Efficacy and Safety of COVID-19 Convalescent Plasma in Hospitalized Patients
et al., JAMA Internal Medicine, doi:10.1001/jamainternmed.2021.6850, CONTAIN COVID-19, NCT04364737, Dec 2021
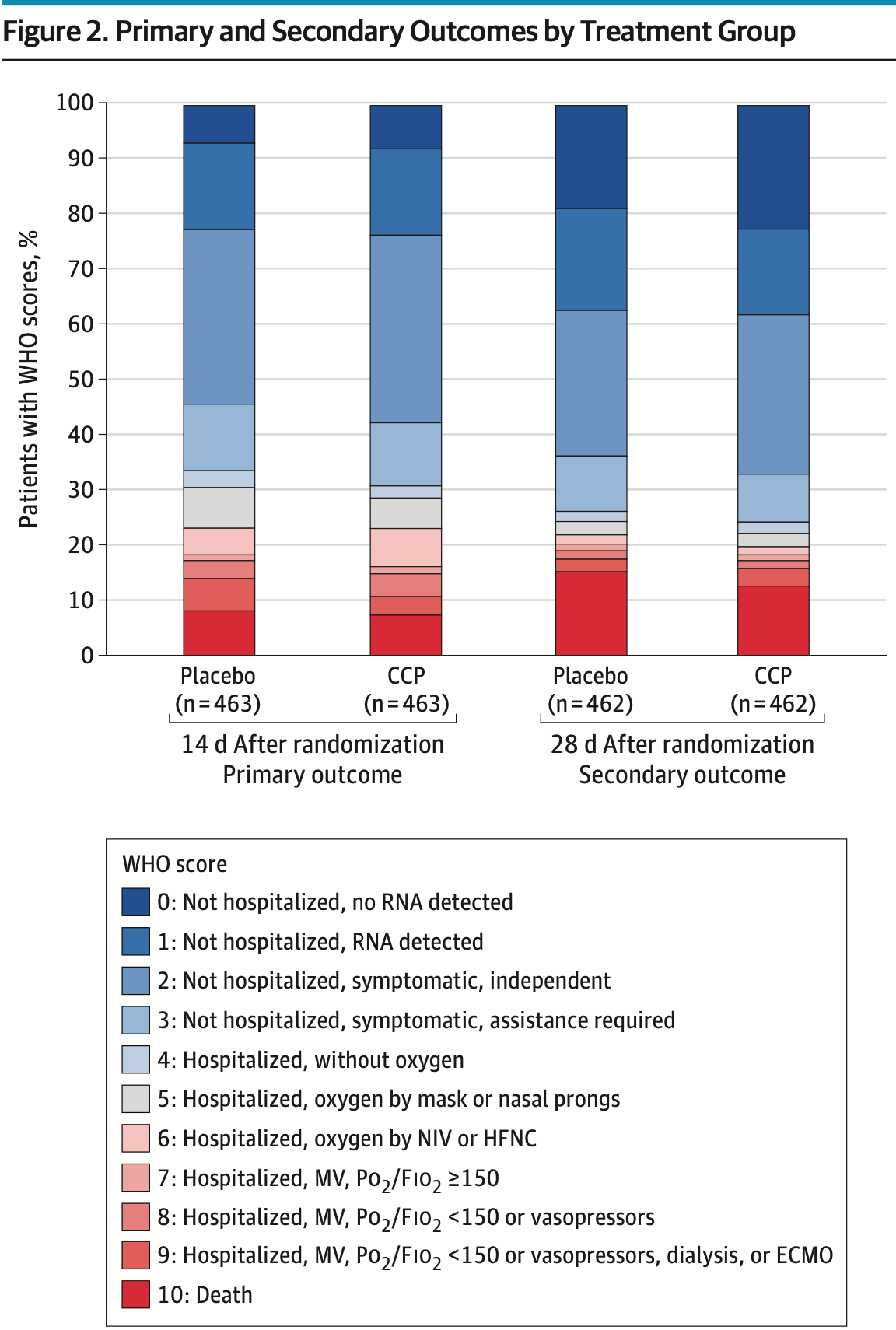
RCT 941 hospitalized patients in the USA, showing no significant difference with convalescent plasma treatment. PASC results are from Yoon et al.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments2.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 11.8% lower, RR 0.88, p = 0.45, treatment 59 of 462 (12.8%), control 71 of 462 (15.4%), NNT 39, odds ratio converted to relative risk, day 28.
|
|
risk of death, 1.3% lower, RR 0.99, p = 0.95, treatment 35 of 463 (7.6%), control 39 of 463 (8.4%), NNT 116, odds ratio converted to relative risk, day 14.
|
|
WHO scale, 7.6% lower, OR 0.92, p = 0.50, treatment 468, control 473, day 28, RR approximated with OR.
|
|
WHO scale, 6.4% lower, OR 0.94, p = 0.58, treatment 468, control 473, day 14, primary outcome, RR approximated with OR.
|
|
risk of long COVID, 2.4% higher, RR 1.02, p = 0.88, treatment 141, control 140, all categories combined.
|
|
risk of long COVID, 5.0% lower, OR 0.95, p = 0.87, treatment 141, control 140, general, RR approximated with OR.
|
|
risk of long COVID, 15.0% higher, OR 1.15, p = 0.70, treatment 141, control 140, gastrointestinal, RR approximated with OR.
|
|
risk of long COVID, 18.0% lower, OR 0.82, p = 0.54, treatment 141, control 140, neurological, RR approximated with OR.
|
|
risk of long COVID, 18.0% higher, OR 1.18, p = 0.53, treatment 141, control 140, respiratory, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Ortigoza et al., 13 Dec 2021, Double Blind Randomized Controlled Trial, placebo-controlled, USA, peer-reviewed, median age 63.0, 268 authors, study period 17 April, 2020 - 15 March, 2021, average treatment delay 7.0 days, trial NCT04364737 (history) (CONTAIN COVID-19).
Contact: l.pirofski@einsteinmed.org.
Efficacy and Safety of COVID-19 Convalescent Plasma in Hospitalized Patients
JAMA Internal Medicine, doi:10.1001/jamainternmed.2021.6850
and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group IMPORTANCE There is clinical equipoise for COVID-19 convalescent plasma (CCP) use in patients hospitalized with COVID-19. OBJECTIVE To determine the safety and efficacy of CCP compared with placebo in hospitalized patients with COVID-19 receiving noninvasive supplemental oxygen. DESIGN, SETTING, AND PARTICIPANTS CONTAIN COVID-19, a randomized, double-blind, placebo-controlled trial of CCP in hospitalized adults with COVID-19, was conducted at 21 US hospitals from April 17, 2020, to March 15, 2021. The trial enrolled 941 participants who were hospitalized for 3 or less days or presented 7 or less days after symptom onset and required noninvasive oxygen supplementation. INTERVENTIONS A unit of approximately 250 mL of CCP or equivalent volume of placebo (normal saline).
MAIN OUTCOMES AND MEASURES The primary outcome was participant scores on the 11-point World Health Organization (WHO) Ordinal Scale for Clinical Improvement on day 14 after randomization; the secondary outcome was WHO scores determined on day 28. Subgroups were analyzed with respect to age, baseline WHO score, concomitant medications, symptom duration, CCP SARS-CoV-2 titer, baseline SARS-CoV-2 serostatus, and enrollment quarter. Outcomes were analyzed using a bayesian proportional cumulative odds model. Efficacy of CCP was defined as a cumulative adjusted odds ratio (cOR) less than 1 and a clinically meaningful effect as cOR less than 0.8.
RESULTS Of 941 participants randomized (473 to placebo and 468 to CCP), 556 were men (59.1%); median age was 63 years (IQR, 52-73); 373 (39.6%) were Hispanic and 132 (14.0%) were non-Hispanic Black. The cOR for the primary outcome adjusted for site, baseline risk, WHO score, age, sex, and symptom duration was 0.94 (95% credible interval [CrI], 0.75-1.18) with posterior probability (P[cOR<1] = 72%); the cOR for the secondary adjusted outcome was 0.92 (95% CrI, 0.74-1.16; P[cOR<1] = 76%). Exploratory subgroup analyses suggested heterogeneity of treatment effect: at day 28, cORs were 0.72 (95% CrI, 0.46-1.13; P[cOR<1] = 93%) for participants enrolled in April-June 2020 and 0.65 (95% CrI, 0.41 to 1.02; P[cOR<1] = 97%) for those not receiving remdesivir and not receiving corticosteroids at randomization. Median CCP SARS-CoV-2 neutralizing titer used in April to June 2020 was 1:175 (IQR, 76-379). Any adverse events (excluding transfusion reactions) were reported for 39 (8.2%) placebo recipients and 44 (9.4%) CCP recipients (P = .57). Transfusion reactions occurred in 2 (0.4) placebo recipients and 8 (1.7) CCP recipients (P = .06).
CONCLUSIONS AND RELEVANCE In this trial, CCP did not meet the prespecified primary and secondary outcomes for CCP efficacy. However, high-titer CCP may have benefited participants early in the pandemic when remdesivir and corticosteroids were not in use.
References
Acquisition, Ortigoza, Yoon, Goldfeld, Troxel et al., Critical revision of the manuscript for important intellectual content
Agarwal, Mukherjee, Kumar, Chatterjee, Bhatnagar et al., Convalescent plasma in the management of moderate Covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial), BMJ, doi:10.1136/bmj.m3939
Avendaño-Solá, Ramos-Martínez, Muñez-Rubio, A multicenter randomized open-label clinical trial for convalescent plasma in patients hospitalized with COVID-19 pneumonia, J Clin Invest, doi:10.1172/JCI152740
Beigel, Tomashek, Dodd, ACTT-1 Study Group Members. Remdesivir for the treatment of Covid-19 -final report, N Engl J Med, doi:10.1056/NEJMoa2007764
Bortz, Iii, Florez, Laudermilch, Single-dilution COVID-19 antibody test with qualitative and quantitative readouts, mSphere, doi:10.1128/mSphere.00224-21
Bégin, Callum, Jamula, 1 Study Group. Convalescent plasma for hospitalized patients with COVID-19: an open-label, randomized controlled trial, Nat Med, doi:10.1038/s41591-021-01488-2
Covid-19, Consortium, Association of convalescent plasma therapy with survival in patients with hematologic cancers and COVID-19, JAMA Oncol, doi:10.1001/jamaoncol.2021.1799?utm_campaign=articlePDF%26utm_medium=articlePDFlink%26utm_source=articlePDF%26utm_content=jamainternmed.2021.6850
Dieterle, Haslwanter, Bortz, Iii, A replication-competent vesicular stomatitis virus for studies of SARS-CoV-2 spike-mediated cell entry and its inhibition, Cell Host Microbe, doi:10.1016/j.chom.2020.06.020
Fibiger, Om Serumbehandling af Difteri
Focosi, Franchini, Pirofski, COVID-19 convalescent plasma and randomized clinical trials: rebuilding confidence by explaining failures and finding signals of efficacy, medRxiv, doi:10.1101/2021.09.07.21263194
Gelman, John, Carlin, Bayesian Data Analysis, doi:10.1201/b16018
Goldfeld, Wu, Tarpey, Prospective individual patient data meta-analysis: evaluating convalescent plasma for COVID-19, Stat Med, doi:10.1002/sim.9115
Hamilton, Lee, Arnold, Lilford, Hemming, Is convalescent plasma futile in COVID-19? A Bayesian re-analysis of the RECOVERY randomized controlled trial, Int J Infect Dis, doi:10.1016/j.ijid.2021.06.034
Herrera, Morano, Celikgil, Characterization of the SARS-CoV-2 S protein: biophysical, biochemical, structural, and antigenic analysis, ACS Omega, doi:10.1021/acsomega.0c03512
Horby, Lim, Emberson, None
Hróbjartsson, Gøtzsche, Gluud, The controlled clinical trial turns 100 years: Fibiger's trial of serum treatment of diphtheria, BMJ, doi:10.1136/bmj.317.7167.1243
Joyner, Carter, Senefeld, Convalescent plasma antibody levels and the risk of death from Covid-19, N Engl J Med, doi:10.1056/NEJMoa2031893
Kraft, Hewlett, Koepsell, None
Kunze, Johnson, Van Helmond, Mortality in individuals treated with COVID-19 convalescent plasma varies with the geographic provenance of donors, Nat Commun, doi:10.1038/s41467-021-25113-5
Körper, Weiss, Zickler, Results of the CAPSID randomized trial for high-dose convalescent plasma in severe COVID-19 patients, J Clin Invest, doi:10.1172/JCI152264
Liu, Lin, Baine, Convalescent plasma treatment of severe COVID-19: a propensity Efficacy and Safety of COVID-19 Convalescent Plasma in Hospitalized Patients Original Investigation Research jamainternalmedicine.com, JAMA Internal Medicine
Luke, Kilbane, Jackson, Hoffman, Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment?, Ann Intern Med, doi:10.7326/0003-4819-145-8-200610170-00139
Maiztegui, Fernandez, De Damilano, Efficacy of immune plasma in treatment of Argentine haemorrhagic fever and association between treatment and a late neurological syndrome, Lancet, doi:10.1016/S0140-6736(79)92335-3
Mcguire, Redden, The use of convalescent human serum in influenza pneumonia-a preliminary report, Am J Public Health, doi:10.2105/AJPH.8.10.741
Petkova, Antman, Troxel, Pooling data from individual clinical trials in the COVID-19 era, JAMA, doi:10.1001/jama.2020.13042?utm_campaign=articlePDF%26utm_medium=articlePDFlink%26utm_source=articlePDF%26utm_content=jamainternmed.2021.6850
Plasma, Group on the Clinical Characterisation and Management of COVID-19 infection. A minimal common outcome measure set for COVID-19 clinical research, Lancet Infect Dis, doi:10.1016/S1473-3099(20)30483-7
Simonovich, Pratx, Scibona, Group. A randomized trial of convalescent plasma in Covid-19 severe pneumonia, N Engl J Med, doi:10.1056/NEJMoa2031304
Thompson, Baumgartner, Pichardo, COVID-19 Outbreak-New York City, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm6946a2
Thompson, Henderson, Shah, None
Thompson, Hughes, Ngai, Rapid emergence and epidemiologic characteristics of the SARS-CoV-2 B.1.526 variant-New, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm7019e1
Wrapp, Wang, Corbett, Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation, Science, doi:10.1126/science.abb2507
Wu, Zhao, Yu, A new coronavirus associated with human respiratory disease in China, Nature, doi:10.1038/s41586-020-2008-3
Yoon, Bartash, Gendlina, Treatment of severe COVID-19 with convalescent plasma in Bronx, NYC, doi:10.1172/jci.insight.142270
DOI record:
{
"DOI": "10.1001/jamainternmed.2021.6850",
"ISSN": [
"2168-6106"
],
"URL": "http://dx.doi.org/10.1001/jamainternmed.2021.6850",
"author": [
{
"affiliation": [
{
"name": "Division of Infectious Disease, Department of Medicine, NYU Grossman School of Medicine, New York, New York"
},
{
"name": "Department of Microbiology, NYU Grossman School of Medicine, New York, New York"
}
],
"family": "Ortigoza",
"given": "Mila B.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Division of Infectious Disease, Department of Medicine, Albert Einstein College of Medicine, Montefiore Medical Center, Bronx, New York"
}
],
"family": "Yoon",
"given": "Hyunah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Population Health, NYU Grossman School of Medicine, New York, New York"
}
],
"family": "Goldfeld",
"given": "Keith S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Population Health, NYU Grossman School of Medicine, New York, New York"
}
],
"family": "Troxel",
"given": "Andrea B.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Disease, Department of Medicine, Albert Einstein College of Medicine, Montefiore Medical Center, Bronx, New York"
},
{
"name": "Department of Microbiology and Immunology, Albert Einstein College of Medicine, Bronx, New York"
}
],
"family": "Daily",
"given": "Johanna P.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Population Health, NYU Grossman School of Medicine, New York, New York"
}
],
"family": "Wu",
"given": "Yinxiang",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Population Health, NYU Grossman School of Medicine, New York, New York"
}
],
"family": "Li",
"given": "Yi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Population Health, NYU Grossman School of Medicine, New York, New York"
}
],
"family": "Wu",
"given": "Danni",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, NYU Grossman School of Medicine, New York, New York"
}
],
"family": "Cobb",
"given": "Gia F.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Surgery, NYU Grossman School of Medicine, New York, New York"
}
],
"family": "Baptiste",
"given": "Gillian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, NYU Long Island School of Medicine, Mineola, New York"
}
],
"family": "O’Keeffe",
"given": "Mary",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Disease, Department of Medicine, Albert Einstein College of Medicine, Montefiore Medical Center, Bronx, New York"
}
],
"family": "Corpuz",
"given": "Marilou O.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Disease, Department of Internal Medicine, The University of Texas Health Science Center at Houston, McGovern Medical School, Houston"
}
],
"family": "Ostrosky-Zeichner",
"given": "Luis",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Emergency Medicine, The University of Texas Health Science Center at Houston, McGovern Medical School, Houston"
}
],
"family": "Amin",
"given": "Amee",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Disease, Department of Medicine, NYU Grossman School of Medicine, New York, New York"
}
],
"family": "Zacharioudakis",
"given": "Ioannis M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Disease, Department of Medicine, University of Miami Miller School of Medicine, Miami, Florida"
},
{
"name": "Miami Clinical and Translational Science Institute, University of Miami Miller School of Medicine Miami, Florida"
}
],
"family": "Jayaweera",
"given": "Dushyantha T.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pathology, University of Miami Miller School of Medicine, Miami, Florida"
}
],
"family": "Wu",
"given": "Yanyun",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, The University of Texas Health Science Center at Tyler, UTHealth East Texas, Tyler"
}
],
"family": "Philley",
"given": "Julie V.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, The University of Texas Health Science Center at Tyler, UTHealth East Texas, Tyler"
}
],
"family": "Devine",
"given": "Megan S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Section of Infectious Diseases, Department of Internal Medicine, Yale University School of Medicine, New Haven, Connecticut"
}
],
"family": "Desruisseaux",
"given": "Mahalia S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Obstetrics, Gynecology, and Reproductive Sciences, Yale University School of Medicine, New Haven, Connecticut"
}
],
"family": "Santin",
"given": "Alessandro D.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Disease, Department of Medicine, University of Miami Miller School of Medicine, Miami, Florida"
}
],
"family": "Anjan",
"given": "Shweta",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Critical Care, Department of Internal Medicine, The University of Texas Health Science Center at Houston, McGovern Medical School, Houston"
}
],
"family": "Mathew",
"given": "Reeba",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Critical Care, Department of Internal Medicine, The University of Texas Health Science Center at Houston, McGovern Medical School, Houston"
}
],
"family": "Patel",
"given": "Bela",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Disease, Department of Internal Medicine, The University of Texas Health Science Center at Houston, McGovern Medical School, Houston"
}
],
"family": "Nigo",
"given": "Masayuki",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, NYU Grossman School of Medicine, New York, New York"
},
{
"name": "Laura and Isaac Perlmutter Cancer Center, NYU Grossman School of Medicine, New York, New York"
}
],
"family": "Upadhyay",
"given": "Rabi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Disease, Department of Medicine, NYU Grossman School of Medicine, New York, New York"
}
],
"family": "Kupferman",
"given": "Tania",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, The University of Texas Rio Grande Valley, Edinburg"
}
],
"family": "Dentino",
"given": "Andrew N.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Pulmonary, Critical Care, and Sleep Medicine, Department of Medicine, Medical College of Wisconsin, Milwaukee"
}
],
"family": "Nanchal",
"given": "Rahul",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Pulmonary and Critical Care Medicine, Department of Medicine, Johns Hopkins University, Baltimore, Maryland"
}
],
"family": "Merlo",
"given": "Christian A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Pulmonary and Critical Care Medicine, Department of Medicine, Johns Hopkins University, Baltimore, Maryland"
}
],
"family": "Hager",
"given": "David N.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Microbiology and Immunology, Albert Einstein College of Medicine, Bronx, New York"
}
],
"family": "Chandran",
"given": "Kartik",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biochemistry, Albert Einstein College of Medicine, Bronx, New York"
}
],
"family": "Lai",
"given": "Jonathan R.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Disease, Department of Medicine, Albert Einstein College of Medicine, Montefiore Medical Center, Bronx, New York"
},
{
"name": "Department of Microbiology and Immunology, Albert Einstein College of Medicine, Bronx, New York"
}
],
"family": "Rivera",
"given": "Johanna",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biochemistry, Albert Einstein College of Medicine, Bronx, New York"
}
],
"family": "Bikash",
"given": "Chowdhury R.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Microbiology and Immunology, Albert Einstein College of Medicine, Bronx, New York"
}
],
"family": "Lasso",
"given": "Gorka",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pathology, NYU Grossman School of Medicine, New York, New York"
}
],
"family": "Hilbert",
"given": "Timothy P.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pathology, Albert Einstein College of Medicine, Bronx, New York"
}
],
"family": "Paroder",
"given": "Monika",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Disease, Department of Medicine, Albert Einstein College of Medicine, Montefiore Medical Center, Bronx, New York"
}
],
"family": "Asencio",
"given": "Andrea A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Population Health, NYU Grossman School of Medicine, New York, New York"
},
{
"name": "Department of Environmental Health, NYU Grossman School of Medicine, New York, New York"
}
],
"family": "Liu",
"given": "Mengling",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Population Health, NYU Grossman School of Medicine, New York, New York"
},
{
"name": "Department of Child and Adolescent Psychiatry, NYU Grossman School of Medicine, New York"
},
{
"name": "Nathan S. Kline Institute for Psychiatric Research, Orangeburg, New York"
}
],
"family": "Petkova",
"given": "Eva",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Research Information Technology and Informatics, NYU Grossman School of Medicine, New York, New York"
}
],
"family": "Bragat",
"given": "Alexander",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical and Translational Science Institute of Southern Wisconsin, Medical College of Wisconsin Milwaukee"
}
],
"family": "Shaker",
"given": "Reza",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Center for Clinical and Translational Sciences, Division of Cardiovascular Medicine, Department of Internal Medicine, The University of Texas Health Science Center at Houston, McGovern Medical School, Houston"
}
],
"family": "McPherson",
"given": "David D.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Miami Clinical and Translational Science Institute, University of Miami Miller School of Medicine Miami, Florida"
}
],
"family": "Sacco",
"given": "Ralph L.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Disease, Department of Medicine, Albert Einstein College of Medicine, Montefiore Medical Center, Bronx, New York"
},
{
"name": "Harold and Muriel Block Institute for Clinical and Translational Research, Albert Einstein College of Medicine and Montefiore Medical Center Bronx, New York"
}
],
"family": "Keller",
"given": "Marla J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Ronald O. Perelman Department of Emergency Medicine, NYU Grossman School of Medicine, New York, New York"
},
{
"name": "NYC Health and Hospitals Corporation Clinical and Translational Science Institute, NYU Grossman School of Medicine, New York, New York"
}
],
"family": "Grudzen",
"given": "Corita R.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "NYC Health and Hospitals Corporation Clinical and Translational Science Institute, NYU Grossman School of Medicine, New York, New York"
},
{
"name": "Leon H. Charney Division of Cardiology, Department of Medicine, NYU Grossman School of Medicine, New York, New York"
}
],
"family": "Hochman",
"given": "Judith S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Disease, Department of Medicine, Albert Einstein College of Medicine, Montefiore Medical Center, Bronx, New York"
},
{
"name": "Department of Microbiology and Immunology, Albert Einstein College of Medicine, Bronx, New York"
}
],
"family": "Pirofski",
"given": "Liise-anne",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Rahman",
"given": "Fatema Z",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Ajayi",
"given": "Adeyinka O",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Rodriguez",
"given": "Sara L",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Ledesma",
"given": "Ana G",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Keeling",
"given": "Deborah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Rappoport",
"given": "Norka",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Ebel",
"given": "Sam F",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Kim",
"given": "Jayne",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Chang",
"given": "Michelle",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Chan",
"given": "Kevin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Patel",
"given": "Payal",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Martocci",
"given": "Anne",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Dave",
"given": "Shivang",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Darwish",
"given": "Yousef",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Taveras",
"given": "Monica",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Shoyelu",
"given": "Victoria",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Xin",
"given": "Patrick",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Iturrate",
"given": "Eduardo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Moldolsky",
"given": "Lee C",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Raimondo",
"given": "Brian J",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Mendez",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Hughes",
"given": "Patricia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Sterling",
"given": "Stephanie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Lord",
"given": "Aaron S",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Yaghi",
"given": "Shadi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Veloso",
"given": "Karen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Sheikh",
"given": "Masooma",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Visconti-Ferrara",
"given": "Erica",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Fleming",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Youn",
"given": "Heekoung",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Jane Fran",
"given": "Baby",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Medina",
"given": "Rosario",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "McKell",
"given": "Renee",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Khan",
"given": "Saila",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Hamilton",
"given": "Tanya",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Sanchez",
"given": "Carlos J",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Patel",
"given": "Nandini H",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Cleare",
"given": "Levi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Vergnolle",
"given": "Olivia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Nakouzi",
"given": "Antonio",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Quevedo",
"given": "Gregory",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Bortz",
"given": "Robert H",
"sequence": "additional",
"suffix": "III"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Wirchnianski",
"given": "Ariel S",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Florez",
"given": "Catalina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Babb",
"given": "Rachelle",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Ayala",
"given": "Jennifer",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Tsagaris",
"given": "K. Zoe",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "James",
"given": "Andria",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Eke",
"given": "Isaiah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Obeidallah",
"given": "Aisha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Sandu",
"given": "Oana A",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Sohval",
"given": "Sophie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Serrano-Rahman",
"given": "Leana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Uehlinger",
"given": "Joan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Bartash",
"given": "Rachel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Al-Abduladheem",
"given": "Aya",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Gendlina",
"given": "Inessa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Sheridan",
"given": "Carol",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Bortnick",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Eichler",
"given": "Jeremy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Kaufman",
"given": "Rachel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Yukelis",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Pennock",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Goggin",
"given": "Michelle",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Shen",
"given": "Christine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Annam",
"given": "Jayabhargav",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Khokhar",
"given": "Ahmed",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Barboto",
"given": "Daniel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Lally",
"given": "Brianna J",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Lee",
"given": "Audrey",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Lee",
"given": "Max",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Yang",
"given": "Xiuyi A",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Allen",
"given": "Stephanie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Malaviya",
"given": "Avinash",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Moussa",
"given": "Omar",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Park",
"given": "Rosa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Sample",
"given": "Reise",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Bae",
"given": "Andrea",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Benoni",
"given": "Galit",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Boerger",
"given": "Lindsie L",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Baker",
"given": "Lisa D",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Luther",
"given": "Martha A",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Ameti",
"given": "Lirim S",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Briggs",
"given": "Neima",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Golden",
"given": "Marjorie R",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Gormally",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Huang",
"given": "Gloria S",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Johnson",
"given": "Raymond M",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Morrison",
"given": "Alyssa R",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Montagna-Hill",
"given": "Michele",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Rivera",
"given": "Brooke N",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Cortezzo",
"given": "Grace M",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Debski",
"given": "Kay B",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Nicoletti",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "DeBenedictis",
"given": "Kerry",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Davis",
"given": "Rivcah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Marshall",
"given": "Christi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Duque Cuartas",
"given": "Miriam Andrea",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Beauchamps",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Bertran-Lopez",
"given": "Jovanna",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Gonzales Zamora",
"given": "Jose",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Delgado-Lelievre",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Dominguez",
"given": "Sheela",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Lee",
"given": "Chin Chin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Kusack",
"given": "Halina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Karakeshishyan",
"given": "Vela",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Hajaz",
"given": "Americo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Deniz",
"given": "Dasmany",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Garcia",
"given": "Giovanni",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Dae",
"given": "Katheryn",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Blenet",
"given": "Patricia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Jaffe",
"given": "Deborah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Olson",
"given": "Lourdes",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Sabogal",
"given": "Diane",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Blust",
"given": "Olivia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Del Prete Perez",
"given": "Veronica",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Bornia",
"given": "Claudia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Rodriguez-Perez",
"given": "Vanessa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Calderon",
"given": "Vivian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Ramdev",
"given": "Rajan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Jolly",
"given": "Aaliyah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Guzman",
"given": "Ivonne",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Guerra",
"given": "Richard",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Brito",
"given": "Sebastian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Hobbs",
"given": "Rhonda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Denham",
"given": "Rebecca",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Dick",
"given": "John",
"sequence": "additional",
"suffix": "II"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Hernandez",
"given": "Maria D",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Nielsen",
"given": "Laura E",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Anjum",
"given": "Sami M",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Mader",
"given": "Shelby C",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Stutz",
"given": "Taylor P",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Mammadova",
"given": "Mehriban",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Nichols",
"given": "Pamela",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Khan",
"given": "Tanya S",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Boktour",
"given": "Maha R",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Castaneda",
"given": "Brenda L",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Benitez",
"given": "Brenda D",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Hinojosa",
"given": "Erik",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Guerra",
"given": "Brenda C",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Ortiz",
"given": "Armando",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Hebbeler-Clark",
"given": "Renee S",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "McShane",
"given": "Pamela J",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Hibbard",
"given": "Rebekah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Hawkins",
"given": "Benji E",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Dohanich",
"given": "Elizabeth R",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Wadle",
"given": "Carly",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Greenlee",
"given": "Kimberly L",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Brooks",
"given": "Jennifer",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Herrick",
"given": "Christopher D",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Gode",
"given": "Amit",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Bergl",
"given": "Paul",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Hu",
"given": "Kurt",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Patel",
"given": "Jayshil",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Srinivasan",
"given": "Shankar",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Graf",
"given": "Jeanette",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Klis",
"given": "Char",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Reimer",
"given": "Kyersten",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Carpenter",
"given": "Erica",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Naczek",
"given": "Christine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Petersen",
"given": "Rae",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Dex",
"given": "Renee",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Drossart",
"given": "Jennifer",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Zelten",
"given": "James",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Brummitt",
"given": "Charles",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Liang",
"given": "Mengyao",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Yanny",
"given": "Lynda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Dennison",
"given": "Gary",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Runningen",
"given": "Phyllis",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Brzezinski",
"given": "Brian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Fiebig",
"given": "Stephen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Naczek",
"given": "Chris",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "and the CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group"
}
],
"family": "Kasdorf",
"given": "Michelle",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Disease, Department of Medicine, NYU Grossman School of Medicine, New York, New York"
}
],
"family": "Parameswaran",
"given": "Lalitha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Urology, NYU Long Island School of Medicine, Mineola, New York"
}
],
"family": "Corcoran",
"given": "Anthony T.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, NYU Long Island School of Medicine, Mineola, New York"
}
],
"family": "Rohatgi",
"given": "Abhinav",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, NYU Long Island School of Medicine, Mineola, New York"
}
],
"family": "Wronska",
"given": "Marta W.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, NYU Grossman School of Medicine, New York, New York"
}
],
"family": "Wu",
"given": "Xinyuan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pediatrics, NYU Grossman School of Medicine, New York, New York"
}
],
"family": "Srinivasan",
"given": "Ranjini",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pathology, NYU Grossman School of Medicine, New York, New York"
}
],
"family": "Deng",
"given": "Fang-Ming",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Disease, Department of Medicine, NYU Grossman School of Medicine, New York, New York"
}
],
"family": "Filardo",
"given": "Thomas D.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, NYU Grossman School of Medicine, New York, New York"
}
],
"family": "Pendse",
"given": "Jay",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, NYU Grossman School of Medicine, New York, New York"
}
],
"family": "Blaser",
"given": "Simone B.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, NYU Grossman School of Medicine, New York, New York"
}
],
"family": "Whyte",
"given": "Olga",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, NYU Grossman School of Medicine, New York, New York"
}
],
"family": "Gallagher",
"given": "Jacqueline M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, NYU Grossman School of Medicine, New York, New York"
}
],
"family": "Thomas",
"given": "Ololade E.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, NYU Grossman School of Medicine, New York, New York"
}
],
"family": "Ramos",
"given": "Danibel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, NYU Grossman School of Medicine, New York, New York"
}
],
"family": "Sturm-Reganato",
"given": "Caroline L.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, NYU Grossman School of Medicine, New York, New York"
}
],
"family": "Fong",
"given": "Charlotte C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, NYU Grossman School of Medicine, New York, New York"
}
],
"family": "Daus",
"given": "Ivy M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, NYU Grossman School of Medicine, New York, New York"
}
],
"family": "Payoen",
"given": "Arianne Gisselle",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pathology, NYU Long Island School of Medicine, Mineola, New York"
}
],
"family": "Chiofolo",
"given": "Joseph T.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pathology, NYU Long Island School of Medicine, Mineola, New York"
}
],
"family": "Friedman",
"given": "Mark T.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pathology, NYU Grossman School of Medicine, New York, New York"
}
],
"family": "Wu",
"given": "Ding Wen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pathology, NYU Grossman School of Medicine, New York, New York"
}
],
"family": "Jacobson",
"given": "Jessica L.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, NYU Long Island School of Medicine, Mineola, New York"
}
],
"family": "Schneider",
"given": "Jeffrey G.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Disease, Department of Medicine, Albert Einstein College of Medicine, Montefiore Medical Center, Bronx, New York"
},
{
"name": "Pfizer Vaccine Clinical Research and Development, Pfizer Inc, Pearl River, New York"
}
],
"family": "Sarwar",
"given": "Uzma N.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Emergency Medicine, The University of Texas Health Science Center at Houston, McGovern Medical School, Houston"
},
{
"name": "Department of Emergency Medicine, The Ohio State University, Ohio"
}
],
"family": "Wang",
"given": "Henry E.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Emergency Medicine, The University of Texas Health Science Center at Houston, McGovern Medical School, Houston"
}
],
"family": "Huebinger",
"given": "Ryan M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Critical Care, Department of Internal Medicine, The University of Texas Health Science Center at Houston, McGovern Medical School, Houston"
}
],
"family": "Dronavalli",
"given": "Goutham",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pathology and Laboratory Medicine, The University of Texas Health Science Center at Houston, McGovern Medical School, Houston"
}
],
"family": "Bai",
"given": "Yu",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Disease, Department of Internal Medicine, The University of Texas Health Science Center at Houston, McGovern Medical School, Houston"
}
],
"family": "Grimes",
"given": "Carolyn Z.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pathology and Laboratory Medicine, The University of Texas Health Science Center at Houston, McGovern Medical School, Houston"
}
],
"family": "Eldin",
"given": "Karen W.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Disease, Department of Internal Medicine, The University of Texas Health Science Center at Houston, McGovern Medical School, Houston"
}
],
"family": "Umana",
"given": "Virginia E",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, The University of Texas Rio Grande Valley, Edinburg"
}
],
"family": "Martin",
"given": "Jessica G.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, The University of Texas Rio Grande Valley, Edinburg"
}
],
"family": "Heath",
"given": "Timothy R.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, The University of Texas Rio Grande Valley, Edinburg"
}
],
"family": "Bello",
"given": "Fatimah O.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Miami Clinical and Translational Science Institute, University of Miami Miller School of Medicine Miami, Florida"
}
],
"family": "Ransford",
"given": "Daru Lane",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Section of Infectious Diseases, Department of Internal Medicine, Yale University School of Medicine, New Haven, Connecticut"
}
],
"family": "Laurent-Rolle",
"given": "Maudry",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Section of Infectious Diseases, Department of Internal Medicine, Yale University School of Medicine, New Haven, Connecticut"
}
],
"family": "Shenoi",
"given": "Sheela V.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Section of Infectious Diseases, Department of Internal Medicine, Yale University School of Medicine, New Haven, Connecticut"
}
],
"family": "Akide-Ndunge",
"given": "Oscar Bate",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Medical College of Wisconsin, Milwaukee"
}
],
"family": "Thapa",
"given": "Bipin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Pulmonary, Critical Care, and Sleep Medicine, Department of Medicine, Medical College of Wisconsin, Milwaukee"
}
],
"family": "Peterson",
"given": "Jennifer L.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical and Translational Science Institute of Southern Wisconsin, Medical College of Wisconsin Milwaukee"
}
],
"family": "Knauf",
"given": "Kelly",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Pulmonary and Critical Care Medicine, Department of Medicine, Johns Hopkins University, Baltimore, Maryland"
}
],
"family": "Patel",
"given": "Shivani U.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Disease, Department of Medicine, Albert Einstein College of Medicine, Montefiore Medical Center, Bronx, New York"
}
],
"family": "Cheney",
"given": "Laura L.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Laboratory Medicine, Yale University School of Medicine, New Haven, Connecticut"
}
],
"family": "Tormey",
"given": "Christopher A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Laboratory Medicine, Yale University School of Medicine, New Haven, Connecticut"
},
{
"name": "Department of Pediatrics, Yale University School of Medicine, New Haven, Connecticut"
}
],
"family": "Hendrickson",
"given": "Jeanne E.",
"sequence": "additional"
},
{
"affiliation": [],
"name": "CONTAIN COVID-19 Consortium for the CONTAIN COVID-19 Study Group",
"sequence": "additional"
}
],
"container-title": "JAMA Internal Medicine",
"container-title-short": "JAMA Intern Med",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
12,
13
]
],
"date-time": "2021-12-13T16:00:52Z",
"timestamp": 1639411252000
},
"deposited": {
"date-parts": [
[
2022,
2,
7
]
],
"date-time": "2022-02-07T16:16:36Z",
"timestamp": 1644250596000
},
"indexed": {
"date-parts": [
[
2022,
11,
4
]
],
"date-time": "2022-11-04T10:31:18Z",
"timestamp": 1667557878219
},
"is-referenced-by-count": 19,
"issue": "2",
"issued": {
"date-parts": [
[
2022,
2,
1
]
]
},
"journal-issue": {
"issue": "2",
"published-print": {
"date-parts": [
[
2022,
2,
1
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://jamanetwork.com/journals/jamainternalmedicine/articlepdf/2787090/jamainternal_ortigoza_2021_oi_210071_1643648313.72397.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "10",
"original-title": [],
"page": "115",
"prefix": "10.1001",
"published": {
"date-parts": [
[
2022,
2,
1
]
]
},
"published-print": {
"date-parts": [
[
2022,
2,
1
]
]
},
"publisher": "American Medical Association (AMA)",
"reference": [
{
"DOI": "10.1038/s41586-020-2008-3",
"article-title": "A new coronavirus associated with human respiratory disease in China.",
"author": "Wu",
"doi-asserted-by": "publisher",
"first-page": "265",
"issue": "7798",
"journal-title": "Nature",
"key": "ioi210071r1",
"volume": "579",
"year": "2020"
},
{
"DOI": "10.15585/mmwr.mm6946a2",
"article-title": "COVID-19 Outbreak—New York City, February 29-June 1, 2020.",
"author": "Thompson",
"doi-asserted-by": "publisher",
"first-page": "1725",
"issue": "46",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "ioi210071r2",
"volume": "69",
"year": "2020"
},
{
"DOI": "10.2105/AJPH.8.10.741",
"article-title": "The use of convalescent human serum in influenza pneumonia—a preliminary report.",
"author": "McGuire",
"doi-asserted-by": "publisher",
"first-page": "741",
"issue": "10",
"journal-title": "Am J Public Health (N Y)",
"key": "ioi210071r3",
"volume": "8",
"year": "1918"
},
{
"DOI": "10.7326/0003-4819-145-8-200610170-00139",
"article-title": "Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment?",
"author": "Luke",
"doi-asserted-by": "publisher",
"first-page": "599",
"issue": "8",
"journal-title": "Ann Intern Med",
"key": "ioi210071r4",
"volume": "145",
"year": "2006"
},
{
"DOI": "10.1093/cid/civ334",
"article-title": "The use of TKM-100802 and convalescent plasma in 2 patients with Ebola virus disease in the United States.",
"author": "Kraft",
"doi-asserted-by": "publisher",
"first-page": "496",
"issue": "4",
"journal-title": "Clin Infect Dis",
"key": "ioi210071r5",
"volume": "61",
"year": "2015"
},
{
"article-title": "Om Serumbehandling af Difteri [published in Danish].",
"author": "Fibiger",
"first-page": "309",
"journal-title": "Hospitalstidende",
"key": "ioi210071r6",
"volume": "6",
"year": "1898"
},
{
"DOI": "10.1136/bmj.317.7167.1243",
"article-title": "The controlled clinical trial turns 100 years: Fibiger’s trial of serum treatment of diphtheria.",
"author": "Hróbjartsson",
"doi-asserted-by": "publisher",
"first-page": "1243",
"issue": "7167",
"journal-title": "BMJ",
"key": "ioi210071r7",
"volume": "317",
"year": "1998"
},
{
"DOI": "10.1016/S0140-6736(79)92335-3",
"article-title": "Efficacy of immune plasma in treatment of Argentine haemorrhagic fever and association between treatment and a late neurological syndrome.",
"author": "Maiztegui",
"doi-asserted-by": "publisher",
"first-page": "1216",
"issue": "8154",
"journal-title": "Lancet",
"key": "ioi210071r8",
"volume": "2",
"year": "1979"
},
{
"DOI": "10.1056/NEJMoa2031893",
"article-title": "Convalescent plasma antibody levels and the risk of death from Covid-19.",
"author": "Joyner",
"doi-asserted-by": "publisher",
"first-page": "1015",
"issue": "11",
"journal-title": "N Engl J Med",
"key": "ioi210071r9",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1038/s41591-020-1088-9",
"article-title": "Convalescent plasma treatment of severe COVID-19: a propensity score–matched control study.",
"author": "Liu",
"doi-asserted-by": "publisher",
"first-page": "1708",
"issue": "11",
"journal-title": "Nat Med",
"key": "ioi210071r10",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1016/j.ajpath.2020.10.008",
"article-title": "Significantly decreased mortality in a large cohort of coronavirus disease 2019 (COVID-19) patients transfused early with convalescent plasma containing high-titer anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein IgG.",
"author": "Salazar",
"doi-asserted-by": "publisher",
"first-page": "90",
"issue": "1",
"journal-title": "Am J Pathol",
"key": "ioi210071r11",
"volume": "191",
"year": "2021"
},
{
"DOI": "10.1371/journal.pone.0254453",
"article-title": "Early but not late convalescent plasma is associated with better survival in moderate-to-severe COVID-19.",
"author": "Briggs",
"doi-asserted-by": "crossref",
"issue": "7",
"journal-title": "PLoS One",
"key": "ioi210071r12",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1172/JCI150646",
"article-title": "A randomized double-blind controlled trial of convalescent plasma in adults with severe COVID-19.",
"author": "O’Donnell",
"doi-asserted-by": "crossref",
"issue": "13",
"journal-title": "J Clin Invest",
"key": "ioi210071r13",
"volume": "131",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.10044",
"article-title": "Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial.",
"author": "Li",
"doi-asserted-by": "publisher",
"first-page": "460",
"issue": "5",
"journal-title": "JAMA",
"key": "ioi210071r14",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2031304",
"article-title": "A randomized trial of convalescent plasma in Covid-19 severe pneumonia.",
"author": "Simonovich",
"doi-asserted-by": "publisher",
"first-page": "619",
"issue": "7",
"journal-title": "N Engl J Med",
"key": "ioi210071r15",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1136/bmj.m3939",
"article-title": "Convalescent plasma in the management of moderate Covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial).",
"author": "Agarwal",
"doi-asserted-by": "crossref",
"first-page": "m3939",
"journal-title": "BMJ",
"key": "ioi210071r16",
"volume": "371",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(21)00897-7",
"article-title": "Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial.",
"author": "RECOVERY Collaborative Group",
"doi-asserted-by": "publisher",
"first-page": "2049",
"issue": "10289",
"journal-title": "Lancet",
"key": "ioi210071r17",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1172/JCI152264",
"article-title": "Results of the CAPSID randomized trial for high-dose convalescent plasma in severe COVID-19 patients.",
"author": "Körper",
"doi-asserted-by": "crossref",
"journal-title": "J Clin Invest",
"key": "ioi210071r18",
"year": "2021"
},
{
"DOI": "10.1172/JCI152740",
"article-title": "A multicenter randomized open-label clinical trial for convalescent plasma in patients hospitalized with COVID-19 pneumonia.",
"author": "Avendaño-Solá",
"doi-asserted-by": "crossref",
"journal-title": "J Clin Invest",
"key": "ioi210071r19",
"year": "2021"
},
{
"DOI": "10.1128/mSphere.00224-21",
"article-title": "Single-dilution COVID-19 antibody test with qualitative and quantitative readouts.",
"author": "Bortz",
"doi-asserted-by": "crossref",
"issue": "2",
"journal-title": "mSphere",
"key": "ioi210071r21",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1016/j.chom.2020.06.020",
"article-title": "A replication-competent vesicular stomatitis virus for studies of SARS-CoV-2 spike-mediated cell entry and its inhibition.",
"author": "Dieterle",
"doi-asserted-by": "publisher",
"first-page": "486",
"issue": "3",
"journal-title": "Cell Host Microbe",
"key": "ioi210071r22",
"volume": "28",
"year": "2020"
},
{
"article-title": "Treatment of severe COVID-19 with convalescent plasma in Bronx, NYC.",
"author": "Yoon",
"issue": "4",
"journal-title": "JCI Insight",
"key": "ioi210071r23",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1016/S1473-3099(20)30483-7",
"article-title": "A minimal common outcome measure set for COVID-19 clinical research.",
"author": "WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection",
"doi-asserted-by": "publisher",
"first-page": "e192",
"issue": "8",
"journal-title": "Lancet Infect Dis",
"key": "ioi210071r26",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1126/science.abb2507",
"article-title": "Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation.",
"author": "Wrapp",
"doi-asserted-by": "publisher",
"first-page": "1260",
"issue": "6483",
"journal-title": "Science",
"key": "ioi210071r27",
"volume": "367",
"year": "2020"
},
{
"DOI": "10.1021/acsomega.0c03512",
"article-title": "Characterization of the SARS-CoV-2 S protein: biophysical, biochemical, structural, and antigenic analysis.",
"author": "Herrera",
"doi-asserted-by": "publisher",
"first-page": "85",
"issue": "1",
"journal-title": "ACS Omega",
"key": "ioi210071r28",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2021436",
"article-title": "Dexamethasone in hospitalized patients with Covid-19.",
"author": "Horby",
"doi-asserted-by": "crossref",
"first-page": "693",
"issue": "8",
"journal-title": "N Engl J Med",
"key": "ioi210071r30",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the treatment of Covid-19 —final report.",
"author": "Beigel",
"doi-asserted-by": "publisher",
"first-page": "1813",
"issue": "19",
"journal-title": "N Engl J Med",
"key": "ioi210071r32",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1001/jamaoncol.2021.1799",
"article-title": "Association of convalescent plasma therapy with survival in patients with hematologic cancers and COVID-19.",
"author": "Thompson",
"doi-asserted-by": "crossref",
"journal-title": "JAMA Oncol",
"key": "ioi210071r33",
"year": "2021"
},
{
"DOI": "10.1038/s41591-021-01488-2",
"article-title": "Convalescent plasma for hospitalized patients with COVID-19: an open-label, randomized controlled trial.",
"author": "Bégin",
"doi-asserted-by": "crossref",
"journal-title": "Nat Med",
"key": "ioi210071r36",
"year": "2021"
},
{
"DOI": "10.15585/mmwr.mm7019e1",
"article-title": "Rapid emergence and epidemiologic characteristics of the SARS-CoV-2 B.1.526 variant—New York City, New York, January 1-April 5, 2021.",
"author": "Thompson",
"doi-asserted-by": "publisher",
"first-page": "712",
"issue": "19",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "ioi210071r37",
"volume": "70",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.13042",
"article-title": "Pooling data from individual clinical trials in the COVID-19 era.",
"author": "Petkova",
"doi-asserted-by": "publisher",
"first-page": "543",
"issue": "6",
"journal-title": "JAMA",
"key": "ioi210071r39",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1002/sim.9115",
"article-title": "Prospective individual patient data meta-analysis: evaluating convalescent plasma for COVID-19.",
"author": "Goldfeld",
"doi-asserted-by": "crossref",
"journal-title": "Stat Med",
"key": "ioi210071r40",
"year": "2021"
},
{
"DOI": "10.1038/s41467-021-25113-5",
"article-title": "Mortality in individuals treated with COVID-19 convalescent plasma varies with the geographic provenance of donors.",
"author": "Kunze",
"doi-asserted-by": "publisher",
"first-page": "4864",
"issue": "1",
"journal-title": "Nat Commun",
"key": "ioi210071r41",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1016/j.ijid.2021.06.034",
"article-title": "Is convalescent plasma futile in COVID-19? A Bayesian re-analysis of the RECOVERY randomized controlled trial.",
"author": "Hamilton",
"doi-asserted-by": "publisher",
"first-page": "114",
"journal-title": "Int J Infect Dis",
"key": "ioi210071r42",
"volume": "109",
"year": "2021"
},
{
"DOI": "10.1201/b16018",
"author": "Gelman",
"doi-asserted-by": "crossref",
"key": "ioi210071r29",
"volume-title": "Bayesian Data Analysis",
"year": "2013"
},
{
"key": "ioi210071r20",
"unstructured": "Centers for Disease Control and Prevention. People with certain medical conditions. October 14, 2021. Accessed September 22, 2021. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html"
},
{
"key": "ioi210071r24",
"unstructured": "New York SARSCoV Microsphere Immunoassay EUA Summary. Accelerated emergency use authorization (EUA) summary: New York SARS-COV microsphere immunoassay for antibody detection (Wadsworth Center at the New York State Department of Health). New York SARS-CoV microsphere immunoassay for antibody detection. Accessed September 22, 2021. https://www.fda.gov/media/137541/download"
},
{
"key": "ioi210071r25",
"unstructured": "US Food and Drug Administration. COVID-19 Convalescent Plasma. 2020. Accessed September 22, 2021. https://www.fda.govmedia/141480/download"
},
{
"DOI": "10.1002/cpu.30542",
"doi-asserted-by": "crossref",
"key": "ioi210071r31",
"unstructured": "FDA approves first treatment for COVID-19. October 22, 2021. Accessed September 22, 2021. https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-covid-19"
},
{
"DOI": "10.1101/2021.09.07.21263194",
"doi-asserted-by": "crossref",
"key": "ioi210071r34",
"unstructured": "Focosi? D, Franchini? M, Pirofski? L-a, ? COVID-19 convalescent plasma and randomized clinical trials: rebuilding confidence by explaining failures and finding signals of efficacy.? medRxiv. 2021:2021.2009.2007.21263194. doi:10.1101/2021.09.07.21263194"
},
{
"key": "ioi210071r35",
"unstructured": "US Food and Drug Administration. Convalescent plasma EUA letter of authorization. March 9, 2021. Accessed September 22, 2021. https://www.fda.gov/media/141477/download"
},
{
"key": "ioi210071r38",
"unstructured": "Centers for Disease Control and Prevention. COVID Data Tracker. Variant proportions. 2021. Accessed September 22, 2021. https://covid.cdc.gov/covid-data-tracker/#variant-proportions"
}
],
"reference-count": 42,
"references-count": 42,
"relation": {},
"resource": {
"primary": {
"URL": "https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2787090"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Internal Medicine"
],
"subtitle": [
"A Randomized Clinical Trial"
],
"title": "Efficacy and Safety of COVID-19 Convalescent Plasma in Hospitalized Patients",
"type": "journal-article",
"volume": "182"
}