Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats
et al., Scientific Reports, doi:10.1038/s41598-025-31048-4, Jan 2026
Animal study analyzing potential cardiopulmonary harm with molnupiravir (MOL), favipiravir (FAVI), hydroxychloroquine (HCQL), and dexamethasone (DEX) in healthy Wistar albino rats.
In summary:
Data suggests molnupiravir may have the highest risk for arrhythmogenic potential and long-term cardiovascular disease, with the strongest evidence for electrical instability and vascular dysfunction.
Data suggests favipiravir may have the highest activation of cell death pathways (apoptosis/necroptosis) across tissues, which could indicate risk for progressive tissue damage.
Data suggests hydroxychloroquine may have the highest risk for exacerbating respiratory failure, reinforcing existing data recommending against late-stage use in COVID-19.
Increased risks were seen with specific combinations.
Details:
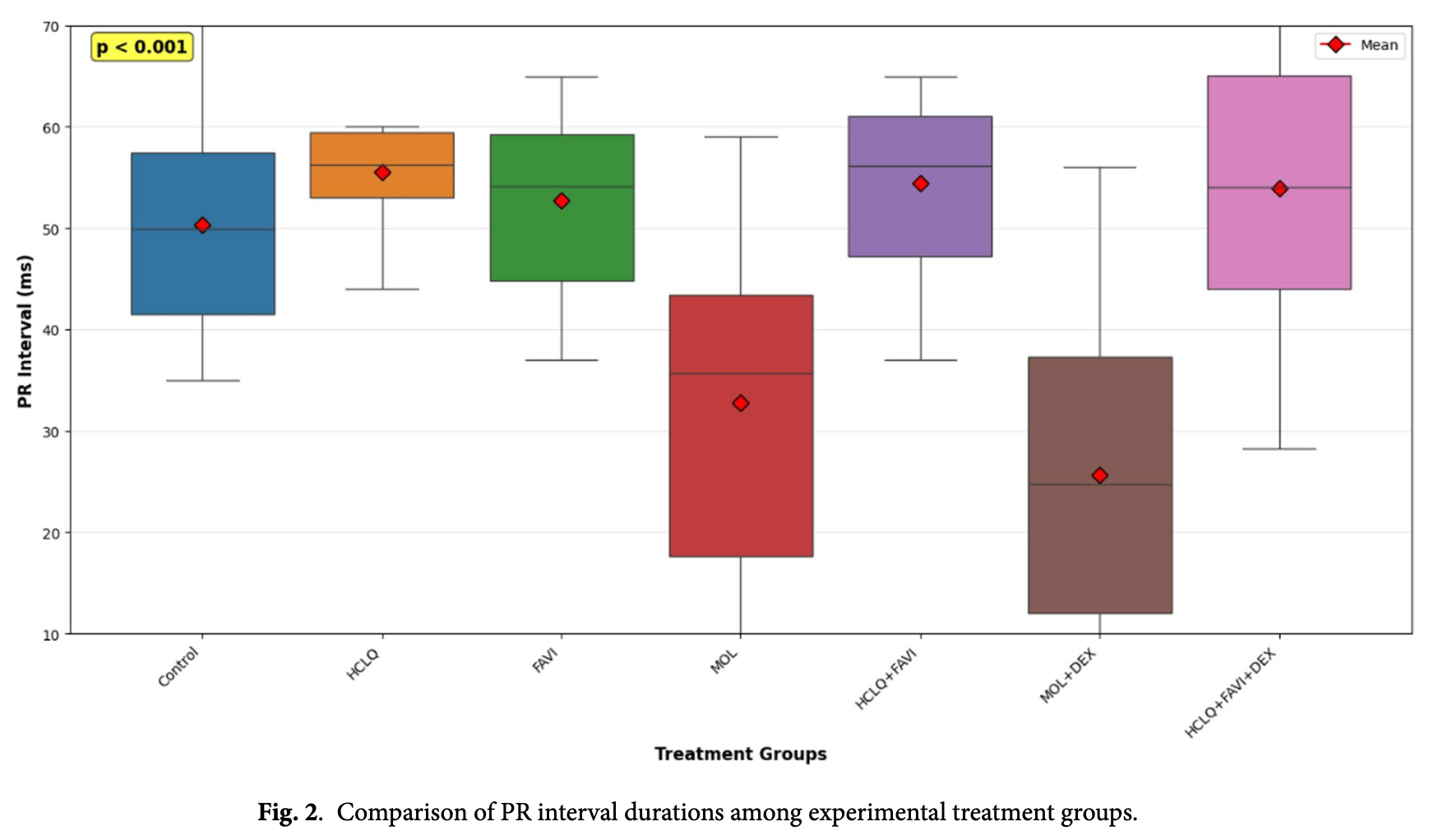
The PR interval showed statistically significant shortening in the MOL and MOL + DEX groups, indicating that molnupiravir significantly alters cardiac electrical conduction by accelerating the impulse transmission between the atria and ventricles. The alteration was drastic - while the control group had a median PR interval of 53 ms, the MOL group dropped to 32 ms, and the MOL+DEX group dropped further to 24 ms. This is a marker of molnupiravir-induced cardiovascular toxicity.
The combination of HCLQ+FAVI+DEX showed significant myocyte degeneration and interstitial edema.
The HCLQ, HCLQ+FAVI, and HCLQ+FAVI+DEX groups showed increased infiltration, alveolar septal thickening, and interstitial edema, indicating an inflammatory response in the lung.
Caspase-3 levels were significantly higher in the FAVI group, indicating cell death in heart tissue.
The combination of FAVI+HCQL showed increased RIPK3, indicating necroptosis in heart tissue.
NO levels were significantly lower in the MOL, MOL+DEX, and HCLQ+FAVI+DEX groups in cardiac tissue.
IL-6 and TNF-α (major inflammatory cytokines) were significantly elevated in the heart tissues of FAVI, MOL, and DEX groups, but were highest in the MOL+DEX and HCLQ+FAVI+DEX combinations.
Vimentin immunoreactivity (a marker of stress and potential fibrosis) was significantly higher in the MOL, HCLQ+FAVI, and MOL+DEX groups in heart tissue, suggesting the heart tissue was undergoing a mesenchymal or fibrotic activation in response to drug toxicity.
Ozhan et al., 14 Jan 2026, Turkey, peer-reviewed, 9 authors.
Contact: onural.ozhan@inonu.edu.tr.
Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats
Scientific Reports, doi:10.1038/s41598-025-31048-4
Hydroxychloroquine (HCLQ), favipiravir (FAVI), molnupiravir (MOL) and dexamethasone (DEX) are recently used drugs, some of which are currently used in the treatment of Coronavirus Disease (COVID-19). We aimed to investigate the cardiovascular and pulmonary effects of MOL, HCLQ, FAVI and DEX-drugs repurposed or used in COVID-19 treatment-independently of SARS-CoV-2 infection, using a healthy rat model. Wistar albino rats were divided into seven groups by simple randomization. (1) Control, (2) HCLQ, (3) FAVI, (4) MOL, (5) HCLQ + FAVI, (6) MOL + DEX, (7) HCLQ + FAVI + DEX. The doses of drugs to be administered to the experimental groups were adapted to rat doses with reference to the clinical treatment protocol. At the end of the experimental period, hemodynamic parameters of the rats were measured invasively. After that, the heart, lung and thoracic aortic tissues of the rats were removed and evaluated biochemically, histopathologically and immunohistochemically. When the hemodynamic parameters of the rats were compared, a statistically significant difference was found between the groups only in the PR interval (p < 0.001). Compared to the control group, the histopathologic changes observed in the HCLQ + FAVI + DEX group were significantly higher (p < 0.05), while all other groups had a normal histologic appearance similar to the control group. Vimentin immunoreactivity was significantly higher in MOL, HCLQ + FAVI and MOL + DEX groups compared to the other groups (p < 0.05). Receptor interacting protein kinase 3 immunoreactivity observed in the cytoplasm of cardiomyocytes was significantly higher in the HCLQ + FAVI group compared to all other groups except the FAVI group (p < 0.05). In contrast, caspase-3 immunoreactivity was found to be significantly higher in the FAVI group compared to the control group (p < 0.05). Drugs used alone or in combination in the treatment of COVID-19 show immunoreactions using different pathways related to apoptosis and necroptosis. Further studies are needed to elucidate the effects of these drugs.
Author contributions OO: Supervision, Conceptualization, Software, Visualization, Data curation, Writing-Original draft preparation, Writing-Reviewing and Editing. AY: Investigation, Methodology. BB: Investigation, Methodology. AU: Investigation, Methodology, Visualization. ZK: Investigation, Methodology, Visualization. EK: Investigation, Methodology, Visualization, NV: Supervision, Writing-Reviewing and Editing. BA: Supervision, Writing-Reviewing and Editing. HP: Supervision, Writing-Reviewing and Editing.
Declarations
Competing interests The authors declare no competing interests.
Ethics approval
An application was made to Inonu University Faculty of Medicine Animal Experiments Local Ethics Committee for ethical approval and ethics committee permission was obtained at the meeting dated 06.01.2022 with ethical approval number 2021/1-6.
Additional information
Supplementary Information The online version contains supplementary material available at h t t p s : / / d o i . o r g / 1 0 . 1 0 3 8 / s 4 1 5 9 8 -0 2 5 -3 1 0 4 8 -4 . Correspondence and requests for materials should be addressed to O.O. Reprints and permissions information is available at www.nature.com/reprints .
Publisher's note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any..
References
Ahmed, Hassan, Dexamethasone for the treatment of coronavirus disease (COVID-19): a review, SN Compr. Clin. Med, doi:10.1007/s42399-020-00610-8
Al-Rohaimi, Al Otaibi, Novel SARS-CoV-2 outbreak and COVID19 disease; a systemic review on the global pandemic, Genes Dis, doi:10.1016/j.gendis.2020.06.004
Bachnas, Placental damage comparison between preeclampsia with COVID-19, COVID-19, and preeclampsia: analysis of caspase-3, caspase-1, and TNF-alpha expression, AJOG Global Rep, doi:10.1016/j.xagr.2023.100234
Bekheit, Panda, Girgis, Potential RNA-dependent RNA polymerase (RdRp) inhibitors as prospective drug candidates for SARS-CoV-2, Eur. J. Med. Chem, doi:10.1016/j.ejmech.2023.115292
Bradford, A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding, Anal. Biochem, doi:10.1016/0003-2697(76)90527-3
Chen, Potential adverse effects of dexamethasone therapy on COVID-19 patients: review and recommendations, Infect. Dis. Therapy, doi:10.1007/s40121-021-00500-z
Erbaş, Celep, Tekiner, Genç, Gedikli, Assessment of toxicological effects of favipiravir (T-705) on the lung tissue of rats: an experimental study, J. Biochem. Mol. Toxicol, doi:10.1002/jbt.23536
Esmaeili-Nadimi, Total Antioxidant Capacity and Total Oxidant Status and Disease Severity in a Cohort Study of COVID-19 Patients, Clin. Lab, doi:10.7754/Clin.Lab.2022.220416
Fischer, A phase 2a clinical trial of molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus, Science translational medicine, doi:10.1126/scitranslmed.abl7430
Furuta, Komeno, Nakamura, Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase, Proc. Jpn. Acad. B, doi:10.2183/pjab.93.027
Ghofrani Nezhad, Jami, Kooshkaki, Chamani, Naghizadeh, The role of inflammatory cytokines (Interleukin-1 and Interleukin-6) as a potential biomarker in the different stages of COVID-19 (Mild, Severe, and Critical), J. Int. Soc. Interferon Cytokine Res, doi:10.1089/jir.2022.0185
Ghosh, Joseph, Anil, Nitric oxide in the management of respiratory consequences in COVID-19: A scoping review of a different treatment approach, Cureus, doi:10.7759/cureus.23852
Goud, Bai, Abu-Soud, A Multiple-Hit hypothesis involving reactive oxygen species and myeloperoxidase explains clinical deterioration and fatality in COVID-19, Int. J. Biol. Sci, doi:10.7150/ijbs.51811
Horby, Dexamethasone in hospitalized patients with Covid-19, N. Engl. J. Med, doi:10.1056/NEJMoa2021436
Infante, Ricordi, Alejandro, Caprio, Fabbri, Hydroxychloroquine in the COVID-19 pandemic era: in pursuit of a rational use for prophylaxis of SARS-CoV-2 infection, Expert Rev. anti-infective Therapy, doi:10.1080/14787210.2020.1799785
Jani, Munoz, Govender, Williams, Cabrales, Implications of microvascular dysfunction and nitric oxide mediated inflammation in severe COVID-19 infection, Am. J. Med. Sci, doi:10.1016/j.amjms.2022.04.015
Jindal, Lehl, Jaswal, Bhardwaj, Gupta, Total antioxidant status and oxidative stress in patients with COVID-19 infection, J. Assoc. Phys. India, doi:10.59556/japi.72.0575
Joshi, Role of favipiravir in the treatment of COVID-19, Int. J. Infect. Dis, doi:10.1016/j.ijid.2020.10.069
Karabulut Uzuncakmak, Dirican, Naldan, Kesmez Can, Halıcı, Investigation of CYP2E1 and Caspase-3 gene expressions in COVID-19 patients, Gene Rep, doi:10.1016/j.genrep.2022.101497
Karkhanei, Talebi Ghane, Mehri, Evaluation of oxidative stress level: total antioxidant capacity, total oxidant status and glutathione activity in patients with COVID-19, New Microbes New Infections, doi:10.1016/j.nmni.2021.100897
Khuroo, Chloroquine and hydroxychloroquine in coronavirus disease 2019 (COVID-19). Facts, fiction and the hype: a critical appraisal, Int. J. Antimicrob. Agents, doi:10.1016/j.ijantimicag.2020.106101
Korula, Favipiravir for treating COVID-19, The Cochrane database of systematic reviews, doi:10.1002/14651858.CD015219.pub2
Li, A Vimentin-Targeting Oral Compound with Host-Directed Antiviral and Anti-Inflammatory Actions Addresses Multiple Features of COVID-19 and Related Diseases, mBio, doi:10.1128/mBio.02542-21
Li, Paulin, Lacolley, Coletti, Agbulut, Vimentin as a target for the treatment of COVID-19, BMJ open. respiratory Res, doi:10.1136/bmjresp-2020-000623
Mohd Zawawi, Prospective Roles of Tumor Necrosis Factor-Alpha (TNF-α) in COVID-19: Prognosis, Therapeutic and Management, Int. J. Mol. Sci, doi:10.3390/ijms24076142
Nair, Jacob, A simple practice guide for dose conversion between animals and human, J. basic. Clin. Pharm, doi:10.4103/0976-0105.177703
Ozhan, Acute and subacute cardiovascular effects of synthetic cannabinoid JWH-018 in rat, Forensic Toxicol, doi:10.1007/s11419-025-00720-9
Ozhan, Parlakpinar, Acet, Comparison of the effects of losartan, captopril, angiotensin II type 2 receptor agonist compound 21, and MAS receptor agonist AVE 0991 on myocardial ischemia-reperfusion necrosis in rats, Fundam. Clin. Pharmacol, doi:10.1111/fcp.12599
Plocque, Should we interfere with the Interleukin-6 receptor during COVID-19: what do we know so far?, Drugs, doi:10.1007/s40265-022-01803-2
Ramezani, Altered serum and cerebrospinal fluid TNF-α, caspase 3, and IL 1β in COVID-19 disease, Caspian J. Intern. Med, doi:10.22088/cjim.13.0.264
Ricciardolo, Bertolini, Carriero, Högman, Nitric oxide's physiologic effects and potential as a therapeutic agent against COVID-19, J. Breath Res, doi:10.1088/1752-7163/abc302
Ruskowski, Persistently elevated plasma concentrations of RIPK3, MLKL, HMGB1, and RIPK1 in patients with COVID-19 in the intensive care unit, Am. J. Respir. Cell Mol. Biol, doi:10.1165/rcmb.2022-0039LE
Sanderson, A molnupiravir-associated mutational signature in global SARS-CoV-2 genomes, Nature, doi:10.1038/s41586-023-06649-6
Seydi, Hassani, Naderpour, Arjmand, Pourahmad, Cardiotoxicity of chloroquine and hydroxychloroquine through mitochondrial pathway, BMC Pharmacol. Toxicol, doi:10.1186/s40360-023-00666-x
Thakur, Exploring the magic bullets to identify achilles' heel in SARS-CoV-2: delving deeper into the sea of possible therapeutic options in Covid-19 disease: an update, Food Chem. Toxicology, doi:10.1016/j.fct.2020.111887
Tian, Molnupiravir and Its Antiviral Activity Against COVID-19, Front. Immunol, doi:10.3389/fimmu.2022.855496
Udomsinprasert, Jittikoon, Sangroongruangsri, Chaikledkaew, Circulating levels of Interleukin-6 and Interleukin-10, but not tumor necrosis Factor-Alpha, as potential biomarkers of severity and mortality for COVID-19: systematic review with Meta-analysis, J. Clin. Immunol, doi:10.1007/s10875-020-00899-z
Vijayvargiya, Treatment considerations for COVID-19: A critical review of the evidence (or lack Thereof), Mayo Clin. Proc, doi:10.1016/j.mayocp.2020.04.027
Walker, The Lambeth conventions: guidelines for the study of arrhythmias in ischaemia infarction, and reperfusion, Cardiovascular. Res, doi:10.1093/cvr/22.7.447
Yao, In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), Clin. Infect. Diseases: Official Publication Infect. Dis. Soc. Am, doi:10.1093/cid/ciaa237
Yildiz, Effects of drugs commonly used in Sars-CoV-2 infection on renal tissue in rats, Ann. Med. Res, doi:10.5455/annalsmedres.2023.08.180
Yilmaz, Yilmaz, Cacan, Severe and post-COVID-19 are associated with high expression of vimentin and reduced expression of N-cadherin, Sci. Rep, doi:10.1038/s41598-024-72192-7
Yin, Increased interleukin-6 is associated with long COVID-19: a systematic review and meta-analysis, Infect. Dis. poverty, doi:10.1186/s40249-023-01086-z
Zhang, Zhang, Hua, Chen, Saying no to SARS-CoV-2: the potential of nitric oxide in the treatment of COVID-19 pneumonia, Med. Gas Res, doi:10.4103/2045-9912.385414
Zhao, Inhaled nitric oxide: can it serve as a savior for COVID-19 and related respiratory and cardiovascular diseases?, Front. Microbiol, doi:10.3389/fmicb.2023.1277552
Zhou, A pneumonia outbreak associated with a new coronavirus of probable Bat origin, Nature, doi:10.1038/s41586-020-2012-7
Zhou, Dai, Tong, COVID-19: a recommendation to examine the effect of hydroxychloroquine in preventing infection and progression, J. Antimicrob. Chemother, doi:10.1093/jac/dkaa114
Çolak, Parlakpınar, Hayvan, deneyleri: in vivo denemelerin bildirimi: ARRIVE Kılavuzu-Derleme, J. Turgut Ozal Med. Cent, doi:10.7247/jiumf.19.2.14
DOI record:
{
"DOI": "10.1038/s41598-025-31048-4",
"ISSN": [
"2045-2322"
],
"URL": "http://dx.doi.org/10.1038/s41598-025-31048-4",
"alternative-id": [
"31048"
],
"article-number": "1770",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "20 March 2025"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "28 November 2025"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "14 January 2026"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "The authors declare no competing interests."
},
{
"group": {
"label": "Ethics approval",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "An application was made to Inonu University Faculty of Medicine Animal Experiments Local Ethics Committee for ethical approval and ethics committee permission was obtained at the meeting dated 06.01.2022 with ethical approval number 2021/1–6."
}
],
"author": [
{
"ORCID": "https://orcid.org/0000-0001-9018-7849",
"affiliation": [],
"authenticated-orcid": false,
"family": "Ozhan",
"given": "Onural",
"sequence": "first"
},
{
"ORCID": "https://orcid.org/0000-0001-5686-7867",
"affiliation": [],
"authenticated-orcid": false,
"family": "Yildiz",
"given": "Azibe",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-7793-1119",
"affiliation": [],
"authenticated-orcid": false,
"family": "Bakar",
"given": "Busra",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-4447-6233",
"affiliation": [],
"authenticated-orcid": false,
"family": "Ulu",
"given": "Ahmet",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-7956-9272",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kucukakcali",
"given": "Zeynep",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-1723-2556",
"affiliation": [],
"authenticated-orcid": false,
"family": "Karaca",
"given": "Elif",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-0576-1696",
"affiliation": [],
"authenticated-orcid": false,
"family": "Vardi",
"given": "Nigar",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-6080-229X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Ates",
"given": "Burhan",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-9497-3468",
"affiliation": [],
"authenticated-orcid": false,
"family": "Parlakpinar",
"given": "Hakan",
"sequence": "additional"
}
],
"container-title": "Scientific Reports",
"container-title-short": "Sci Rep",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2026,
1,
14
]
],
"date-time": "2026-01-14T06:44:41Z",
"timestamp": 1768373081000
},
"deposited": {
"date-parts": [
[
2026,
1,
14
]
],
"date-time": "2026-01-14T12:58:28Z",
"timestamp": 1768395508000
},
"funder": [
{
"DOI": "10.13039/100031253",
"award": [
"TOA-2020-2347"
],
"award-info": [
{
"award-number": [
"TOA-2020-2347"
]
}
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/100031253",
"id-type": "DOI"
}
],
"name": "İnönü Üniversitesi"
}
],
"indexed": {
"date-parts": [
[
2026,
1,
14
]
],
"date-time": "2026-01-14T23:24:26Z",
"timestamp": 1768433066923,
"version": "3.49.0"
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2026,
1,
14
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2026,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2026,
1,
14
]
],
"date-time": "2026-01-14T00:00:00Z",
"timestamp": 1768348800000
}
},
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2026,
1,
14
]
],
"date-time": "2026-01-14T00:00:00Z",
"timestamp": 1768348800000
}
}
],
"link": [
{
"URL": "https://www.nature.com/articles/s41598-025-31048-4",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-025-31048-4.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-025-31048-4.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1038",
"published": {
"date-parts": [
[
2026,
1,
14
]
]
},
"published-online": {
"date-parts": [
[
2026,
1,
14
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1016/j.gendis.2020.06.004",
"author": "AH Al-Rohaimi",
"doi-asserted-by": "publisher",
"first-page": "491",
"journal-title": "Genes Dis.",
"key": "31048_CR1",
"unstructured": "Al-Rohaimi, A. H., Al Otaibi, F. & Novel SARS-CoV-2 outbreak and COVID19 disease; a systemic review on the global pandemic. Genes Dis. 7, 491–501. https://doi.org/10.1016/j.gendis.2020.06.004 (2020).",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2012-7",
"author": "P Zhou",
"doi-asserted-by": "publisher",
"first-page": "270",
"journal-title": "Nature",
"key": "31048_CR2",
"unstructured": "Zhou, P. et al. A pneumonia outbreak associated with a new coronavirus of probable Bat origin. Nature 579, 270–273. https://doi.org/10.1038/s41586-020-2012-7 (2020).",
"volume": "579",
"year": "2020"
},
{
"DOI": "10.1016/j.mayocp.2020.04.027",
"author": "P Vijayvargiya",
"doi-asserted-by": "publisher",
"first-page": "1454",
"journal-title": "Mayo Clin. Proc.",
"key": "31048_CR3",
"unstructured": "Vijayvargiya, P. et al. Treatment considerations for COVID-19: A critical review of the evidence (or lack Thereof). Mayo Clin. Proc. 95, 1454–1466. https://doi.org/10.1016/j.mayocp.2020.04.027 (2020).",
"volume": "95",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2022.855496",
"doi-asserted-by": "publisher",
"key": "31048_CR4",
"unstructured": "Tian, L. et al. Molnupiravir and Its Antiviral Activity Against COVID-19. Front. Immunol. 13, https://doi.org/10.3389/fimmu.2022.855496 (2022)."
},
{
"DOI": "10.1002/14651858.CD015219.pub2",
"doi-asserted-by": "publisher",
"key": "31048_CR5",
"unstructured": "Korula, P. et al. Favipiravir for treating COVID-19. The Cochrane database of systematic reviews 2, Cd015219, (2024). https://doi.org/10.1002/14651858.CD015219.pub2"
},
{
"DOI": "10.1186/s40360-023-00666-x",
"doi-asserted-by": "publisher",
"key": "31048_CR6",
"unstructured": "Seydi, E., Hassani, M. K., Naderpour, S., Arjmand, A. & Pourahmad, J. Cardiotoxicity of chloroquine and hydroxychloroquine through mitochondrial pathway. BMC Pharmacol. Toxicol. 24, https://doi.org/10.1186/s40360-023-00666-x (2023)."
},
{
"DOI": "10.1080/14787210.2020.1799785",
"author": "M Infante",
"doi-asserted-by": "publisher",
"first-page": "5",
"journal-title": "Expert Rev. anti-infective Therapy",
"key": "31048_CR7",
"unstructured": "Infante, M., Ricordi, C., Alejandro, R., Caprio, M. & Fabbri, A. Hydroxychloroquine in the COVID-19 pandemic era: in pursuit of a rational use for prophylaxis of SARS-CoV-2 infection. Expert Rev. anti-infective Therapy. 19, 5–16. https://doi.org/10.1080/14787210.2020.1799785 (2021).",
"volume": "19",
"year": "2021"
},
{
"DOI": "10.1093/jac/dkaa114",
"author": "D Zhou",
"doi-asserted-by": "publisher",
"first-page": "1667",
"journal-title": "J. Antimicrob. Chemother.",
"key": "31048_CR8",
"unstructured": "Zhou, D., Dai, S. M. & Tong, Q. COVID-19: a recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. J. Antimicrob. Chemother. 75, 1667–1670. https://doi.org/10.1093/jac/dkaa114 (2020).",
"volume": "75",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa237",
"author": "X Yao",
"doi-asserted-by": "publisher",
"first-page": "732",
"journal-title": "Clin. Infect. Diseases: Official Publication Infect. Dis. Soc. Am.",
"key": "31048_CR9",
"unstructured": "Yao, X. et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin. Infect. Diseases: Official Publication Infect. Dis. Soc. Am. 71, 732–739. https://doi.org/10.1093/cid/ciaa237 (2020).",
"volume": "71",
"year": "2020"
},
{
"DOI": "10.2183/pjab.93.027",
"author": "Y Furuta",
"doi-asserted-by": "publisher",
"first-page": "449",
"journal-title": "Proc. Jpn. Acad. B",
"key": "31048_CR10",
"unstructured": "Furuta, Y., Komeno, T. & Nakamura, T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc. Jpn. Acad. B. 93, 449–463. https://doi.org/10.2183/pjab.93.027 (2017).",
"volume": "93",
"year": "2017"
},
{
"DOI": "10.1016/j.ijid.2020.10.069",
"author": "S Joshi",
"doi-asserted-by": "publisher",
"first-page": "501",
"journal-title": "Int. J. Infect. Dis.",
"key": "31048_CR11",
"unstructured": "Joshi, S. et al. Role of favipiravir in the treatment of COVID-19. Int. J. Infect. Dis. 102, 501–508. https://doi.org/10.1016/j.ijid.2020.10.069 (2021).",
"volume": "102",
"year": "2021"
},
{
"DOI": "10.1016/j.ejmech.2023.115292",
"author": "MS Bekheit",
"doi-asserted-by": "publisher",
"first-page": "115292",
"journal-title": "Eur. J. Med. Chem.",
"key": "31048_CR12",
"unstructured": "Bekheit, M. S., Panda, S. S. & Girgis, A. S. Potential RNA-dependent RNA polymerase (RdRp) inhibitors as prospective drug candidates for SARS-CoV-2. Eur. J. Med. Chem. 252, 115292. https://doi.org/10.1016/j.ejmech.2023.115292 (2023).",
"volume": "252",
"year": "2023"
},
{
"DOI": "10.1126/scitranslmed.abl7430",
"doi-asserted-by": "publisher",
"key": "31048_CR13",
"unstructured": "Fischer, W. A. et al. A phase 2a clinical trial of molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus. Science translational medicine 14, eabl7430, (2022). https://doi.org/10.1126/scitranslmed.abl7430"
},
{
"DOI": "10.1016/j.fct.2020.111887",
"author": "S Thakur",
"doi-asserted-by": "publisher",
"first-page": "111887",
"journal-title": "Food Chem. Toxicology: Int. J. Published Br. Industrial Biol. Res. Association",
"key": "31048_CR14",
"unstructured": "Thakur, S. et al. Exploring the magic bullets to identify achilles’ heel in SARS-CoV-2: delving deeper into the sea of possible therapeutic options in Covid-19 disease: an update. Food Chem. Toxicology. 147, 111887. https://doi.org/10.1016/j.fct.2020.111887 (2021).",
"volume": "147",
"year": "2021"
},
{
"DOI": "10.1016/j.ijantimicag.2020.106101",
"author": "MS Khuroo",
"doi-asserted-by": "publisher",
"first-page": "106101",
"journal-title": "Int. J. Antimicrob. Agents",
"key": "31048_CR15",
"unstructured": "Khuroo, M. S. Chloroquine and hydroxychloroquine in coronavirus disease 2019 (COVID-19). Facts, fiction and the hype: a critical appraisal. Int. J. Antimicrob. Agents. 56, 106101. https://doi.org/10.1016/j.ijantimicag.2020.106101 (2020).",
"volume": "56",
"year": "2020"
},
{
"DOI": "10.1007/s40121-021-00500-z",
"author": "F Chen",
"doi-asserted-by": "publisher",
"first-page": "1907",
"journal-title": "Infect. Dis. Therapy",
"key": "31048_CR16",
"unstructured": "Chen, F. et al. Potential adverse effects of dexamethasone therapy on COVID-19 patients: review and recommendations. Infect. Dis. Therapy. 10, 1907–1931. https://doi.org/10.1007/s40121-021-00500-z (2021).",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1007/s42399-020-00610-8",
"author": "MH Ahmed",
"doi-asserted-by": "publisher",
"first-page": "2637",
"journal-title": "SN Compr. Clin. Med.",
"key": "31048_CR17",
"unstructured": "Ahmed, M. H. & Hassan, A. Dexamethasone for the treatment of coronavirus disease (COVID-19): a review. SN Compr. Clin. Med. 2, 2637–2646. https://doi.org/10.1007/s42399-020-00610-8 (2020).",
"volume": "2",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2021436",
"author": "P Horby",
"doi-asserted-by": "publisher",
"first-page": "693",
"journal-title": "N. Engl. J. Med.",
"key": "31048_CR18",
"unstructured": "Horby, P. et al. Dexamethasone in hospitalized patients with Covid-19. N. Engl. J. Med. 384, 693–704. https://doi.org/10.1056/NEJMoa2021436 (2021).",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.7247/jiumf.19.2.14",
"doi-asserted-by": "crossref",
"key": "31048_CR19",
"unstructured": "Çolak, C. & Parlakpınar, H. Hayvan deneyleri: in vivo denemelerin bildirimi: ARRIVE Kılavuzu-Derleme. J. Turgut Ozal Med. Cent. 19, 128–131 https://doi.org/10.7247/jiumf.19.2.14 (2012)."
},
{
"DOI": "10.4103/0976-0105.177703",
"author": "AB Nair",
"doi-asserted-by": "publisher",
"first-page": "27",
"journal-title": "J. basic. Clin. Pharm.",
"key": "31048_CR20",
"unstructured": "Nair, A. B. & Jacob, S. A simple practice guide for dose conversion between animals and human. J. basic. Clin. Pharm. 7, 27–31. https://doi.org/10.4103/0976-0105.177703 (2016).",
"volume": "7",
"year": "2016"
},
{
"DOI": "10.1111/fcp.12599",
"author": "O Ozhan",
"doi-asserted-by": "publisher",
"first-page": "669",
"journal-title": "Fundam. Clin. Pharmacol.",
"key": "31048_CR21",
"unstructured": "Ozhan, O., Parlakpinar, H. & Acet, A. Comparison of the effects of losartan, captopril, angiotensin II type 2 receptor agonist compound 21, and MAS receptor agonist AVE 0991 on myocardial ischemia-reperfusion necrosis in rats. Fundam. Clin. Pharmacol. 35, 669–680. https://doi.org/10.1111/fcp.12599 (2021).",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1007/s11419-025-00720-9",
"author": "O Ozhan",
"doi-asserted-by": "publisher",
"first-page": "266",
"journal-title": "Forensic Toxicol.",
"key": "31048_CR22",
"unstructured": "Ozhan, O. et al. Acute and subacute cardiovascular effects of synthetic cannabinoid JWH-018 in rat. Forensic Toxicol. 43, 266–279. https://doi.org/10.1007/s11419-025-00720-9 (2025).",
"volume": "43",
"year": "2025"
},
{
"DOI": "10.1093/cvr/22.7.447",
"author": "MJ Walker",
"doi-asserted-by": "publisher",
"first-page": "447",
"journal-title": "Cardiovascular. Res.",
"key": "31048_CR23",
"unstructured": "Walker, M. J. et al. The Lambeth conventions: guidelines for the study of arrhythmias in ischaemia infarction, and reperfusion. Cardiovascular. Res. 22, 447–455. https://doi.org/10.1093/cvr/22.7.447 (1988).",
"volume": "22",
"year": "1988"
},
{
"DOI": "10.5455/annalsmedres.2023.08.180",
"doi-asserted-by": "crossref",
"key": "31048_CR24",
"unstructured": "Yildiz, A. et al. Effects of drugs commonly used in Sars-CoV-2 infection on renal tissue in rats. Ann. Med. Res. 30, 1209-1216. https://doi.org/10.5455/annalsmedres.2023.08.180 (2023)."
},
{
"DOI": "10.1016/0003-2697(76)90527-3",
"author": "MM Bradford",
"doi-asserted-by": "publisher",
"first-page": "248",
"journal-title": "Anal. Biochem.",
"key": "31048_CR25",
"unstructured": "Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254. https://doi.org/10.1016/0003-2697(76)90527-3 (1976).",
"volume": "72",
"year": "1976"
},
{
"DOI": "10.1136/bmjresp-2020-000623",
"doi-asserted-by": "publisher",
"key": "31048_CR26",
"unstructured": "Li, Z., Paulin, D., Lacolley, P., Coletti, D. & Agbulut, O. Vimentin as a target for the treatment of COVID-19. BMJ open. respiratory Res. 7, https://doi.org/10.1136/bmjresp-2020-000623 (2020)."
},
{
"DOI": "10.1128/mBio.02542-21",
"doi-asserted-by": "publisher",
"key": "31048_CR27",
"unstructured": "Li, Z. et al. A Vimentin-Targeting Oral Compound with Host-Directed Antiviral and Anti-Inflammatory Actions Addresses Multiple Features of COVID-19 and Related Diseases. mBio. 12, e0254221, (2021). https://doi.org/10.1128/mBio.02542-21"
},
{
"DOI": "10.1038/s41598-024-72192-7",
"author": "E Yilmaz",
"doi-asserted-by": "publisher",
"first-page": "29256",
"journal-title": "Sci. Rep.",
"key": "31048_CR28",
"unstructured": "Yilmaz, E., Yilmaz, D. & Cacan, E. Severe and post-COVID-19 are associated with high expression of vimentin and reduced expression of N-cadherin. Sci. Rep. 14, 29256. https://doi.org/10.1038/s41598-024-72192-7 (2024).",
"volume": "14",
"year": "2024"
},
{
"DOI": "10.1165/rcmb.2022-0039LE",
"author": "K Ruskowski",
"doi-asserted-by": "publisher",
"first-page": "405",
"journal-title": "Am. J. Respir. Cell Mol. Biol.",
"key": "31048_CR29",
"unstructured": "Ruskowski, K. et al. Persistently elevated plasma concentrations of RIPK3, MLKL, HMGB1, and RIPK1 in patients with COVID-19 in the intensive care unit. Am. J. Respir. Cell Mol. Biol. 67, 405–408. https://doi.org/10.1165/rcmb.2022-0039LE (2022).",
"volume": "67",
"year": "2022"
},
{
"DOI": "10.1016/j.genrep.2022.101497",
"author": "S Karabulut Uzuncakmak",
"doi-asserted-by": "publisher",
"first-page": "101497",
"journal-title": "Gene Rep.",
"key": "31048_CR30",
"unstructured": "Karabulut Uzuncakmak, S., Dirican, E., Naldan, M. E., Kesmez Can, F. & Halıcı, Z. Investigation of CYP2E1 and Caspase-3 gene expressions in COVID-19 patients. Gene Rep. 26, 101497. https://doi.org/10.1016/j.genrep.2022.101497 (2022).",
"volume": "26",
"year": "2022"
},
{
"DOI": "10.1016/j.xagr.2023.100234",
"author": "MA Bachnas",
"doi-asserted-by": "publisher",
"first-page": "100234",
"journal-title": "AJOG Global Rep.",
"key": "31048_CR31",
"unstructured": "Bachnas, M. A. et al. Placental damage comparison between preeclampsia with COVID-19, COVID-19, and preeclampsia: analysis of caspase-3, caspase-1, and TNF-alpha expression. AJOG Global Rep. 3, 100234. https://doi.org/10.1016/j.xagr.2023.100234 (2023).",
"volume": "3",
"year": "2023"
},
{
"DOI": "10.22088/cjim.13.0.264",
"author": "M Ramezani",
"doi-asserted-by": "publisher",
"first-page": "264",
"journal-title": "Caspian J. Intern. Med.",
"key": "31048_CR32",
"unstructured": "Ramezani, M. et al. Altered serum and cerebrospinal fluid TNF-α, caspase 3, and IL 1β in COVID-19 disease. Caspian J. Intern. Med. 13, 264–269. https://doi.org/10.22088/cjim.13.0.264 (2022).",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1186/s40249-023-01086-z",
"doi-asserted-by": "publisher",
"key": "31048_CR33",
"unstructured": "Yin, J. X. et al. Increased interleukin-6 is associated with long COVID-19: a systematic review and meta-analysis. Infect. Dis. poverty. 12, https://doi.org/10.1186/s40249-023-01086-z (2023)."
},
{
"DOI": "10.1089/jir.2022.0185",
"author": "M Ghofrani Nezhad",
"doi-asserted-by": "publisher",
"first-page": "147",
"journal-title": "J. Interferon Cytokine Research: Official J. Int. Soc. Interferon Cytokine Res.",
"key": "31048_CR34",
"unstructured": "Ghofrani Nezhad, M., Jami, G., Kooshkaki, O., Chamani, S. & Naghizadeh, A. The role of inflammatory cytokines (Interleukin-1 and Interleukin-6) as a potential biomarker in the different stages of COVID-19 (Mild, Severe, and Critical). J. Int. Soc. Interferon Cytokine Res. 43, 147–163. https://doi.org/10.1089/jir.2022.0185 (2023).",
"volume": "43",
"year": "2023"
},
{
"DOI": "10.3390/ijms24076142",
"doi-asserted-by": "publisher",
"key": "31048_CR35",
"unstructured": "Mohd Zawawi, Z. et al. Prospective Roles of Tumor Necrosis Factor-Alpha (TNF-α) in COVID-19: Prognosis, Therapeutic and Management. Int. J. Mol. Sci. 24, https://doi.org/10.3390/ijms24076142 (2023)."
},
{
"DOI": "10.1007/s40265-022-01803-2",
"author": "A Plocque",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Drugs",
"key": "31048_CR36",
"unstructured": "Plocque, A. et al. Should we interfere with the Interleukin-6 receptor during COVID-19: what do we know so far? Drugs. 83, 1–36. https://doi.org/10.1007/s40265-022-01803-2 (2023).",
"volume": "83",
"year": "2023"
},
{
"DOI": "10.1007/s10875-020-00899-z",
"author": "W Udomsinprasert",
"doi-asserted-by": "publisher",
"first-page": "11",
"journal-title": "J. Clin. Immunol.",
"key": "31048_CR37",
"unstructured": "Udomsinprasert, W., Jittikoon, J., Sangroongruangsri, S. & Chaikledkaew, U. Circulating levels of Interleukin-6 and Interleukin-10, but not tumor necrosis Factor-Alpha, as potential biomarkers of severity and mortality for COVID-19: systematic review with Meta-analysis. J. Clin. Immunol. 41, 11–22. https://doi.org/10.1007/s10875-020-00899-z (2021).",
"volume": "41",
"year": "2021"
},
{
"DOI": "10.7150/ijbs.51811",
"author": "PT Goud",
"doi-asserted-by": "publisher",
"first-page": "62",
"journal-title": "Int. J. Biol. Sci.",
"key": "31048_CR38",
"unstructured": "Goud, P. T., Bai, D. & Abu-Soud, H. M. A Multiple-Hit hypothesis involving reactive oxygen species and myeloperoxidase explains clinical deterioration and fatality in COVID-19. Int. J. Biol. Sci. 17, 62–72. https://doi.org/10.7150/ijbs.51811 (2021).",
"volume": "17",
"year": "2021"
},
{
"DOI": "10.7754/Clin.Lab.2022.220416",
"doi-asserted-by": "publisher",
"key": "31048_CR39",
"unstructured": "Esmaeili-Nadimi, A. et al. Total Antioxidant Capacity and Total Oxidant Status and Disease Severity in a Cohort Study of COVID-19 Patients. Clin. Lab. 69, https://doi.org/10.7754/Clin.Lab.2022.220416 (2023)."
},
{
"DOI": "10.1016/j.nmni.2021.100897",
"author": "B Karkhanei",
"doi-asserted-by": "publisher",
"first-page": "100897",
"journal-title": "New. Microbes new. Infections",
"key": "31048_CR40",
"unstructured": "Karkhanei, B., Talebi Ghane, E. & Mehri, F. Evaluation of oxidative stress level: total antioxidant capacity, total oxidant status and glutathione activity in patients with COVID-19. New Microbes New Infections. 42, 100897. https://doi.org/10.1016/j.nmni.2021.100897 (2021).",
"volume": "42",
"year": "2021"
},
{
"DOI": "10.59556/japi.72.0575",
"author": "M Jindal",
"doi-asserted-by": "publisher",
"first-page": "36",
"journal-title": "J. Assoc. Phys. India",
"key": "31048_CR41",
"unstructured": "Jindal, M., Lehl, S. S., Jaswal, S., Bhardwaj, N. & Gupta, M. Total antioxidant status and oxidative stress in patients with COVID-19 infection. J. Assoc. Phys. India. 72, 36–39. https://doi.org/10.59556/japi.72.0575 (2024).",
"volume": "72",
"year": "2024"
},
{
"DOI": "10.4103/2045-9912.385414",
"author": "H Zhang",
"doi-asserted-by": "publisher",
"first-page": "39",
"journal-title": "Med. Gas Res.",
"key": "31048_CR42",
"unstructured": "Zhang, H., Zhang, C., Hua, W. & Chen, J. Saying no to SARS-CoV-2: the potential of nitric oxide in the treatment of COVID-19 pneumonia. Med. Gas Res. 14, 39–47. https://doi.org/10.4103/2045-9912.385414 (2024).",
"volume": "14",
"year": "2024"
},
{
"DOI": "10.3389/fmicb.2023.1277552",
"author": "Y Zhao",
"doi-asserted-by": "publisher",
"first-page": "1277552",
"journal-title": "Front. Microbiol.",
"key": "31048_CR43",
"unstructured": "Zhao, Y. et al. Inhaled nitric oxide: can it serve as a savior for COVID-19 and related respiratory and cardiovascular diseases? Front. Microbiol. 14, 1277552. https://doi.org/10.3389/fmicb.2023.1277552 (2023).",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.1016/j.amjms.2022.04.015",
"author": "VP Jani",
"doi-asserted-by": "publisher",
"first-page": "251",
"journal-title": "Am. J. Med. Sci.",
"key": "31048_CR44",
"unstructured": "Jani, V. P., Munoz, C. J., Govender, K., Williams, A. T. & Cabrales, P. Implications of microvascular dysfunction and nitric oxide mediated inflammation in severe COVID-19 infection. Am. J. Med. Sci. 364, 251–256. https://doi.org/10.1016/j.amjms.2022.04.015 (2022).",
"volume": "364",
"year": "2022"
},
{
"DOI": "10.7759/cureus.23852",
"author": "A Ghosh",
"doi-asserted-by": "publisher",
"first-page": "e23852",
"journal-title": "Cureus",
"key": "31048_CR45",
"unstructured": "Ghosh, A., Joseph, B. & Anil, S. Nitric oxide in the management of respiratory consequences in COVID-19: A scoping review of a different treatment approach. Cureus 14, e23852. https://doi.org/10.7759/cureus.23852 (2022).",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1088/1752-7163/abc302",
"author": "FLM Ricciardolo",
"doi-asserted-by": "publisher",
"first-page": "014001",
"journal-title": "J. Breath Res.",
"key": "31048_CR46",
"unstructured": "Ricciardolo, F. L. M., Bertolini, F., Carriero, V. & Högman, M. Nitric oxide’s physiologic effects and potential as a therapeutic agent against COVID-19. J. Breath Res. 15, 014001. https://doi.org/10.1088/1752-7163/abc302 (2020).",
"volume": "15",
"year": "2020"
},
{
"DOI": "10.1002/jbt.23536",
"author": "E Erbaş",
"doi-asserted-by": "publisher",
"first-page": "e23536",
"journal-title": "J. Biochem. Mol. Toxicol.",
"key": "31048_CR47",
"unstructured": "Erbaş, E., Celep, N. A., Tekiner, D., Genç, A. & Gedikli, S. Assessment of toxicological effects of favipiravir (T-705) on the lung tissue of rats: an experimental study. J. Biochem. Mol. Toxicol. 38, e23536. https://doi.org/10.1002/jbt.23536 (2024).",
"volume": "38",
"year": "2024"
},
{
"DOI": "10.1038/s41586-023-06649-6",
"author": "T Sanderson",
"doi-asserted-by": "publisher",
"first-page": "594",
"journal-title": "Nature",
"key": "31048_CR48",
"unstructured": "Sanderson, T. et al. A molnupiravir-associated mutational signature in global SARS-CoV-2 genomes. Nature 623, 594–600. https://doi.org/10.1038/s41586-023-06649-6 (2023).",
"volume": "623",
"year": "2023"
}
],
"reference-count": 48,
"references-count": 48,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.nature.com/articles/s41598-025-31048-4"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats",
"type": "journal-article",
"update-policy": "https://doi.org/10.1007/springer_crossmark_policy",
"volume": "16"
}
ozhan