Clinical Prognosis of Patients With Mild COVID-19 Treated With Casirivimab/Imdevimab in Japan
et al., Cureus, doi:10.7759/cureus.21882, Feb 2022
19th treatment shown to reduce risk in
March 2021, now with p = 0.000095 from 34 studies, recognized in 52 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
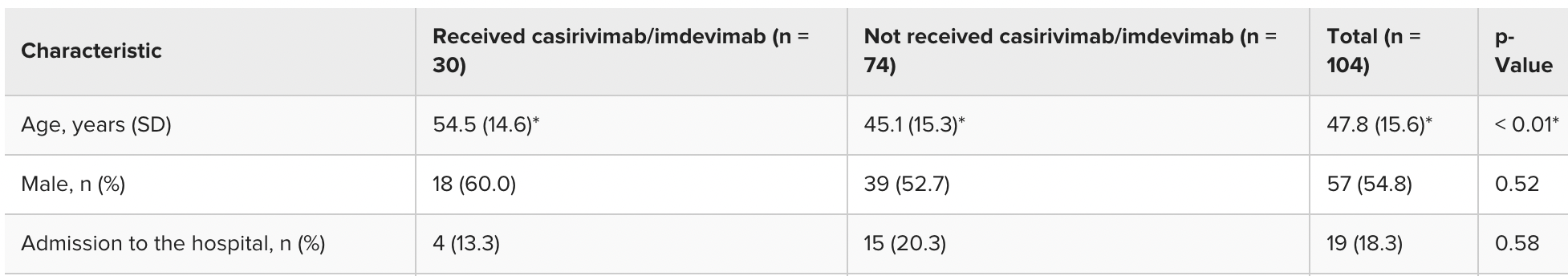
Retrospective 104 outpatients in Japan, 30 treated with casirivimab/imdevimab, showing no significant difference in hospitalization.
Efficacy is variant dependent. In Vitro research suggests a lack of efficacy for many omicron variants1-7.
Standard of Care (SOC) for COVID-19 in the study country,
Japan, is very poor with very low average efficacy for approved treatments8.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of hospitalization, 24.0% lower, HR 0.76, p = 0.65, treatment 4 of 30 (13.3%), control 15 of 74 (20.3%), adjusted per study, multivariable, Cox proportional hazards.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
2.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
3.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
4.
Tatham et al., Lack of Ronapreve (REGN-CoV; casirivimab and imdevimab) virological efficacy against the SARS-CoV 2 Omicron variant (B.1.1.529) in K18-hACE2 mice, bioRxiv, doi:10.1101/2022.01.23.477397.
5.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
6.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
Osugi et al., 3 Feb 2022, retrospective, Japan, peer-reviewed, mean age 47.8, 5 authors, study period 31 August, 2021 - 27 September, 2021.
Clinical Prognosis of Patients With Mild COVID-19 Treated With Casirivimab/Imdevimab in Japan
Cureus, doi:10.7759/cureus.21882
Aim: This study aimed to report clinical courses of patients who had mild coronavirus disease 2019 (COVID-19), defined as SpO 2 of 96 or higher, and treated with/without casirivimab/imdevimab in Japan, where mortality and number of severe patients were very limited compared to other resource-rich countries.
Additional Information Disclosures Human subjects: Consent was obtained or waived by all participants in this study. The Ethical Committee of Toyota Regional Medical Center issued approval #2021-kenrin13. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
References
Arentz, Yim, Klaff, Lokhandwala, Riedo et al., Characteristics and outcomes of 21 critically Ill patients with COVID-19 in Washington state, JAMA, doi:10.1001/jama.2020.4326
Dougan, Nirula, Azizad, Bamlanivimab plus etesevimab in mild or moderate COVID-19, N Engl J Med, doi:10.1056/NEJMoa2102685
Gupta, Gonzalez-Rojas, Juarez, Early treatment for COVID-19 with SARS-CoV-2 neutralizing antibody sotrovimab, N Engl J Med, doi:10.1056/NEJMoa2107934
Liu, Fang, Deng, Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei province, Chin Med J (Engl), doi:10.1097/CM9.0000000000000744
Osugi, None, Cureus, doi:10.7759/cureus.218825of5
Weinreich, Sivapalasingam, Norton, REGEN-COV antibody combination and outcomes in outpatients with COVID-19, N Engl J Med, doi:10.1056/NEJMoa2108163
DOI record:
{
"DOI": "10.7759/cureus.21882",
"ISSN": [
"2168-8184"
],
"URL": "http://dx.doi.org/10.7759/cureus.21882",
"author": [
{
"affiliation": [],
"family": "Osugi",
"given": "Yasuhiro",
"sequence": "first"
},
{
"affiliation": [],
"family": "Iwata",
"given": "Hitoshi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Imai",
"given": "Yasushi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kobayashi",
"given": "Daiki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hirashima",
"given": "Ryutaro",
"sequence": "additional"
}
],
"container-title": [
"Cureus"
],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
2,
3
]
],
"date-time": "2022-02-03T20:28:16Z",
"timestamp": 1643920096000
},
"deposited": {
"date-parts": [
[
2022,
2,
3
]
],
"date-time": "2022-02-03T20:28:20Z",
"timestamp": 1643920100000
},
"indexed": {
"date-parts": [
[
2022,
2,
5
]
],
"date-time": "2022-02-05T03:14:52Z",
"timestamp": 1644030892122
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "print",
"value": "2168-8184"
}
],
"issued": {
"date-parts": [
[
2022,
2,
3
]
]
},
"language": "en",
"link": [
{
"URL": "https://www.cureus.com/articles/83767-clinical-prognosis-of-patients-with-mild-covid-19-treated-with-casirivimabimdevimab-in-japan",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "4492",
"original-title": [],
"prefix": "10.7759",
"published": {
"date-parts": [
[
2022,
2,
3
]
]
},
"published-print": {
"date-parts": [
[
2022,
2,
3
]
]
},
"publisher": "Cureus, Inc.",
"reference": [
{
"DOI": "10.1056/NEJMoa2108163",
"article-title": "REGEN-COV antibody combination and outcomes in outpatients with COVID-19",
"author": "Weinreich DM",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "ref1",
"unstructured": "Weinreich DM, Sivapalasingam S, Norton T, et al.. REGEN-COV antibody combination and outcomes in outpatients with COVID-19. N Engl J Med. 2021, 385:10.1056/NEJMoa2108163",
"volume": "385",
"year": "2021"
},
{
"key": "ref2",
"unstructured": "Japan becomes first country to approve Regeneron antibody cocktail (casirivimab and imdevimab) for the treatment of mild to moderate COVID-19. (2021). Accessed. January 16, 2022: https://newsroom.regeneron.com/news-releases/news-release-details/japan-becomes-first-country-approve-regeneron-antib...."
},
{
"key": "ref3",
"unstructured": "WHO coronavirus (COVID-19) dashboard. (2022). Accessed. January 12, 2022: https://covid19.who.int/."
},
{
"key": "ref4",
"unstructured": "Underlying medical conditions associated with higher risk for severe COVID-19. information for healthcare providers. (2021). Accessed: January 12, 2022: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html."
},
{
"DOI": "10.1097/CM9.0000000000000744",
"article-title": "Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei province",
"author": "Liu K",
"doi-asserted-by": "publisher",
"journal-title": "Chin Med J (Engl)",
"key": "ref5",
"unstructured": "Liu K, Fang YY, Deng Y, et al.. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei province. Chin Med J (Engl). 2020, 133:1025-31. 10.1097/CM9.0000000000000744",
"volume": "133",
"year": "2020"
},
{
"DOI": "10.15585/mmwr.mm6912e2",
"doi-asserted-by": "crossref",
"key": "ref6",
"unstructured": "Severe outcomes among patients with coronavirus disease 2019 (COVID-19) - United States, February 12-March 16, 2020. (2020). Accessed. January 16, 2022: https://www.cdc.gov/mmwr/volumes/69/wr/mm6912e2.htm."
},
{
"DOI": "10.1001/jama.2020.4326",
"article-title": "Characteristics and outcomes of 21 critically Ill patients with COVID-19 in Washington state",
"author": "Arentz M",
"doi-asserted-by": "publisher",
"journal-title": "JAMA",
"key": "ref7",
"unstructured": "Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, Lee M. Characteristics and outcomes of 21 critically Ill patients with COVID-19 in Washington state. JAMA. 2020, 323:1612-4. 10.1001/jama.2020.4326",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2107934",
"article-title": "Early treatment for COVID-19 with SARS-CoV-2 neutralizing antibody sotrovimab",
"author": "Gupta A",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "ref8",
"unstructured": "Gupta A, Gonzalez-Rojas Y, Juarez E, et al.. Early treatment for COVID-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021, 385:1941-50. 10.1056/NEJMoa2107934",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2102685",
"article-title": "Bamlanivimab plus etesevimab in mild or moderate COVID-19",
"author": "Dougan M",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "ref9",
"unstructured": "Dougan M, Nirula A, Azizad M, et al.. Bamlanivimab plus etesevimab in mild or moderate COVID-19. N Engl J Med. 2021, 385:1382-92. 10.1056/NEJMoa2102685",
"volume": "385",
"year": "2021"
},
{
"key": "ref10",
"unstructured": "Implications of the emergence and spread of the SARS-CoV-2 B.1.1. 529 variant of concern (Omicron) for the EU/EEA. (2021). Accessed. January 16, 2022: https://www.ecdc.europa.eu/sites/default/files/documents/Implications-emergence-spread-SARS-CoV-2%20B.1.1.529-variant...."
},
{
"key": "ref11",
"unstructured": "Enhancing response to Omicron SARS-CoV-2 variant. (2022). Accessed. January 16, 2022: https://www.who.int/publications/m/item/enhancing-readiness-for-omicron-(b.1.1.529)-technical-brief-and-priority-acti...."
}
],
"reference-count": 11,
"references-count": 11,
"relation": {},
"score": 1,
"short-container-title": [],
"short-title": [],
"source": "Crossref",
"subject": [
"Aerospace Engineering"
],
"subtitle": [],
"title": [
"Clinical Prognosis of Patients With Mild COVID-19 Treated With Casirivimab/Imdevimab in Japan"
],
"type": "journal-article"
}
