COVID-19 Convalescent Plasma Therapy: Long Term Implications
et al., Open Forum Infectious Diseases, doi:10.1093/ofid/ofad686, CONTAIN-Extend, NCT04364737, Dec 2023
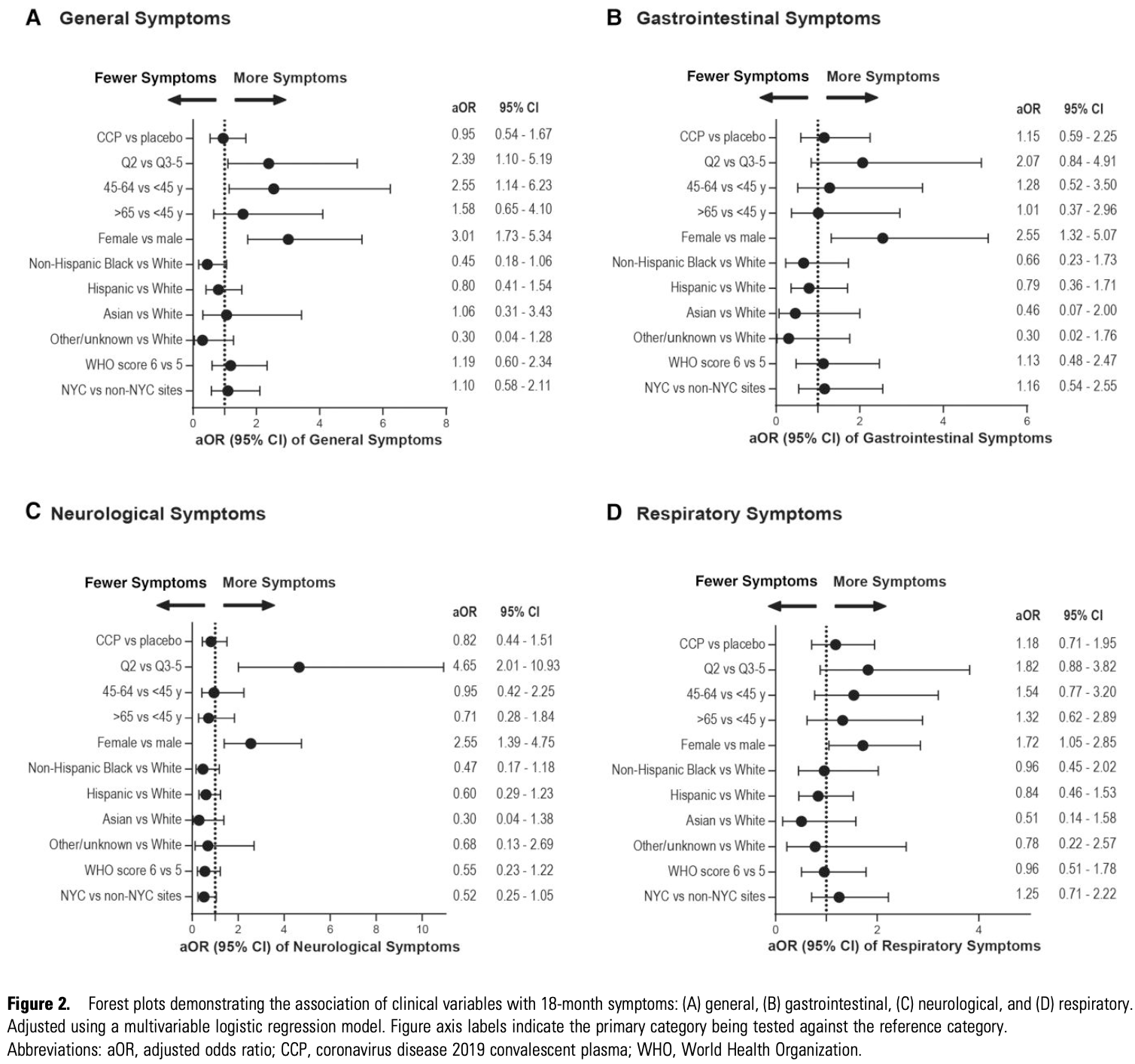
Long COVID (PASC) results for Ortigoza et al. showing no significant difference with convalescent plasma treatment.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments2.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of long COVID, 2.4% higher, RR 1.02, p = 0.88, treatment 141, control 140, all categories combined.
|
|
risk of long COVID, 5.0% lower, OR 0.95, p = 0.87, treatment 141, control 140, general, RR approximated with OR.
|
|
risk of long COVID, 15.0% higher, OR 1.15, p = 0.70, treatment 141, control 140, gastrointestinal, RR approximated with OR.
|
|
risk of long COVID, 18.0% lower, OR 0.82, p = 0.54, treatment 141, control 140, neurological, RR approximated with OR.
|
|
risk of long COVID, 18.0% higher, OR 1.18, p = 0.53, treatment 141, control 140, respiratory, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Yoon et al., 29 Dec 2023, Double Blind Randomized Controlled Trial, placebo-controlled, USA, peer-reviewed, 91 authors, average treatment delay 7.0 days, trial NCT04364737 (history) (CONTAIN-Extend).
COVID-19 Convalescent Plasma Therapy: Long Term Implications
Open Forum Infectious Diseases, doi:10.1093/ofid/ofad686
Background. The long-term effect of coronavirus disease 2019 (COVID-19) acute treatments on postacute sequelae of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (PASC) is unknown. The CONTAIN-Extend study explores the long-term impact of COVID-19 convalescent plasma (CCP) therapy on postacute sequelae of SARS-CoV-2 infection (PASC) symptoms and general health 18 months following hospitalization. Methods. The CONTAIN-Extend study examined 281 participants from the original CONTAIN COVID-19 trial (CONTAIN-RCT, NCT04364737) at 18 months post-hospitalization for acute COVID-19. Symptom surveys, global health assessments, and biospecimen collection were performed from November 2021 to October 2022. Multivariable logistic and linear regression estimated associations between the randomization arms and self-reported symptoms and Patient-Reported Outcomes Measurement Information System (PROMIS) scores and adjusted for covariables, including age, sex, race/ethnicity, disease severity, and CONTAIN enrollment quarter and sites. Results. There were no differences in symptoms or PROMIS scores between CCP and placebo (adjusted odds ratio [aOR] of general symptoms, 0.95; 95% CI, 0.54-1.67). However, females (aOR, 3.01; 95% CI, 1.73-5.34), those 45-64 years (aOR, 2.55; 95% CI, 1.14-6.23), and April-June 2020 enrollees (aOR, 2.39; 95% CI, 1.10-5.19) were more likely to report general symptoms and have poorer PROMIS physical health scores than their respective reference groups. Hispanic participants (difference, -3.05; 95% CI, -5.82 to -0.27) and Black participants (-4.48; 95% CI, -7.94 to -1.02) had poorer PROMIS physical health than White participants. Conclusions. CCP demonstrated no lasting effect on PASC symptoms or overall health in comparison to the placebo. This study underscores the significance of demographic factors, including sex, age, and timing of acute infection, in influencing symptom reporting 18 months after acute hypoxic COVID-19 hospitalization.
Supplementary Data Supplementary materials are available online. Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
References
Adjaye-Gbewonyo, Vahratian, Perrine, Bertolli, Long COVID in adults: United States, NCHS Data Brief, doi:10.15620/cdc:132417
Agarwal, Mukherjee, Kumar, Chatterjee, Bhatnagar et al., Convalescent plasma in the management of moderate COVID-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID trial), BMJ
Altmann, Whettlock, Liu, Arachchillage, Boyton, The immunology of long COVID, Nat Rev Immunol
Apple, Oddi, Peluso, Risk factors and abnormal cerebrospinal fluid associate with cognitive symptoms after mild COVID-19, Ann Clin Transl Neurol
Bai, Tomasoni, Falcinella, Female gender is associated with long COVID syndrome: a prospective cohort study, Clin Microbiol Infect
Boglione, Meli, Poletti, Risk factors and incidence of long-COVID syndrome in hospitalized patients: does remdesivir have a protective effect?, QJM
Bramante, Buse, Liebovitz, Outpatient treatment of COVID-19 and incidence of post-COVID-19 condition over 10 months (COVID-OUT): a multicentre, randomised, quadruple-blind, parallel-group, phase 3 trial, Lancet Infect Dis
Brannock, Chew, Preiss, Long COVID risk and pre-COVID vaccination in an EHR-based cohort study from the RECOVER program, Nat Commun
Casadevall, Pirofski, Misinterpretation of clinical research findings and COVID-19 mortality, Ann Intern Med
Cella, Riley, Stone, The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult selfreported health outcome item banks: 2005-2008, J Clin Epidemiol
Chemaitelly, Tang, Coyle, Protection against reinfection with the Omicron BA.2.75 subvariant, N Engl J Med
Davis, Mccorkell, Vogel, Topol, Long COVID: major findings, mechanisms and recommendations, Nat Rev Microbiol
Frontera, Simon, Bridging knowledge gaps in the diagnosis and management of neuropsychiatric sequelae of COVID-19, JAMA Psychiatry
Ganesh, Ghosh, Nyman, PROMIS scales for assessment of persistent post-COVID symptoms: a cross sectional study, J Prim Care Community Health
García-Abellán, Padilla, Fernández-González, Antibody response to SARS-CoV-2 is associated with long-term clinical outcome in patients with COVID-19: a longitudinal study, J Clin Immunol
Gebo, Heath, Fukuta, Early antibody treatment, inflammation, and risk of post-COVID conditions, mBio
Griffith, Morris, Tudball, Collider bias undermines our understanding of COVID-19 disease risk and severity, Nat Commun
Hays, Bjorner, Revicki, Spritzer, Cella, Development of physical and mental health summary scores from the Patient-Reported Outcomes Measurement Information System (PROMIS) global items, Qual Life Res
Healy, Liu, Winston-Khan, Weiner, Chitnis et al., Association between PROMIS10, SF-36 and NeuroQoL in persons with multiple sclerosis, Mult Scler Relat Disord
Herman, Wang, Burke, Nucleocapsid-specific antibody function is associated with therapeutic benefit from COVID-19 convalescent plasma therapy, Cell Rep Med
Jia, Cao, Lee, Anti-nucleocapsid antibody levels and pulmonary comorbid conditions are linked to post-COVID-19 syndrome, JCI Insight
Khullar, Zhang, Zang, Racial/ethnic disparities in post-acute sequelae of SARS-CoV-2 infection in New York: an EHR-based cohort study from the RECOVER program, J Gen Intern Med
Klein, Wood, Jaycox, Distinguishing features of long COVID identified through immune profiling, Nature
Korchut, Rejdak, Late neurological consequences of SARS-CoV-2 infection: new challenges for the neurologist, Front Neurosci
Korte, Buljan, Rösslein, SARS-CoV-2 IgG and IgA antibody response is gender dependent; and IgG antibodies rapidly decline early on, J Infect
Levin, Lustig, Cohen, Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months, N Engl J Med
Liu, Cella, Representativeness of the Patient-Reported Outcomes Measurement Information System internet panel, J Clin Epidemiol
Long, None
Nevalainen, Horstia, Laakkonen, Effect of remdesivir post hospitalization for COVID-19 infection from the randomized SOLIDARITY Finland trial, Nat Commun
Ortigoza, Yoon, Goldfeld, Efficacy and safety of COVID-19 convalescent plasma in hospitalized patients: a randomized clinical trial, JAMA Intern Med
Perumal, Shunmugam, Naidoo, Long COVID: a review and proposed visualization of the complexity of long COVID, Front Immunol
Phetsouphanh, Darley, Wilson, Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection, Nat Immunol
Proal, Vanelzakker, Long COVID or post-acute sequelae of COVID-19 (PASC): an overview of biological factors that may contribute to persistent symptoms, Front Microbiol
Richard, Pollett, Fries, Persistent COVID-19 symptoms at 6 months after onset and the role of vaccination before or after SARS-CoV-2 infection, JAMA Netw Open
Robertson, Shamsunder, Brazier, Racial/ethnic disparities in exposure, disease susceptibility, and clinical outcomes during COVID-19 pandemic in national cohort of adults, United States, Emerg Infect Dis
Rodríguez-Grande, Estévez, Palomino-Cabrera, Early SARS-CoV-2 reinfections involving the same or different genomic lineages, Spain, Emerg Infect Dis
Sigfrid, Drake, Pauley, Long COVID in adults discharged from UK hospitals after COVID-19: a prospective, multicentre cohort study using the ISARIC WHO clinical characterisation protocol, Lancet Reg Health Eur
Soriano, Murthy, Marshall, Relan, Diaz, A clinical case definition of post-COVID-19 condition by a Delphi consensus, Lancet Infect Dis
Sudre, Murray, Varsavsky, Attributes and predictors of long COVID, Nat Med
Swank, Senussi, Manickas-Hill, Persistent circulating severe acute respiratory syndrome coronavirus 2 spike is associated with post-acute coronavirus disease 2019 sequelae, Clin Infect Dis
Tabacof, Tosto-Mancuso, Wood, Post-acute COVID-19 syndrome negatively impacts physical function, cognitive function, health-related quality of life, and participation, Am J Phys Med Rehabil
Taquet, Sillett, Zhu, Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: an analysis of 2-year retrospective cohort studies including 1 284 437 patients, Lancet Psychiatry
Taquet, Skorniewska, Hampshire, Acute blood biomarker profiles predict cognitive deficits 6 and 12 months after COVID-19 hospitalization, Nat Med
Thaweethai, Jolley, Karlson, Development of a definition of postacute sequelae of SARS-CoV-2 infection, JAMA
Watanabe, Iwagami, Yasuhara, Takagi, Kuno, Protective effect of COVID-19 vaccination against long COVID syndrome: a systematic review and meta-analysis, Vaccine
Wisk, Gottlieb, Spatz, Association of initial SARS-CoV-2 test positivity with patient-reported well-being 3 months after a symptomatic illness, JAMA Netw Open
Xie, Choi, Al-Aly, Association of treatment with nirmatrelvir and the risk of post-COVID-19 condition, JAMA Intern Med
Yuan, Zhao, Zhang, COVID-19-related stigma and its sociodemographic correlates: a comparative study, Global Health
Zeng, Dai, Cai, A comparison study of SARS-CoV-2 IgG antibody between male and female COVID-19 patients: a possible reason underlying different outcome between sex, J Med Virol
DOI record:
{
"DOI": "10.1093/ofid/ofad686",
"ISSN": [
"2328-8957"
],
"URL": "http://dx.doi.org/10.1093/ofid/ofad686",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>The long-term effect of Coronavirus Disease 2019 (COVID-19) acute treatments on post-acute sequelae of SARS-CoV-2 infection (PASC) is unknown. The CONTAIN-Extend study explores the long-term impact of COVID-19 convalescent plasma (CCP) therapy on Post-Acute Sequelae of SARS-CoV-2 infection (PASC) symptoms and general health 18 months following hospitalization.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>The CONTAIN-Extend study examined 281 participants from the original CONTAIN COVID-19 trial (CONTAIN-RCT, NCT04364737) at 18 months post-hospitalization for acute COVID-19. Symptom surveys, global health assessments, and biospecimen collection was performed from November 2021 to October 2022. Multivariable logistic and linear regression estimated associations between the randomization arms and self-reported symptoms and PROMIS scores, adjusted for covariables, including age, sex, race/ethnicity, disease severity, and CONTAIN enrollment quarter and sites.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>There were no differences in symptoms or PROMIS scores between CCP and placebo (adjusted odds ratio of general symptoms, 0.95; 95% confidence intervals, 0.54, 1.67). However, females (3.01; 1.73, 5.34), those 45-64 years (2.55; 1.14, 6.23), and April-June 2020 enrollees (2.39; 1.10, 5.19) were more likely to report general symptoms and have poorer PROMIS physical health scores than their respective reference groups. Hispanic participants (difference, -3.05; 95% CI, -5.82, -0.27) and Black participants (-4.48; -7.94, -1.02) had poorer PROMIS physical health than White participants.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>CCP demonstrated no lasting effect on PASC symptoms or overall health in comparison to the placebo. This study underscores the significance of demographic factors, including sex, age, and the timing of acute infection, in influencing symptom reporting 18 months after acute hypoxic COVID-19 hospitalization.</jats:p>\n </jats:sec>",
"author": [
{
"affiliation": [
{
"name": "Division of Infectious Diseases, Department of Medicine, Albert Einstein College of Medicine, Montefiore Medical Center , Bronx, New York"
}
],
"family": "Yoon",
"given": "Hyunah",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Division of Biostatistics, Department of Population Health, NYU Grossman School of Medicine , New York, New York"
}
],
"family": "Li",
"given": "Yi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Biostatistics, Department of Population Health, NYU Grossman School of Medicine , New York, New York"
}
],
"family": "Goldfeld",
"given": "Keith S",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, NYU Grossman School of Medicine , New York, New York"
}
],
"family": "Cobb",
"given": "Gia F",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, NYU Grossman School of Medicine , New York, New York"
}
],
"family": "Sturm-Reganato",
"given": "Caroline L",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4784-7589",
"affiliation": [
{
"name": "Division of Infectious Diseases, Department of Internal Medicine, The University of Texas Health Science Center at Houston, McGovern Medical School , Houston"
}
],
"authenticated-orcid": false,
"family": "Ostrosky-Zeichner",
"given": "Luis",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Diseases, Department of Medicine, University of Miami Miller School of Medicine , Miami, Florida"
},
{
"name": "Miami Clinical and Translational Science Institute, University of Miami Miller School of Medicine Miami , Florida"
}
],
"family": "Jayaweera",
"given": "Dushyantha T",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, The University of Texas Health Science Center at Tyler , UTHealth East Texas, Tyler"
}
],
"family": "Philley",
"given": "Julie V",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Section of Infectious Diseases, Department of Internal Medicine, Yale University School of Medicine , New Haven, Connecticut"
}
],
"family": "Desruisseaux",
"given": "Mahalia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Diseases, Department of Medicine, Albert Einstein College of Medicine, Montefiore Medical Center , Bronx, New York"
},
{
"name": "Harold and Muriel Block Institute for Clinical and Translational Research, Albert Einstein College of Medicine and Montefiore Medical Center , Bronx, New York"
}
],
"family": "Keller",
"given": "Marla J",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "NYC Health and Hospitals Corporation Clinical and Translational Science Institute, NYU Grossman School of Medicine , New York, New York"
},
{
"name": "Leon H. Charney Division of Cardiology, Department of Medicine, NYU Grossman School of Medicine , New York, New York"
}
],
"family": "Hochman",
"given": "Judith S",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Diseases, Department of Medicine, Albert Einstein College of Medicine, Montefiore Medical Center , Bronx, New York"
},
{
"name": "Department of Microbiology and Immunology, Albert Einstein College of Medicine , Bronx, New York"
}
],
"family": "Pirofski",
"given": "Liise-anne",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9428-1469",
"affiliation": [
{
"name": "Division of Infectious Diseases, Department of Medicine, NYU Grossman School of Medicine , New York, New York"
},
{
"name": "Department of Microbiology, NYU Grossman School of Medicine , New York, New York"
}
],
"authenticated-orcid": false,
"family": "Ortigoza",
"given": "Mila B",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hochman",
"given": "Judith S",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cronstein",
"given": "Bruce N",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Keeling",
"given": "Deborah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rappoport",
"given": "Norka",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saraga",
"given": "Jenna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Holahan",
"given": "James",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ortigoza",
"given": "Mila B",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pirofski",
"given": "Liise-anne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yoon",
"given": "Hyunah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sturm-Reganato",
"given": "Caroline L",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cobb",
"given": "Gia F",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Andela",
"given": "Rakshit",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Darwish",
"given": "Yousef",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Taveras",
"given": "Monica R",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Xin",
"given": "Patrick S",
"sequence": "additional"
},
{
"affiliation": [],
"family": "LaFleur",
"given": "Jeff",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cleare",
"given": "Levi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Goldfeld",
"given": "Keith S",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Yi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ortigoza",
"given": "Mila B",
"sequence": "additional"
},
{
"affiliation": [],
"family": "O’Keeffe",
"given": "Mary L",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cobb",
"given": "Gia F",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sturm-Reganato",
"given": "Caroline L",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rahman",
"given": "Fatema Z",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ajayi",
"given": "Adeyinka O",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rodriguez",
"given": "Sara L",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Iturrate",
"given": "Eduardo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gallagher",
"given": "Jacqueline M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thomas",
"given": "Ololade E",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ramos",
"given": "Danibel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fong",
"given": "Charlotte C",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pirofski",
"given": "Liise-anne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yoon",
"given": "Hyunah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Keller",
"given": "Marla J",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Asencio",
"given": "Andrea A",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Eke",
"given": "Isaiah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Castro",
"given": "James",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shan",
"given": "Jidong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chalco",
"given": "Alex",
"sequence": "additional"
},
{
"affiliation": [],
"family": "LaFleur",
"given": "Jeff",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cleare",
"given": "Levi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Desruisseaux",
"given": "Mahalia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cortezzo",
"given": "Grace M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rocco",
"given": "Erica",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ndunge",
"given": "Oscar Bate Akide",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Parmelee",
"given": "Catherine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Solomon",
"given": "Gina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cahil",
"given": "Staci",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jayaweera",
"given": "Dushyantha T",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lee",
"given": "Chin Chin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ransford",
"given": "Daru L",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dasmany",
"given": "Deniz",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Corona",
"given": "Andres",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moreno",
"given": "Kenia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Martinez",
"given": "Gledys L",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Otero",
"given": "Christopher",
"sequence": "additional"
},
{
"affiliation": [],
"family": "McPherson",
"given": "David D",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ostrosky-Zeichner",
"given": "Luis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Patel",
"given": "Bela",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nigo",
"given": "Masayuki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Huebinger",
"given": "Ryan M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dronavalli",
"given": "Goutham",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Grimes",
"given": "Carolyn Z",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Umana",
"given": "Virginia E",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hernandez",
"given": "Maria D",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nielsen",
"given": "Laura E",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Stutz",
"given": "Taylor P",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mammadova",
"given": "Mehriban",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dentino",
"given": "Andrew N",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Heath",
"given": "Timothy R",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Martin",
"given": "Jessica G",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bello",
"given": "Fatimah O",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hinojosa",
"given": "Erik",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Philley",
"given": "Julie V",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Devine",
"given": "Megan S",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hibbard",
"given": "Rebekah L",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ford",
"given": "Anne M",
"sequence": "additional"
},
{
"affiliation": [],
"name": "for the CONTAIN-Extend Study Group",
"sequence": "additional"
}
],
"container-title": "Open Forum Infectious Diseases",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
12,
29
]
],
"date-time": "2023-12-29T07:23:25Z",
"timestamp": 1703834605000
},
"deposited": {
"date-parts": [
[
2023,
12,
29
]
],
"date-time": "2023-12-29T07:23:25Z",
"timestamp": 1703834605000
},
"indexed": {
"date-parts": [
[
2023,
12,
29
]
],
"date-time": "2023-12-29T12:41:54Z",
"timestamp": 1703853714617
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
12,
29
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "am",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
12,
29
]
],
"date-time": "2023-12-29T00:00:00Z",
"timestamp": 1703808000000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/ofid/advance-article-pdf/doi/10.1093/ofid/ofad686/54919495/ofad686.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/ofid/advance-article-pdf/doi/10.1093/ofid/ofad686/54919495/ofad686.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2023,
12,
29
]
]
},
"published-online": {
"date-parts": [
[
2023,
12,
29
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/ofid/advance-article/doi/10.1093/ofid/ofad686/7503511"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Oncology"
],
"subtitle": [],
"title": "COVID-19 Convalescent Plasma Therapy: Long Term Implications",
"type": "journal-article"
}