "1267",
"journal-title": "Drugs",
"key": "ref8",
"volume": "80",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2021436",
"doi-asserted-by": "crossref",
"key": "ref9",
"unstructured": "Anon. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693–704. doi:10.1056/NEJMoa2021436"
},
{
"DOI": "10.1016/S0140-6736(21)00676-0",
"author": "Abani",
"doi-asserted-by": "crossref",
"first-page": "1637",
"journal-title": "Lancet",
"key": "ref10",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1093/rheumatology/kex453",
"author": "Slobodnick",
"doi-asserted-by": "publisher",
"first-page": "i4",
"journal-title": "Rheumatology (Oxford)",
"key": "ref11",
"volume": "57",
"year": "2018"
},
{
"author": "Aimo",
"first-page": "e13464",
"journal-title": "Eur J Clin Invest",
"key": "ref12",
"volume": "51",
"year": "2020"
},
{
"DOI": "10.1038/nature02393",
"author": "Ravelli",
"doi-asserted-by": "publisher",
"first-page": "198",
"journal-title": "Nature",
"key": "ref13",
"volume": "428",
"year": "2004"
},
{
"DOI": "10.1038/srep32587",
"author": "Otani",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Sci Rep",
"key": "ref14",
"volume": "6",
"year": "2016"
},
{
"DOI": "10.1002/art.22938",
"author": "Pope",
"doi-asserted-by": "publisher",
"first-page": "3183",
"journal-title": "Arthritis Rheum",
"key": "ref15",
"volume": "56",
"year": "2007"
},
{
"DOI": "10.1016/j.bcp.2020.114296",
"author": "Yousefi",
"doi-asserted-by": "crossref",
"journal-title": "Biochem Pharmacol",
"key": "ref16",
"volume": "183",
"year": "2021"
},
{
"DOI": "10.5114/reum.2020.100088",
"author": "Perricone",
"doi-asserted-by": "publisher",
"first-page": "261",
"journal-title": "Reumatologia",
"key": "ref17",
"volume": "58",
"year": "2020"
},
{
"DOI": "10.1016/j.rmed.2021.106322",
"author": "Vitiello",
"doi-asserted-by": "publisher",
"first-page": "106322",
"journal-title": "Respir Med",
"key": "ref18",
"volume": "178",
"year": "2021"
},
{
"DOI": "10.1001/jamanetworkopen.2020.13136",
"author": "Deftereos",
"doi-asserted-by": "publisher",
"first-page": "e2013136",
"journal-title": "JAMA Netw open",
"key": "ref19",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.1016/j.clim.2020.108490",
"author": "Della-Torre",
"doi-asserted-by": "crossref",
"first-page": "108490",
"journal-title": "Clin Immunol",
"key": "ref20",
"volume": "217",
"year": "2020"
},
{
"author": "Tardif",
"first-page": "1",
"journal-title": "Lancet Respir Med",
"key": "ref21",
"volume": "19",
"year": "2021"
},
{
"author": "Landray",
"journal-title": "medRxiv",
"key": "ref22",
"year": "2021"
},
{
"key": "ref23",
"unstructured": "World Health Organization. R&D blueprint and COVID-19. World Health Organization; 2020. Available from: https://www.who.int/teams/blueprint/covid-19. Accessed February 19, 2021."
},
{
"author": "Lopes",
"journal-title": "medRxiv",
"key": "ref24",
"year": "2020"
},
{
"DOI": "10.1136/annrheumdis-2020-217712",
"author": "Scarsi",
"doi-asserted-by": "publisher",
"first-page": "1286",
"journal-title": "Ann Rheum Dis",
"key": "ref25",
"volume": "79",
"year": "2020"
},
{
"DOI": "10.1155/2020/8865954",
"author": "Sandhu",
"doi-asserted-by": "crossref",
"journal-title": "Can J Infect Dis Med Microbiol",
"key": "ref26",
"volume": "2020",
"year": "2020"
},
{
"DOI": "10.3390/jcm9092961",
"author": "Brunetti",
"doi-asserted-by": "publisher",
"first-page": "2961",
"journal-title": "J Clin Med",
"key": "ref27",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1016/j.obmed.2020.100290",
"author": "Ivan",
"doi-asserted-by": "publisher",
"first-page": "100290",
"journal-title": "Obes Med",
"key": "ref28",
"volume": "19",
"year": "2020"
},
{
"author": "Ivan",
"first-page": "823",
"journal-title": "Clin Exp Pharmacol Physiol",
"key": "ref29",
"volume": "6",
"year": "2021"
}
],
"reference-count": 29,
"references-count": 29,
"relation": {},
"score": 1,
"short-container-title": [
"IJGM"
],
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": [
"Colchicine in Recently Hospitalized Patients with COVID-19: A Randomized Controlled Trial (COL-COVID)"
],
"type": "journal-article",
"volume": "Volume 14"
}
