Feb 21 |
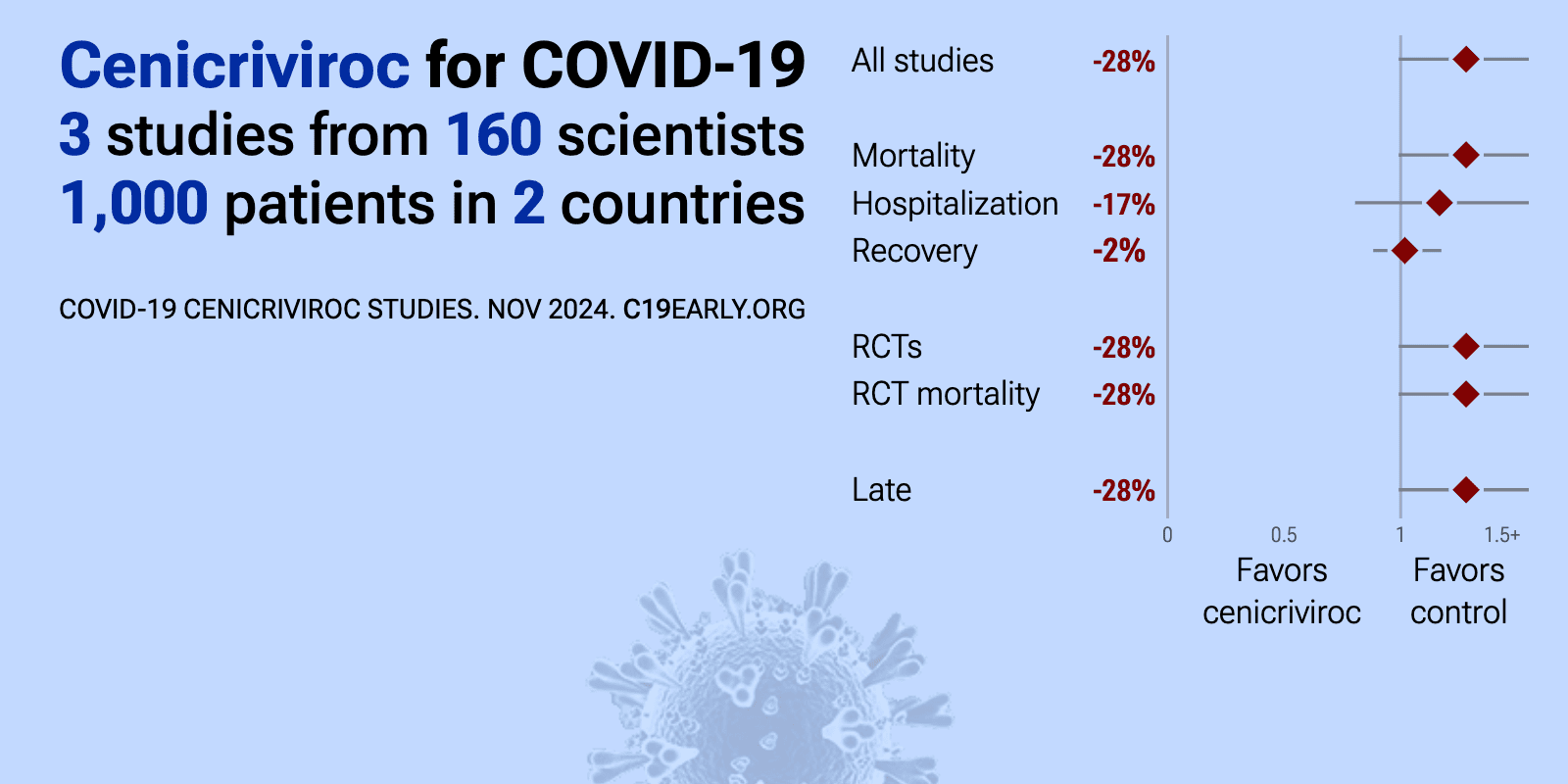
Meta-analysis of cenicriviroc studies | |
| Meta-analysis of cenicriviroc studies | ||
Jul 25 2023 |
et al., JAMA, doi:10.1001/jama.2023.11043 | Abatacept, Cenicriviroc, or Infliximab for Treatment of Adults Hospitalized With COVID-19 Pneumonia |
| 30% higher mortality (p=0.15), 8% worse improvement (p=0.62), and 1% improved recovery (p=0.91). RCT 723 hospitalized COVID-19 patients showing no significant difference in outcomes with cenicriviroc treatment. | ||
Mar 31 2023 |
et al., Journal of Global Antimicrobial Resistance, doi:10.1016/j.jgar.2022.12.004 | Cenicriviroc for the treatment of COVID-19: first interim results of a randomised, placebo-controlled, investigator-initiated, double-blind phase II trial |
| 500% higher need for oxygen therapy (p=0.26), 100% worse recovery (p=0.55), and 17% longer hospitalization (p=0.42). RCT 30 hospitalized COVID-19 patients showing no significant difference in clinical improvement with cenicriviroc (CVC) treatment compared to placebo. | ||
Mar 3 2023 |
et al., eClinicalMedicine, doi:10.1016/j.eclinm.2023.101889 | Report of the first seven agents in the I-SPY COVID trial: a phase 2, open label, adaptive platform randomised controlled trial |
| 24% higher mortality (p=0.28) and 14% worse recovery (p=0.46). RCT 261 severe COVID-19 patients showing no significant difference in outcomes with cenicriviroc. | ||