Abatacept, Cenicriviroc, or Infliximab for Treatment of Adults Hospitalized With COVID-19 Pneumonia
et al., JAMA, doi:10.1001/jama.2023.11043, ACTIV-1 IM, NCT04593940, Jul 2023
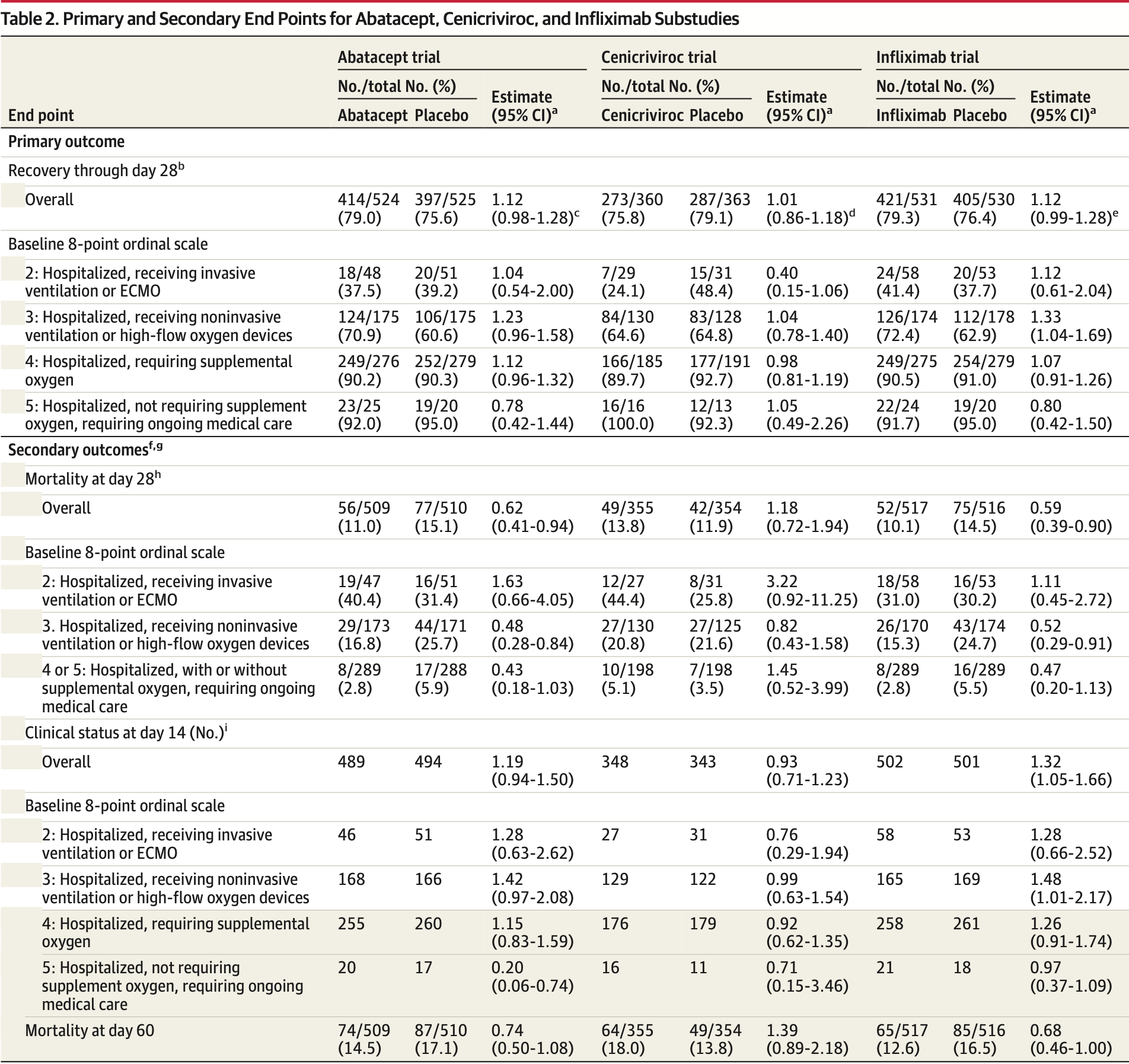
RCT 723 hospitalized COVID-19 patients showing no significant difference in outcomes with cenicriviroc treatment.
|
risk of death, 30.2% higher, RR 1.30, p = 0.15, treatment 64 of 355 (18.0%), control 49 of 354 (13.8%), day 60.
|
|
risk of death, 16.3% higher, RR 1.16, p = 0.50, treatment 49 of 355 (13.8%), control 42 of 354 (11.9%), day 28.
|
|
risk of no improvement, 7.5% higher, OR 1.08, p = 0.62, treatment 348, control 343, inverted to make OR<1 favor treatment, clinical status, day 14, RR approximated with OR.
|
|
risk of no recovery, 1.0% lower, RR 0.99, p = 0.91, treatment 360, control 363, inverted to make RR<1 favor treatment, day 28.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
O’Halloran et al., 25 Jul 2023, Double Blind Randomized Controlled Trial, placebo-controlled, multiple countries, peer-reviewed, mean age 54.8, 51 authors, study period 16 October, 2020 - 31 December, 2021, average treatment delay 9.8 days, trial NCT04593940 (history) (ACTIV-1 IM).
Contact: jrutter@nih.gov, cschmitt@niaid.nih.gov, mkurilla@niaid.nih.gov.
Abatacept, Cenicriviroc, or Infliximab for Treatment of Adults Hospitalized With COVID-19 Pneumonia
JAMA, doi:10.1001/jama.2023.11043
IMPORTANCE Immune dysregulation contributes to poorer outcomes in COVID-19. OBJECTIVE To investigate whether abatacept, cenicriviroc, or infliximab provides benefit when added to standard care for COVID-19 pneumonia. DESIGN, SETTING, AND PARTICIPANTS Randomized, double-masked, placebo-controlled clinical trial using a master protocol to investigate immunomodulators added to standard care for treatment of participants hospitalized with COVID-19 pneumonia. The results of 3 substudies are reported from 95 hospitals at 85 clinical research sites in the US and Latin America. Hospitalized patients 18 years or older with confirmed SARS-CoV-2 infection within 14 days and evidence of pulmonary involvement underwent randomization between October 2020 and December 2021. INTERVENTIONS Single infusion of abatacept (10 mg/kg; maximum dose, 1000 mg) or infliximab (5 mg/kg) or a 28-day oral course of cenicriviroc (300-mg loading dose followed by 150 mg twice per day).
MAIN OUTCOMES AND MEASURES The primary outcome was time to recovery by day 28 evaluated using an 8-point ordinal scale (higher scores indicate better health). Recovery was defined as the first day the participant scored at least 6 on the ordinal scale.
RESULTS Of the 1971 participants randomized across the 3 substudies, the mean (SD) age was 54.8 (14.6) years and 1218 (61.8%) were men. The primary end point of time to recovery from COVID-19 pneumonia was not significantly different for abatacept (recovery rate ratio [RRR], 1.12 [95% CI, 0.98-1.28]; P = .09), cenicriviroc (RRR, 1.01 [95% CI, 0.86-1.18]; P = .94), or infliximab (RRR, 1.12 [95% CI, 0.99-1.28]; P = .08) compared with placebo. All-cause 28-day mortality was 11.0% for abatacept vs 15.1% for placebo (odds ratio [OR], 0.62 [95% CI, 0.41-0.94]), 13.8% for cenicriviroc vs 11.9% for placebo (OR, 1.18 [95% CI 0.72-1.94]), and 10.1% for infliximab vs 14.5% for placebo (OR, 0.59 [95% CI, 0.39-0.90]). Safety outcomes were comparable between active treatment and placebo, including secondary infections, in all 3 substudies. CONCLUSIONS AND RELEVANCE Time to recovery from COVID-19 pneumonia among hospitalized participants was not significantly different for abatacept, cenicriviroc, or infliximab vs placebo.
Role of the Funder/Sponsor: Janssen provided infliximab, Bristol Myers Squibb provided abatacept, and AbbVie provided cenicriviroc for use in this trial but did not provide any financial support. Gilead Sciences provided remdesivir for use in this trial but did not provide any financial support. Employees of Janssen and Gilead Sciences participated in discussions about protocol development and in weekly protocol team calls. The final trial protocol was developed by the protocol chair, Dr William Powderly, the IND sponsor Dr Daniel K. Benjamin Jr, and a protocol development committee including representatives from the National Center for Advancing Translational Sciences (NCATS). NCATS had a collaborative role in the trial design, management, interpretation of the data and the preparation of the manuscript along with the protocol chair, the study statisticians and the study writing group.
Group Information: The ACTIV-1 IM Study Group Members are provided in Supplement 3. William Checkley, MD, PhD (Johns Hopkins University, Baltimore, MD); and Beatriz Grinsztejn, MD (Instituto Nacional de Infectologia Evandro Chagas-Fiocruz, Rio de Janeiro, Brazil), for their oversight; the community advisory board, Larisa Caicedo, Ashish Cowlagi, Anna Davis, Lincoln Larmond, Doug Lindsey, Bob Pearson, and the participants and their families for their altruism in participating in this trial; Elizabeth E.S. Cook for editorial support; and Christopher J. Lindsell for contributions to the..
References
Beigel, Tomashek, Dodd, ACTT-1 Study Group Members. Remdesivir for the treatment of Covid-19: final report, N Engl J Med, doi:10.1056/NEJMoa2007764
Douin, Siegel, Grandits, Evaluating primary endpoints for COVID-19 therapeutic trials to assess recovery, Am J Respir Crit Care Med, doi:10.1164/rccm.202112-2836OC
Ely, Ramanan, Kartman, Efficacy and safety of baricitinib plus standard of care for the treatment of critically ill hospitalised adults with COVID-19 on invasive mechanical ventilation or extracorporeal membrane oxygenation: an exploratory, randomised, placebo-controlled trial, Lancet Respir Med, doi:10.1016/S2213-2600(22)00006-6
Files, Tacke, Sullivan, Dorr, Ferguson et al., Rationale of using the dual chemokine receptor CCR2/CCR5 inhibitor cenicriviroc for the treatment of COVID-19, PLoS Pathog, doi:10.1371/journal.ppat.1010547
Fine, Gray, A proportional hazards model for the subdistribution of a competing risk, J Am Stat Assoc, doi:10.1080/01621459.1999.10474144
Gillings, Koch, The application of the principle of intention-to-treat to the analysis of clinical trials, Drug Inf J, doi:10.1177/009286159102500311
Gordon, Mouncey, Al-Beidh, Interleukin-6 receptor antagonists in critically ill patients with Covid-19
Horby, Lim, Emberson, Dexamethasone in hospitalized patients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2021436
Kalfaoglu, Almeida-Santos, Tye, Satou, Ono, T-cell hyperactivation and paralysis in severe COVID-19 infection revealed by single-cell analysis, Front Immunol, doi:10.3389/fimmu.2020.589380
Kalil, Patterson, Mehta, ACTT-2 Study Group Members. Baricitinib plus remdesivir for hospitalized adults with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2031994
Lan, Demets, Discrete sequential boundaries for clinical trials, Biometrika, doi:10.2307/2336502
Lavange, Adam, Currier, Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV): designing master protocols for evaluation of candidate COVID-19 therapeutics, Ann Intern Med, doi:10.7326/M21-1269
Marconi, Ramanan, De Bono, Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial, Lancet Respir Med, doi:10.1016/S2213-2600(21)00331-3
O'brien, Fleming, A multiple testing procedure for clinical trials, Biometrics, doi:10.2307/2530245
Otsuka, Seino, Macrophage activation syndrome and COVID-19, Inflamm Regen, doi:10.1186/s41232-020-00131-w
Pan, Peto, Henao-Restrepo, Repurposed antiviral drugs for Covid-19: interim WHO solidarity trial results, N Engl J Med, doi:10.1056/NEJMoa2023184
Rüggeberg, Gold, Bayas, Anaphylaxis: case definition and guidelines for data collection, analysis, and presentation of immunization safety data, Vaccine, doi:10.1016/j.vaccine.2007.02.064
Soto, Lim, Evaluating the therapeutic potential of cenicriviroc in the treatment of nonalcoholic steatohepatitis with fibrosis: a brief report on emerging data, Hepat Med, doi:10.2147/HMER.S230613
Tacke, Cenicriviroc for the treatment of non-alcoholic steatohepatitis and liver fibrosis, Expert Opin Investig Drugs, doi:10.1080/13543784.2018.1442436
DOI record:
{
"DOI": "10.1001/jama.2023.11043",
"ISSN": [
"0098-7484"
],
"URL": "http://dx.doi.org/10.1001/jama.2023.11043",
"abstract": "<jats:sec><jats:title>Importance</jats:title><jats:p>Immune dysregulation contributes to poorer outcomes in COVID-19.</jats:p></jats:sec><jats:sec><jats:title>Objective</jats:title><jats:p>To investigate whether abatacept, cenicriviroc, or infliximab provides benefit when added to standard care for COVID-19 pneumonia.</jats:p></jats:sec><jats:sec><jats:title>Design, Setting, and Participants</jats:title><jats:p>Randomized, double-masked, placebo-controlled clinical trial using a master protocol to investigate immunomodulators added to standard care for treatment of participants hospitalized with COVID-19 pneumonia. The results of 3 substudies are reported from 95 hospitals at 85 clinical research sites in the US and Latin America. Hospitalized patients 18 years or older with confirmed SARS-CoV-2 infection within 14 days and evidence of pulmonary involvement underwent randomization between October 2020 and December 2021.</jats:p></jats:sec><jats:sec><jats:title>Interventions</jats:title><jats:p>Single infusion of abatacept (10 mg/kg; maximum dose, 1000 mg) or infliximab (5 mg/kg) or a 28-day oral course of cenicriviroc (300-mg loading dose followed by 150 mg twice per day).</jats:p></jats:sec><jats:sec><jats:title>Main Outcomes and Measures</jats:title><jats:p>The primary outcome was time to recovery by day 28 evaluated using an 8-point ordinal scale (higher scores indicate better health). Recovery was defined as the first day the participant scored at least 6 on the ordinal scale.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>Of the 1971 participants randomized across the 3 substudies, the mean (SD) age was 54.8 (14.6) years and 1218 (61.8%) were men. The primary end point of time to recovery from COVID-19 pneumonia was not significantly different for abatacept (recovery rate ratio [RRR], 1.12 [95% CI, 0.98-1.28]; <jats:italic>P</jats:italic> = .09), cenicriviroc (RRR, 1.01 [95% CI, 0.86-1.18]; <jats:italic>P</jats:italic> = .94), or infliximab (RRR, 1.12 [95% CI, 0.99-1.28]; <jats:italic>P</jats:italic> = .08) compared with placebo. All-cause 28-day mortality was 11.0% for abatacept vs 15.1% for placebo (odds ratio [OR], 0.62 [95% CI, 0.41-0.94]), 13.8% for cenicriviroc vs 11.9% for placebo (OR, 1.18 [95% CI 0.72-1.94]), and 10.1% for infliximab vs 14.5% for placebo (OR, 0.59 [95% CI, 0.39-0.90]). Safety outcomes were comparable between active treatment and placebo, including secondary infections, in all 3 substudies.</jats:p></jats:sec><jats:sec><jats:title>Conclusions and Relevance</jats:title><jats:p>Time to recovery from COVID-19 pneumonia among hospitalized participants was not significantly different for abatacept, cenicriviroc, or infliximab vs placebo.</jats:p></jats:sec><jats:sec><jats:title>Trial Registration</jats:title><jats:p>ClinicalTrials.gov Identifier: <jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"uri\" xlink:href=\"https://classic.clinicaltrials.gov/ct2/show/NCT04593940\">NCT04593940</jats:ext-link></jats:p></jats:sec>",
"author": [
{
"affiliation": [
{
"name": "Washington University St Louis, St Louis, Missouri"
}
],
"family": "O’Halloran",
"given": "Jane A.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Duke University Health System, Durham, North Carolina"
}
],
"family": "Ko",
"given": "Emily R.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of North Carolina, Chapel Hill"
}
],
"family": "Anstrom",
"given": "Kevin J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "St Lawrence Health, Potsdam, New York"
}
],
"family": "Kedar",
"given": "Eyal",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Weill Cornell Medicine, New York, New York"
}
],
"family": "McCarthy",
"given": "Matthew W.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Robert Wood Johnson Medical School, New Brunswick, New Jersey"
}
],
"family": "Panettieri",
"given": "Reynold A.",
"sequence": "additional",
"suffix": "Jr"
},
{
"affiliation": [
{
"name": "Sanatorio Diagnostico, Santa Fe, Argentina"
}
],
"family": "Maillo",
"given": "Martin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Hospital Nacional Hipolito Unanue, Lima, Peru"
}
],
"family": "Nunez",
"given": "Patricia Segura",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of North Carolina, Chapel Hill"
}
],
"family": "Lachiewicz",
"given": "Anne M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "National Center for Advancing Translational Sciences, Bethesda, Maryland"
}
],
"family": "Gonzalez",
"given": "Cynthia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Duke Clinical Research Institute, Duke University School of Medicine, Durham, North Carolina"
}
],
"family": "Smith",
"given": "P. Brian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Hospital Central de la Fuerza Aerea del Peru, Lima, Peru"
}
],
"family": "de Tai",
"given": "Sabina Mendivil-Tuchia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Oregon Health and Science University, Portland"
}
],
"family": "Khan",
"given": "Akram",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Illinois at Chicago"
}
],
"family": "Lora",
"given": "Alfredo J. Mena",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Kansas Medical Center, Kansas City"
}
],
"family": "Salathe",
"given": "Matthias",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Trinitas Hospital, Elizabeth, New Jersey"
}
],
"family": "Capo",
"given": "Gerardo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Nuevo Hospital Civil de Guadalajara Juan I. Menchaca, Guadalajara, Mexico"
}
],
"family": "Gonzalez",
"given": "Daniel Rodríguez",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Texas Health Science Center at San Antonio"
}
],
"family": "Patterson",
"given": "Thomas F.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Rochester School of Medicine and Dentistry, Rochester, New York"
}
],
"family": "Palma",
"given": "Christopher",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinica Central SA, Villa Regina, Argentina"
}
],
"family": "Ariza",
"given": "Horacio",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Hospital e Maternidade Celso Pierro—PUC Campinas, Campinas, Brazil"
}
],
"family": "Lima",
"given": "Maria Patelli",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "MidMichigan Medical Center, Midland"
}
],
"family": "Blamoun",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Sanatorio Britanico, Santa Fe, Argentina"
}
],
"family": "Nannini",
"given": "Esteban C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Hospital de Clinicas de Porto Alegre HCPA, Porto Alegre, Brazil"
}
],
"family": "Sprinz",
"given": "Eduardo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Instituto Medico Platense, La Plata, Argentina"
}
],
"family": "Mykietiuk",
"given": "Analia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Providence Medical Research Center, Spokane, Washington"
}
],
"family": "Alicic",
"given": "Radica",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Washington University St Louis, St Louis, Missouri"
}
],
"family": "Rauseo",
"given": "Adriana M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Duke University Health System, Durham, North Carolina"
}
],
"family": "Wolfe",
"given": "Cameron R.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Weill Cornell Medicine, New York, New York"
}
],
"family": "Witting",
"given": "Britta",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of Massachusetts Medical Center, Worcester"
}
],
"family": "Wang",
"given": "Jennifer P.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Washington University St Louis, St Louis, Missouri"
}
],
"family": "Parra-Rodriguez",
"given": "Luis",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Duke University Health System, Durham, North Carolina"
}
],
"family": "Der",
"given": "Tatyana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Weill Cornell Medicine, New York, New York"
}
],
"family": "Willsey",
"given": "Kate",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Duke Clinical Research Institute, Duke University School of Medicine, Durham, North Carolina"
}
],
"family": "Wen",
"given": "Jun",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Duke Clinical Research Institute, Duke University School of Medicine, Durham, North Carolina"
}
],
"family": "Silverstein",
"given": "Adam",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Duke Clinical Research Institute, Duke University School of Medicine, Durham, North Carolina"
}
],
"family": "O’Brien",
"given": "Sean M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Duke Clinical Research Institute, Duke University School of Medicine, Durham, North Carolina"
}
],
"family": "Al-Khalidi",
"given": "Hussein R.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Bristol Myers Squibb, Philadelphia, Pennsylvania"
}
],
"family": "Maldonado",
"given": "Michael A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Janssen Pharmaceuticals, Leiden, the Netherlands"
}
],
"family": "Melsheimer",
"given": "Richard",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "AbbVie, Inc, North Chicago, Illinois"
}
],
"family": "Ferguson",
"given": "William G.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Duke Clinical Research Institute, Duke University School of Medicine, Durham, North Carolina"
}
],
"family": "McNulty",
"given": "Steven E.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Duke Clinical Research Institute, Duke University School of Medicine, Durham, North Carolina"
}
],
"family": "Zakroysky",
"given": "Pearl",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Duke Clinical Research Institute, Duke University School of Medicine, Durham, North Carolina"
}
],
"family": "Halabi",
"given": "Susan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Duke Clinical Research Institute, Duke University School of Medicine, Durham, North Carolina"
}
],
"family": "Benjamin",
"given": "Daniel K.",
"sequence": "additional",
"suffix": "Jr"
},
{
"affiliation": [
{
"name": "Technical Resources International (TRI), Bethesda, Maryland"
}
],
"family": "Butler",
"given": "Sandra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "National Center for Advancing Translational Sciences, Bethesda, Maryland"
}
],
"family": "Atkinson",
"given": "Jane C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Foundation for the National Institutes of Health, Bethesda, Maryland"
}
],
"family": "Adam",
"given": "Stacey J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "National Center for Advancing Translational Sciences, Bethesda, Maryland"
}
],
"family": "Chang",
"given": "Soju",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "University of North Carolina, Chapel Hill"
}
],
"family": "LaVange",
"given": "Lisa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "National Institute of Allergy and Infectious Diseases, Bethesda, Maryland"
}
],
"family": "Proschan",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "National Center for Advancing Translational Sciences, Bethesda, Maryland"
}
],
"family": "Bozzette",
"given": "Samuel A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Washington University St Louis, St Louis, Missouri"
}
],
"family": "Powderly",
"given": "William G.",
"sequence": "additional"
},
{
"affiliation": [],
"name": "ACTIV-1 IM Study Group Members",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Patel",
"given": "Mahendra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Sanyal",
"given": "Arun",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Green",
"given": "Jason",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Wu",
"given": "Huimin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Linas",
"given": "Benjamin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Grant",
"given": "Philip",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Iyer",
"given": "Vivek",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Yang",
"given": "Otto",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Balani",
"given": "Bindu",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Parnia",
"given": "Sam",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Dare",
"given": "Ryan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Morse",
"given": "Caryn G.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Harris",
"given": "Estelle S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Wortmann",
"given": "Glenn",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Hill",
"given": "Nicholas",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Patel",
"given": "Shama",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Garcia-Diaz",
"given": "Julia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Thapamager",
"given": "Suman",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Devine",
"given": "Megan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Bojanowski",
"given": "Christine M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Meisenberg",
"given": "Barry",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Marshall",
"given": "Gailen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Dandachi",
"given": "Dima",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Sabin",
"given": "Arick",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Breemo",
"given": "Anthony",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Sinha",
"given": "Suman",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Goss",
"given": "Christopher",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Reece",
"given": "Rebecca",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Aouad",
"given": "Arlette",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Glassman",
"given": "Seth",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Morris",
"given": "Peter",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Patel",
"given": "Bela",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Bello",
"given": "Fatimah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Cardozo Fernandes",
"given": "Juliana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Carbajal",
"given": "Oscar",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Ravera",
"given": "Lorena",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Castro",
"given": "Mozar",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Villegas-Chiroque",
"given": "Miguel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Oscar Riera",
"given": "Fernando",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Camacho",
"given": "Adrian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Stadnik",
"given": "Claudio",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Gave",
"given": "Jorge",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Biondi",
"given": "Rodrigo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Gamarra Velarde",
"given": "Ronal",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Cerbino Neto",
"given": "Jose",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Ditondo",
"given": "Juan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Losso",
"given": "Marcelo H.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Dolz",
"given": "Mariano",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "O’Sullivan",
"given": "Alexandra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Gavin",
"given": "Brian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Beumont-Mauviel",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Ca",
"given": "Huyen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Beci",
"given": "Rose",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Molina",
"given": "Daniel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Rao",
"given": "Sandhya",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Stock",
"given": "Thomas",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Erhardt",
"given": "William",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Read",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Springer",
"given": "Jessica",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Presti",
"given": "Rachel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Thompson",
"given": "Ryley",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Gray",
"given": "Kimberly",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Henry",
"given": "Cathy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Haile",
"given": "Alem",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Klebert",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Kessels",
"given": "Lisa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Vehe",
"given": "Kathryn",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Bakos",
"given": "Kristopher",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Spitz",
"given": "Teresa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Hubert",
"given": "Sara",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Alyatim",
"given": "Raghd",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Schneider",
"given": "Brittany",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Ayres",
"given": "Chapelle",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Spec",
"given": "Andrej",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Blair",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Afghanzada",
"given": "Anita",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Schodl",
"given": "Natalie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Wahid",
"given": "Lana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Engemann",
"given": "John J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Pinero",
"given": "Gloria",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "McLendon-Arvik",
"given": "Beth",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Whitt",
"given": "Lynn",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Shroba",
"given": "Jenny",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Salsgiver",
"given": "Elizabeth",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Alleyne",
"given": "Candace",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Gwak",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Pickell",
"given": "Nicholas",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Spagnoletti",
"given": "Jack",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Goh",
"given": "Samson",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Robb",
"given": "Katharine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Cenname",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Small",
"given": "Catherine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Plate",
"given": "Markus",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Burgos",
"given": "Rodrigo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Lindsey",
"given": "Brenna",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Herald",
"given": "Fischer",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Echeverria",
"given": "Stephanie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Lewis",
"given": "Dorendra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Patel",
"given": "Mahesh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Bengtson",
"given": "Charles D.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Schmid",
"given": "Andreas",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Lovell",
"given": "Kimberly",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Lovelett",
"given": "Carly",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Soule",
"given": "Daniel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Jaremczuk",
"given": "Daniel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Flanagan",
"given": "Jennie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Murray",
"given": "Cameron",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Sands",
"given": "Kylie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Flint",
"given": "Kyle",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Mohaddes",
"given": "Sara",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Harrington",
"given": "Caryn",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Broughal",
"given": "Kylie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Sogoian",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Cox",
"given": "Karen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Javeri",
"given": "Heta",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Ponce",
"given": "Philip O.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Dixon",
"given": "Danielle O.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Bowling",
"given": "Jason E.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Patterson",
"given": "Jan E.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Taylor",
"given": "Barbara S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Serrano",
"given": "Ruth C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Sallee",
"given": "Kaylin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Tragus",
"given": "Robin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Catano",
"given": "Gabriel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Scholler",
"given": "Irma",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Barajas",
"given": "Rose Ann",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Garcia",
"given": "Armando",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Soileau",
"given": "Bridgette",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Heard",
"given": "Patricia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Camilo Endo Carvajal",
"given": "Manuel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Ehwarieme",
"given": "Rukevwe",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Chandramohan",
"given": "Divya",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Cabo",
"given": "Alejandro",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Nawwar",
"given": "Abdelhameed",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Quill",
"given": "Caroline M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Choudhury",
"given": "Nayeem",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Arrington",
"given": "Ashley",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Holyfield",
"given": "Isaiah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Smith",
"given": "Abby",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Brown",
"given": "Glenda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Varner",
"given": "Kyle",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Baxter",
"given": "Joni",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Roundy",
"given": "Tracy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Co",
"given": "Mary",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Wessolossky",
"given": "Mireya",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Perez-Velazquez",
"given": "Juan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Holter-Chakrabarty",
"given": "Jennifer",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Karfonta",
"given": "Brittany",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Anjum",
"given": "Juvaria",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Marathe",
"given": "Jai",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Castagne",
"given": "Myriam",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Mompoint",
"given": "Daniel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Schroeder",
"given": "Ryan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Rao",
"given": "Mallika",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Nguyen",
"given": "Johnathan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Plewa",
"given": "Jake",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Donlinger",
"given": "Sue",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Breslin",
"given": "Marylynn",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Dodson",
"given": "Susan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Jenkins",
"given": "Mitch",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Williamson",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Middleton",
"given": "Elizabeth A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Tavadze",
"given": "Mai",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Sebhatu",
"given": "Romai",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Pierobon",
"given": "Jessica",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Miller",
"given": "Nate",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Lee",
"given": "John",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Doshi",
"given": "Pratik",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Dentino",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Martin",
"given": "Jessica",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Hinojosa",
"given": "Erik",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Torres",
"given": "Pablo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Sanchez",
"given": "Ricardo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Murga",
"given": "Gladys",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "de la Gala",
"given": "Silvana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Chaiña",
"given": "Jhon",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Ramos",
"given": "Jorge",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Malca",
"given": "Jenny",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Castillo",
"given": "Kathia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Calderon Galvez",
"given": "Johana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Icochea Perez",
"given": "Maria Lyda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Becerra Nunez",
"given": "Claudia Carolina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Betteta Riondato",
"given": "Sandra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Delgado Málaga",
"given": "Sandra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Barreda Sánchez",
"given": "Cecilia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Sánchez Morales",
"given": "Sylvia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Yaringano Palacios",
"given": "Myriam",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Galarza Cuba",
"given": "Dora",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Hermenegildo",
"given": "Ivan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Falla Benites",
"given": "Mayra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Neyra",
"given": "Stefania",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Hernández",
"given": "Josefina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "García",
"given": "Victoria",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Palacios",
"given": "Katherine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Matos",
"given": "Miluska",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Zuloeta",
"given": "Fiorella",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "del Carpio",
"given": "Fiorella",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Chacaltana",
"given": "Gloria",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "de la Cruz",
"given": "Carmen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Ceriolli Breda",
"given": "Felipe",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Mello Roux Leite",
"given": "Mauricio",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Milbradt",
"given": "Tobias",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Rodeles",
"given": "Luz",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Benzaquen",
"given": "Nadia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Pezzini",
"given": "Sebastian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Alberdi",
"given": "Lucila",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Serravalle",
"given": "Priscila",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Russo",
"given": "Giulia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Ferini",
"given": "Franco",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Guala",
"given": "Maria Eugenia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Crespo",
"given": "Alejandro",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Benitez",
"given": "Agostina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Cristaldi",
"given": "Maria Elena",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Di Renzo",
"given": "Paula",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Gramagalia",
"given": "Corina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Tessini",
"given": "Antonela",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Alonso",
"given": "Joana Evelin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Pic",
"given": "Carmen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Ceraldi",
"given": "Georgina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Mondino",
"given": "Azucena",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Higareda Almaraz",
"given": "Iliana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Madrigal Robles",
"given": "Víctor Hugo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Rosas Ismerio",
"given": "María Fernanda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Rodarte Rodriguez",
"given": "Maria Fernanda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Olmos Meza",
"given": "Norma Esther",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "de la Cruz Barba",
"given": "Norma Esther",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Alba Ponce",
"given": "Ana Maria",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Calderon",
"given": "Juan Manuel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Borsetta",
"given": "Eduardo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Sandoval",
"given": "Noemí",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Vazquez",
"given": "Daniela",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Mansilla",
"given": "Malena",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Molina",
"given": "Marta",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Jara",
"given": "Yamila",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "De Bona",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Eduarda Claus",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Pille",
"given": "Arthur",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Lahitte",
"given": "Matías",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Fenés",
"given": "Mariángeles",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Bianchi",
"given": "Cecilia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Miserere",
"given": "María Emilia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Alzogaray",
"given": "Maria Fernanda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Christian Sanchez Carrillo",
"given": "Halbert",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Mano",
"given": "Aldana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Zuain",
"given": "Myrna",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Toibaro",
"given": "Javier J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Pachioli",
"given": "Valeria",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Chaio",
"given": "Sebastián",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Malamud",
"given": "Natalia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Bharucha",
"given": "David B.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Dorr",
"given": "Patrick",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Sadeh",
"given": "Jonathan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Kelly",
"given": "Sheila",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Stevens",
"given": "Marita",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Cao",
"given": "Huyen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "DeZure",
"given": "Adam",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Juneja",
"given": "Kavita",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Abdelghany",
"given": "Mazin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Jasion",
"given": "Theresa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Olson",
"given": "Rachel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Roebuck",
"given": "Megan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Huvane",
"given": "Jacqueline",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Lindsell",
"given": "Christopher J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Leimberger",
"given": "Jeff",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Yow",
"given": "Eric",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Huang",
"given": "Zhen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Kim",
"given": "Hwasoon",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Anderson",
"given": "Carla",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Elliott",
"given": "Carrie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Swartz",
"given": "Merri",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Garg",
"given": "Jyotsna",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Nelson",
"given": "Neta",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Kalaria",
"given": "Divya",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Philogene",
"given": "Ketty",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Schulz",
"given": "Tim",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Kuek",
"given": "Averie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Bah",
"given": "Fatou",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Mitchell",
"given": "Jarrard",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Polo",
"given": "Elizabeth",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Wong",
"given": "Michelle",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Baldan",
"given": "Sharon",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Mendez",
"given": "Sandra",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Stevens",
"given": "Bradford",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Toledo",
"given": "Marcela",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Abba",
"given": "Talita",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Herrejon",
"given": "Emma",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Gomez",
"given": "Cristina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Mardari",
"given": "Georgeta",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Putta",
"given": "Neeraja",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Mason",
"given": "Robin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Hamilton",
"given": "Holli",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Eisnor",
"given": "Derek",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "O’Rourke",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Patel",
"given": "Aditi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Brody",
"given": "Betty",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Chiang",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Lind",
"given": "Brian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Portilla",
"given": "Lilli M.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Gadhia",
"given": "Ami D.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Vepa",
"given": "Sury",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Carlson Marti",
"given": "Emily",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Gardner",
"given": "Bobbi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Rutter",
"given": "Joni",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Schmitt",
"given": "Clare",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "for the ACTIV-1 IM Study Group Members"
}
],
"family": "Kurilla",
"given": "Michael",
"sequence": "additional"
}
],
"container-title": "JAMA",
"container-title-short": "JAMA",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
7,
10
]
],
"date-time": "2023-07-10T15:01:44Z",
"timestamp": 1689001304000
},
"deposited": {
"date-parts": [
[
2023,
7,
25
]
],
"date-time": "2023-07-25T15:54:18Z",
"timestamp": 1690300458000
},
"indexed": {
"date-parts": [
[
2024,
9,
23
]
],
"date-time": "2024-09-23T04:31:36Z",
"timestamp": 1727065896082
},
"is-referenced-by-count": 34,
"issue": "4",
"issued": {
"date-parts": [
[
2023,
7,
25
]
]
},
"journal-issue": {
"issue": "4",
"published-print": {
"date-parts": [
[
2023,
7,
25
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://jamanetwork.com/journals/jama/articlepdf/2807333/jama_ohalloran_2023_oi_230073_1689353562.4173.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "10",
"original-title": [],
"page": "328",
"prefix": "10.1001",
"published": {
"date-parts": [
[
2023,
7,
25
]
]
},
"published-print": {
"date-parts": [
[
2023,
7,
25
]
]
},
"publisher": "American Medical Association (AMA)",
"reference": [
{
"DOI": "10.3389/fimmu.2020.589380",
"article-title": "T-cell hyperactivation and paralysis in severe COVID-19 infection revealed by single-cell analysis.",
"author": "Kalfaoglu",
"doi-asserted-by": "publisher",
"journal-title": "Front Immunol",
"key": "joi230073r1",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1186/s41232-020-00131-w",
"article-title": "Macrophage activation syndrome and COVID-19.",
"author": "Otsuka",
"doi-asserted-by": "publisher",
"first-page": "19",
"journal-title": "Inflamm Regen",
"key": "joi230073r2",
"volume": "40",
"year": "2020"
},
{
"DOI": "10.1371/journal.ppat.1010547",
"article-title": "Rationale of using the dual chemokine receptor CCR2/CCR5 inhibitor cenicriviroc for the treatment of COVID-19.",
"author": "Files",
"doi-asserted-by": "publisher",
"issue": "6",
"journal-title": "PLoS Pathog",
"key": "joi230073r3",
"volume": "18",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2021436",
"article-title": "Dexamethasone in hospitalized patients with Covid-19.",
"author": "Horby",
"doi-asserted-by": "publisher",
"first-page": "693",
"issue": "8",
"journal-title": "N Engl J Med",
"key": "joi230073r4",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(21)00676-0",
"article-title": "Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial.",
"author": "RECOVERY Collaborative Group",
"doi-asserted-by": "publisher",
"first-page": "1637",
"issue": "10285",
"journal-title": "Lancet",
"key": "joi230073r5",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2100433",
"article-title": "Interleukin-6 receptor antagonists in critically ill patients with Covid-19.",
"author": "Gordon",
"doi-asserted-by": "publisher",
"first-page": "1491",
"issue": "16",
"journal-title": "N Engl J Med",
"key": "joi230073r6",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the treatment of Covid-19: final report.",
"author": "Beigel",
"doi-asserted-by": "publisher",
"first-page": "1813",
"issue": "19",
"journal-title": "N Engl J Med",
"key": "joi230073r7",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1016/S2213-2600(22)00006-6",
"article-title": "Efficacy and safety of baricitinib plus standard of care for the treatment of critically ill hospitalised adults with COVID-19 on invasive mechanical ventilation or extracorporeal membrane oxygenation: an exploratory, randomised, placebo-controlled trial.",
"author": "Ely",
"doi-asserted-by": "publisher",
"first-page": "327",
"issue": "4",
"journal-title": "Lancet Respir Med",
"key": "joi230073r8",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2031994",
"article-title": "Baricitinib plus remdesivir for hospitalized adults with Covid-19.",
"author": "Kalil",
"doi-asserted-by": "publisher",
"first-page": "795",
"issue": "9",
"journal-title": "N Engl J Med",
"key": "joi230073r9",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1080/13543784.2018.1442436",
"article-title": "Cenicriviroc for the treatment of non-alcoholic steatohepatitis and liver fibrosis.",
"author": "Tacke",
"doi-asserted-by": "publisher",
"first-page": "301",
"issue": "3",
"journal-title": "Expert Opin Investig Drugs",
"key": "joi230073r10",
"volume": "27",
"year": "2018"
},
{
"DOI": "10.7326/M21-1269",
"article-title": "Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV): designing master protocols for evaluation of candidate COVID-19 therapeutics.",
"author": "LaVange",
"doi-asserted-by": "publisher",
"first-page": "1293",
"issue": "9",
"journal-title": "Ann Intern Med",
"key": "joi230073r11",
"volume": "174",
"year": "2021"
},
{
"DOI": "10.1080/01621459.1999.10474144",
"article-title": "A proportional hazards model for the subdistribution of a competing risk.",
"author": "Fine",
"doi-asserted-by": "publisher",
"first-page": "496",
"issue": "446",
"journal-title": "J Am Stat Assoc",
"key": "joi230073r12",
"volume": "94",
"year": "1999"
},
{
"DOI": "10.2307/2336502",
"article-title": "Discrete sequential boundaries for clinical trials.",
"author": "Lan",
"doi-asserted-by": "publisher",
"first-page": "659",
"issue": "3",
"journal-title": "Biometrika",
"key": "joi230073r13",
"volume": "70",
"year": "1983"
},
{
"DOI": "10.2307/2530245",
"article-title": "A multiple testing procedure for clinical trials.",
"author": "O’Brien",
"doi-asserted-by": "publisher",
"first-page": "549",
"issue": "3",
"journal-title": "Biometrics",
"key": "joi230073r14",
"volume": "35",
"year": "1979"
},
{
"DOI": "10.1177/009286159102500311",
"article-title": "The application of the principle of intention-to-treat to the analysis of clinical trials.",
"author": "Gillings",
"doi-asserted-by": "publisher",
"first-page": "411",
"issue": "3",
"journal-title": "Drug Inf J",
"key": "joi230073r15",
"volume": "25",
"year": "1991"
},
{
"DOI": "10.1016/j.vaccine.2007.02.064",
"article-title": "Anaphylaxis: case definition and guidelines for data collection, analysis, and presentation of immunization safety data.",
"author": "Rüggeberg",
"doi-asserted-by": "publisher",
"first-page": "5675",
"issue": "31",
"journal-title": "Vaccine",
"key": "joi230073r17",
"volume": "25",
"year": "2007"
},
{
"DOI": "10.1016/S2213-2600(21)00331-3",
"article-title": "Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial.",
"author": "Marconi",
"doi-asserted-by": "publisher",
"first-page": "1407",
"issue": "12",
"journal-title": "Lancet Respir Med",
"key": "joi230073r18",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.2147/HMER.S230613",
"article-title": "Evaluating the therapeutic potential of cenicriviroc in the treatment of nonalcoholic steatohepatitis with fibrosis: a brief report on emerging data.",
"author": "Diaz Soto",
"doi-asserted-by": "publisher",
"first-page": "115",
"journal-title": "Hepat Med",
"key": "joi230073r21",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2023184",
"article-title": "Repurposed antiviral drugs for Covid-19: interim WHO solidarity trial results.",
"author": "Pan",
"doi-asserted-by": "publisher",
"first-page": "497",
"issue": "6",
"journal-title": "N Engl J Med",
"key": "joi230073r22",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1164/rccm.202112-2836OC",
"article-title": "Evaluating primary endpoints for COVID-19 therapeutic trials to assess recovery.",
"author": "Douin",
"doi-asserted-by": "publisher",
"first-page": "730",
"issue": "6",
"journal-title": "Am J Respir Crit Care Med",
"key": "joi230073r23",
"volume": "206",
"year": "2022"
},
{
"key": "joi230073r16",
"unstructured": "US Food and Drug Administration. E9 Statistical Principles for Clinical Trials; 1998. Accessed June 28, 2023. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/e9-statistical-principles-clinical-trials"
},
{
"key": "joi230073r19",
"unstructured": "COVID-19 Treatment Guidelines Panel. Coronavirus disease 2019 (COVID-19) treatment guidelines. National Institutes of Health. Updated May 2, 2023. Accessed June 28, 2023.https://www.covid19treatmentguidelines.nih.gov/"
},
{
"key": "joi230073r20",
"unstructured": "Centers for Disease Control and Prevention. COVID data tracker. Accessed March 20, 2023. https://covid.cdc.gov/covid-data-tracker/#datatracker-home"
}
],
"reference-count": 23,
"references-count": 23,
"relation": {},
"resource": {
"primary": {
"URL": "https://jamanetwork.com/journals/jama/fullarticle/2807333"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [
"A Randomized Clinical Trial"
],
"title": "Abatacept, Cenicriviroc, or Infliximab for Treatment of Adults Hospitalized With COVID-19 Pneumonia",
"type": "journal-article",
"volume": "330"
}