20-Week Study of Clinical Outcomes of Over-the-Counter COVID-19 Prophylaxis and Treatment
et al., Journal of Evidence-Based Integrative Medicine, doi:10.1177/2515690X211026193, Jul 2021
Vitamin C for COVID-19
6th treatment shown to reduce risk in
September 2020, now with p = 0.000000076 from 73 studies, recognized in 22 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
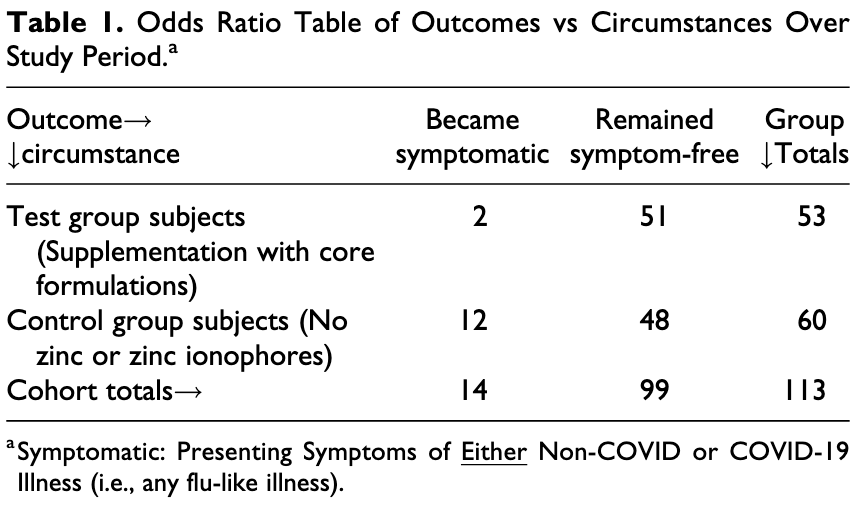
Retrospective 113 outpatients, 53 (patient choice) treated with zinc, quercetin, vitamin C/D/E, l-lysine, and quina, showing lower cases with treatment. Results are subject to selection bias and limited information on the groups is provided. See1.
Treatment assignment was voluntary. Authors note that control subjects declined the regimen due to educational and socio-economic barriers. This results in unadjusted confounders for COVID-19 exposure risk and overall health.
In the acknowledgments, authors reveal that the formulations and methods are US patent pending to Dr. Margolin. However, in the Declaration of Conflicting Interests section immediately following, they declare no potential conflicts of interest.
Arbitrary probability adjustments for confounding: instead of using standard epidemiological adjustments for confounding like logistic regression or propensity matching, authors attempt to adjust for healthy-user bias by arbitrarily assigning a lower hypothetical infection rate to the test group in a binomial expansion model.
Lack of formal baseline characteristic comparisons: authors vaguely state that the groups had roughly the same distribution of comorbidities and age, but provide no baseline data table, standard deviations, or statistical tests to show demographic equivalence between the groups.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments2.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of case, 94.4% lower, RR 0.06, p = 0.003, treatment 0 of 53 (0.0%), control 9 of 60 (15.0%), NNT 6.7, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of COVID-19 or flu-like illness, 81.1% lower, RR 0.19, p = 0.01, treatment 2 of 53 (3.8%), control 12 of 60 (20.0%), NNT 6.2.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Margolin et al., 6 Jul 2021, retrospective, USA, peer-reviewed, 5 authors, this trial uses multiple treatments in the treatment arm (combined with zinc, quercetin, vitamin D/E, l-lysine, and quina) - results of individual treatments may vary.
20-Week Study of Clinical Outcomes of Over-the-Counter COVID-19 Prophylaxis and Treatment
Journal of Evidence-Based Integrative Medicine, doi:10.1177/2515690x211026193
Objectives and Setting. As the lethal COVID-19 pandemic enters its second year, the need for effective modalities of alleviation remains urgent. This includes modalities that can readily be used by the public to reduce disease spread and severity. Such preventive measures and early-stage treatments may temper the immediacy of demand for advanced anti-COVID measures (drugs, antibodies, vaccines) and help relieve strain also on other health system resources. Design and Participants. We present results of a clinical study with a multi-component OTC "core formulation" regimen used in a multiply exposed adult population. Analysis of clinical outcome data from our sample of over 100 subjects À comprised of roughly equal sized regimen-compliant (test) and non-compliant (control) groups meeting equivalent inclusion criteria À demonstrates a strong statistical significance in favor of use of the core formulations. Results. While both groups were moderate in size, the difference between them in outcomes over the 20-week study period was large and stark: Just under 4% of the compliant test group presented flu-like symptoms, but none of the test group was COVID-positive; whereas 20% of the non-compliant control group presented flu-like symptoms, three-quarters of whom (15% overall of the control group) were COVID-positive. Conclusions. Offering a low cost, readily implemented anti-viral approach, the study regimen may serve, at the least, as a stopgap modality and, perhaps, as a useful tool in combatting the pandemic.
Authors' Note This article complies with CPMI guidelines. No PHI is included.
Declaration of Conflicting Interests The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iDs Leon Margolin, MD, PhD https://orcid.org/0000-0002-0642-300X Jeremy Luchins, PhD https://orcid.org/0000-0003-2806-6872
References
Alattar, Ibrahim, Shaar, Tocilizumab for the treatment of severe coronavirus disease 2019, J Med Virol, doi:10.1002/jmv.25964
Arshad, Kilgore, Chaudhry, Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19, Int J Infect Dis, doi:10.1016/j.ijid.2020.06.099
Babaei, Mirzababaei, Nassiri-Asl, Quercetin in food: possible mechanisms of its effect on memory, J Food Sci, doi:10.1111/1750-3841.14317
Caly, Druce, Catton, Jans, Wagstaff, The FDAapproved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro, Antiviral Res, doi:10.1016/j.antiviral.2020.104787
Capodice, Chubak, Traditional Chinese herbal medicinepotential therapeutic application for the treatment of COVID-19, Chin Med, doi:10.1186/s13020-020-00419-6
Carlucci, Ahuja, Petrilli, Zinc sulfate in combination with a zinc ionophore may improve outcomes in hospitalized COVID-19 patients, J Med Microbiol, doi:10.1099/jmm.0.001250
Charoenngam, Holick, Immunologic effects of Vitamin D on human health and disease, Nutrients, doi:10.3390/nu12072097
Chen, Wang, Yi, Epidemiological characteristics of infection in COVID-19 close contacts in Ningbo city
Cheng, Jian, Liu, Contact tracing assessment of Covid-19 transmission dynamics in Taiwan and risk at different exposure periods before and after symptom onset, JAMA Intern Med, doi:10.1001/jamain-ternmed.2020.2020
Cheng, Wu, Huang, Pang, Huang et al., Anti-inflammatory property of quercetin through downregulation of ICAM-1 and MMP-9 in TNF-a-activated retinal pigment epithelial cells, Cytokine, doi:10.1016/j.cyto.2019.01.001
Dabbagh-Bazarbachi, Clergeaud, Quesada, Zinc ionophore activity of quercetin and epigallocatechin-gallate: from Hepa 1-6 cells to a liposome model, J Agric Food Chem, doi:10.1021/jf5014633
Duncan, Yacoubian, Watson, The risk of copper deficiency in patients prescribed zinc supplements, J Clin Pathol, doi:10.1136/jclinpath-2014-202837
Dunnick, Hailey, Toxicity and carcinogenicity studies of quercetin, a natural component of foods, Fundam Appl Toxicol, doi:10.1016/0272-0590(92)90181-G
Eid, Haddad, The antidiabetic potential of quercetin: underlying mechanisms, Curr Med Chem, doi:10.2174/0929867323666160909153707
Fosmire, Zinc toxicity, Am J Clin Nutr, doi:10.1093/ajcn/51.2.225
Gasmi, Noor, Tippairote, Dadar, Menzel et al., Individual risk management strategy and potential therapeutic options for the COVID-19 pandemic, Clin Immunol, doi:10.1016/j.clim.2020.108409
Gautret, Lagier, Parola, Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an openlabel non-randomized clinical trial, Int J Antimicrob Agents, doi:10.1016/j.ijantimicag.2020.105949
Gorton, Jarvis, The effectiveness of vitamin C in preventing and relieving the symptoms of virus-induced respiratory infections, J Manipulative Physiol Ther, doi:10.1016/s0161-4754(99)70005-9
Grein, Ohmagari, Shin, Compassionate use of remdesivir for patients with severe Covid-19, N Engl J Med, doi:10.1056/NEJMoa2007016
Haleagrahara, Miranda-Hernandez, Alim, Hayes, Bird et al., Therapeutic effect of quercetin in collageninduced arthritis, Biomed Pharmacother, doi:10.1016/j.biopha.2017.03.026
Hemila, Chalker, Vitamin C for preventing and treating the common cold, Cochrane Database Syst Rev, doi:10.1002/14651858.CD000980.pub4
Hewings-Martin, How do SARS and MERS compare with COVID-19?, Medical News Today
Higgins, Ng, Danese, Rao, The risk of SARS-CoV-2 in immunosuppressed IBD patients. Crohn's, Colitis, doi:10.1093/crocol/otaa026
Kim, Park, Anti-inflammatory effect of quercetin on raw 264.7 mouse macrophages induced with polyinosinicpolycytidylic acid, Molecules, doi:10.3390/molecules21040450
Kimball, Hatfield, Arons, Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a longterm care Skilled Nursing Facility-King County, Washington, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm6913e1
Klompas, Baker, Rhee, Airborne transmission of sarscov-2: theoretical considerations and available evidence, JAMA, doi:10.1001/jama.2020.12458
Korber, Fischer, Gnanakaran, Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID19 virus, Cell, doi:10.1016/j.cell.2020.06.043
Lauring, Hodcroft, Genetic variants of SARS-CoV-2-what do they mean?, JAMA, doi:10.1001/jama.2020.27124
Lee, Han, The role of vitamin e in immunity, Nutrients, doi:10.3390/nu10111614
Lee, Kim, Lee, Clinical course and molecular viral shedding among asymptomatic and symptomatic patients with SARS-CoV-2 infection in a community treatment center in the Republic of Korea, JAMA Intern Med, doi:10.1001/jamainternmed.2020.3862
Lee, Li, Liu, Traditional Chinese herbal medicine at the forefront battle against COVID-19: clinical experience and scientific basis, Phytomedicine, doi:10.1016/j.phymed.2020.153337
Lewis, Meydani, Wu, Regulatory role of vitamin E in the immune system and inflammation, IUBMB Life, doi:10.1002/iub.1976
Li, Wu, Nie, The impact of mutations in SARS-CoV-2 spike on viral infectivity and antigenicity, Cell, doi:10.1016/j.cell.2020.07.012
Li, Zhang, Liu, Existing bitter medicines for fighting 2019-nCoV-associated infectious diseases, FASEB J, doi:10.1096/fj.202000502
Liu, Lee, Ahn, Effect of quercetin on the anti-tumor activity of cisplatin in EMT6 breast tumor-bearing mice, Obstet Gynecol Sci, doi:10.5468/ogs.2019.62.4.242
Liu, Qi, Liu, Suppression of tumor cell proliferation by quinine via the inhibition of the tumor necrosis factor receptorassociated factor 6-AKT interaction, Mol Med Rep, doi:10.3892/mmr.2016.5492
Liu, Xue, Zhi, None, doi:10.3760/cma.j.cn112338-20200304-00251
Liu, Yu, Ji, Quercetin reduces TNF-a-induced mesangial cell proliferation and inhibits PTX 3 production: involvement of NF-kB signaling pathway, Phytother Res, doi:10.1002/ptr.6430
Martineau, Jolliffe, Greenberg, Vitamin D supplementation to prevent acute respiratory infections: individual participant data meta-analysis, Health Technol Assess, doi:10.3310/hta23020
Marunaka, Marunaka, Sun, Actions of quercetin, a polyphenol, on blood pressure, Molecules, doi:10.3390/molecules22020209
Mccall, Huang, Fierke, Function and mechanism of zinc metalloenzymes, J Nutr, doi:10.1093/jn/130.5.1437S
Meltzer, Best, Zhang, Association of vitamin D status and other clinical characteristics with COVID-19 test results, JAMA Netw Open, doi:10.1001/jamanetworkopen.2020.19722
Ogunlana, Ogunlana, Ademowo, Comparative in vitro assessment of the antiplasmodial activity of quinine-zinc complex and quinine sulphate, Sci Res Essays, doi:10.5897/SRE.9000281
Osterholm, Possible scenarios for the course of the COVID-19 pandemic: are we prepared for at least another 18-24 months of significant COVID-19 activity? VuMedi Presentation
Pal, Squitti, Picozza, Zinc and COVID-19: basis of current clinical trials, Biol Trace Elem Res, doi:10.1007/s12011-020-02437-9
Patel, Mistry, Shinde, Syed, Singh et al., Therapeutic potential of quercetin as a cardiovascular agent, Eur J Med Chem, doi:10.1016/j.ejmech.2018.06.053
Peiffer-Smadja, Lescure, Sallard, Anticovid, a comprehensive open-access real-time platform of registered clinical studies for COVID-19, J Antimicrob Chemother, doi:10.1093/jac/dkaa223
Prasad, Zinc: mechanisms of host defense, J Nutr, doi:10.1093/jn/137.5.1345
Qiu, Kroeker, He, Prophylactic efficacy of quercetin 3-b-o-d-glucoside against ebola virus infection, Antimicrob Agents Chemother, doi:10.1128/AAC.00307-16
Racaniello, Dr, VuMedi presentation (TWIV: This Week in Virology)
Rastogi, Ayurveda co-interventions have supported complete recovery in Severe COVID-19 infection with a chest severity score 18/25: a case report, J Ayurveda Integr Med
Rastogi, Pandey, Singh, COVID-19 pandemic: a pragmatic plan for Ayurveda intervention, J Ayurveda Integr Med, doi:10.1016/j.jaim.2020.04.002
Rastogi, Rastogi, Kharbanda, Time when a physician turned out to be a patient: a case study on how an Ayurvedic physician cured himself from COVID-19, J Ayurveda Integr Med, doi:10.1016/j.jaim.2021.02.002
Razzaque, COVID-19 pandemic: can maintaining optimal zinc balance enhance host resistance?, Tohoku J Exp Med, doi:10.1620/tjem.251.175
Risch, Opinion: early outpatient treatment of symptomatic, high-risk Covid-19 patients that should be ramped-up immediately as key to the pandemic crisis, Am J Epidemiol, doi:10.1093/aje/kwaa093
Roohani, Hurrell, Kelishadi, Zinc and its importance for human health: an integrative review, J Res Med Sci
Sahebnasagh, Saghafi, Avan, The prophylaxis and treatment potential of supplements for COVID-19, Eur J Pharmacol, doi:10.1016/j.ejphar.2020.173530
Shankar, Prasad, Bu ¨low, Dubben, Engelhardt, Zinc-dependent suppression of TNF-alpha production is mediated by protein kinase A-induced inhibition of Raf-1, I kappa B kinase beta, and NF-kappa B, Am J Clin Nutr, doi:10.1093/ajcn/68.2.447
Silverman, Hupert, Washburne, Using influenza surveillance networks to estimate state-specific prevalence of SARS-CoV-2 in the United States, Sci Transl Med, doi:10.1126/scitranslmed.abc1126
Skalny, Rink, Ajsuvakova, Zinc and respiratory tract infections: perspectives for COVID 19 (Review), Int J Mol Med, doi:10.3892/ijmm.2020.4575
Skrajnowska, Bobrowska-Korczak, Role of zinc in immune system and anti-cancer defense mechanisms, Nutrients, doi:10.3390/nu11102273
Smith, Smith, Repurposing therapeutics for COVID-19: supercomputer-based docking to the SARS-CoV-2 viral spike protein and viral spike protein-human ACE2 interface, Chemrxiv. Preprint, doi:10.26434/chemrxiv.11871402
Sugeng, Adriani, Wirjatmadi, The effect of zinc and lysine supplementation on infection rate and cd4 count in elderly, Biochem Physiol, doi:10.4172/2168-9652.S5-002
Szumilas, Explaining odds ratios, J Can Acad Child Adolesc Psychiatry
Tevelthuis, Van Den Worm, She, Sims, Zinc(2þ) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophore block the replication of these viruses in cell culture, PLoS Pathog, doi:10.1371/journal.ppat.1001176
Thorlund, Dron, Park, Hsu, Forrest et al., A realtime dashboard of clinical trials for COVID-19, Lancet Digit Health, doi:10.1018/S2589-7500(20)30086-8
Tillu, Chaturvedi, Chopra, Public health approach of Ayurveda and yoga for COVID-19 prophylaxis, J Altern Complement Med, doi:10.1089/acm.2020.0129
Wang, Cao, Zhang, Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res, doi:10.1038/s41422-020-0282-0
Wessels, Rolles, Rink, The potential impact of zinc supplementation on COVID-19 Pathogenesis, Front Immunol, doi:10.3389/fimmu.2020.01712
Wu, Li, He, Quercetin as an antiviral agent inhibits influenza A virus (IAV) entry, Viruses, doi:10.3390/v8010006
Xue, Moyer, Peng, Chloroquine is a zinc ionophore, PLoS One, doi:10.1371/journal.pone.0109180
Yi, Li, Yuan, Small molecules blocking the entry of severe acute respiratory syndrome coronavirus into host cell, J Virol, doi:10.1128/JVI.78.20.11334-11339.2004
Zhao, Zhai, Zhang, Lysine-fortified wheat flour improves the nutritional and immunological status of wheateating families in northern China, Food Nutr Bull, doi:10.1177/156482650402500203
DOI record:
{
"DOI": "10.1177/2515690x211026193",
"ISSN": [
"2515-690X",
"2515-690X"
],
"URL": "http://dx.doi.org/10.1177/2515690X211026193",
"abstract": "<jats:sec><jats:title>Objectives and Setting.</jats:title><jats:p> As the lethal COVID-19 pandemic enters its second year, the need for effective modalities of alleviation remains urgent. This includes modalities that can readily be used by the public to reduce disease spread and severity. Such preventive measures and early-stage treatments may temper the immediacy of demand for advanced anti-COVID measures (drugs, antibodies, vaccines) and help relieve strain also on other health system resources. </jats:p></jats:sec><jats:sec><jats:title>Design and Participants.</jats:title><jats:p> We present results of a clinical study with a multi-component OTC “core formulation” regimen used in a multiply exposed adult population. Analysis of clinical outcome data from our sample of over 100 subjects − comprised of roughly equal sized regimen-compliant (test) and non-compliant (control) groups meeting equivalent inclusion criteria − demonstrates a strong statistical significance in favor of use of the core formulations. </jats:p></jats:sec><jats:sec><jats:title>Results.</jats:title><jats:p> While both groups were moderate in size, the difference between them in outcomes over the 20-week study period was large and stark: Just under 4% of the compliant test group presented flu-like symptoms, but none of the test group was COVID-positive; whereas 20% of the non-compliant control group presented flu-like symptoms, three-quarters of whom (15% overall of the control group) were COVID-positive. </jats:p></jats:sec><jats:sec><jats:title>Conclusions.</jats:title><jats:p> Offering a low cost, readily implemented anti-viral approach, the study regimen may serve, at the least, as a stopgap modality and, perhaps, as a useful tool in combatting the pandemic. </jats:p></jats:sec>",
"alternative-id": [
"10.1177/2515690X211026193"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-0642-300X",
"affiliation": [
{
"name": "Comprehensive Pain Management Institute, LLC, Columbus, OH, USA"
}
],
"authenticated-orcid": false,
"family": "Margolin",
"given": "Leon",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0003-2806-6872",
"affiliation": [
{
"name": "Comprehensive Pain Management Institute, LLC, Columbus, OH, USA"
}
],
"authenticated-orcid": false,
"family": "Luchins",
"given": "Jeremy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Comprehensive Pain Management Institute, LLC, Columbus, OH, USA"
}
],
"family": "Margolin",
"given": "Daniel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Comprehensive Pain Management Institute, LLC, Columbus, OH, USA"
}
],
"family": "Margolin",
"given": "Michelle",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Comprehensive Pain Management Institute, LLC, Columbus, OH, USA"
}
],
"family": "Lefkowitz",
"given": "Sanford",
"sequence": "additional"
}
],
"container-title": "Journal of Evidence-Based Integrative Medicine",
"container-title-short": "J Evid Based Complementary Altern Med",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"journals.sagepub.com"
]
},
"created": {
"date-parts": [
[
2021,
7,
6
]
],
"date-time": "2021-07-06T07:08:32Z",
"timestamp": 1625555312000
},
"deposited": {
"date-parts": [
[
2021,
7,
6
]
],
"date-time": "2021-07-06T07:08:45Z",
"timestamp": 1625555325000
},
"indexed": {
"date-parts": [
[
2024,
3,
8
]
],
"date-time": "2024-03-08T16:38:12Z",
"timestamp": 1709915892617
},
"is-referenced-by-count": 16,
"issued": {
"date-parts": [
[
2021,
1,
1
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
1,
1
]
],
"date-time": "2021-01-01T00:00:00Z",
"timestamp": 1609459200000
}
}
],
"link": [
{
"URL": "http://journals.sagepub.com/doi/pdf/10.1177/2515690X211026193",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://journals.sagepub.com/doi/full-xml/10.1177/2515690X211026193",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://journals.sagepub.com/doi/pdf/10.1177/2515690X211026193",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "179",
"original-title": [],
"page": "2515690X2110261",
"prefix": "10.1177",
"published": {
"date-parts": [
[
2021,
1,
1
]
]
},
"published-online": {
"date-parts": [
[
2021,
7,
6
]
]
},
"published-print": {
"date-parts": [
[
2021,
1,
1
]
]
},
"publisher": "SAGE Publications",
"reference": [
{
"DOI": "10.15585/mmwr.mm6913e1",
"doi-asserted-by": "publisher",
"key": "bibr1-2515690X211026193"
},
{
"DOI": "10.1001/jamainternmed.2020.3862",
"doi-asserted-by": "publisher",
"key": "bibr2-2515690X211026193"
},
{
"DOI": "10.1016/j.cell.2020.06.043",
"doi-asserted-by": "publisher",
"key": "bibr4-2515690X211026193"
},
{
"DOI": "10.1016/j.cell.2020.07.012",
"doi-asserted-by": "publisher",
"key": "bibr5-2515690X211026193"
},
{
"DOI": "10.1001/jama.2020.27124",
"doi-asserted-by": "publisher",
"key": "bibr6-2515690X211026193"
},
{
"DOI": "10.1016/j.ijantimicag.2020.105949",
"doi-asserted-by": "publisher",
"key": "bibr7-2515690X211026193"
},
{
"DOI": "10.1016/j.ijid.2020.06.099",
"doi-asserted-by": "publisher",
"key": "bibr8-2515690X211026193"
},
{
"DOI": "10.1093/aje/kwaa093",
"doi-asserted-by": "publisher",
"key": "bibr9-2515690X211026193"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"doi-asserted-by": "publisher",
"key": "bibr10-2515690X211026193"
},
{
"DOI": "10.1056/NEJMoa2007016",
"doi-asserted-by": "publisher",
"key": "bibr11-2515690X211026193"
},
{
"DOI": "10.1016/j.antiviral.2020.104787",
"doi-asserted-by": "publisher",
"key": "bibr12-2515690X211026193"
},
{
"DOI": "10.1002/jmv.25964",
"doi-asserted-by": "publisher",
"key": "bibr13-2515690X211026193"
},
{
"DOI": "10.1016/S2589-7500(20)30086-8",
"doi-asserted-by": "publisher",
"key": "bibr14-2515690X211026193"
},
{
"DOI": "10.1093/jac/dkaa223",
"doi-asserted-by": "publisher",
"key": "bibr15-2515690X211026193"
},
{
"DOI": "10.3389/fimmu.2020.01712",
"doi-asserted-by": "publisher",
"key": "bibr16-2515690X211026193"
},
{
"DOI": "10.3390/nu11102273",
"doi-asserted-by": "publisher",
"key": "bibr17-2515690X211026193"
},
{
"DOI": "10.1093/jn/130.5.1437S",
"doi-asserted-by": "publisher",
"key": "bibr18-2515690X211026193"
},
{
"author": "Roohani N",
"first-page": "144",
"issue": "2",
"journal-title": "J Res Med Sci",
"key": "bibr19-2515690X211026193",
"volume": "18",
"year": "2013"
},
{
"DOI": "10.1093/jn/137.5.1345",
"doi-asserted-by": "publisher",
"key": "bibr20-2515690X211026193"
},
{
"DOI": "10.1093/ajcn/68.2.447S",
"doi-asserted-by": "publisher",
"key": "bibr21-2515690X211026193"
},
{
"DOI": "10.4049/jimmunol.179.6.4180",
"doi-asserted-by": "publisher",
"key": "bibr22-2515690X211026193"
},
{
"DOI": "10.1371/journal.ppat.1001176",
"doi-asserted-by": "publisher",
"key": "bibr23-2515690X211026193"
},
{
"DOI": "10.1093/ajcn/51.2.225",
"doi-asserted-by": "publisher",
"key": "bibr24-2515690X211026193"
},
{
"DOI": "10.1136/jclinpath-2014-202837",
"doi-asserted-by": "publisher",
"key": "bibr26-2515690X211026193"
},
{
"author": "Ogunlana OO",
"first-page": "180",
"issue": "3",
"journal-title": "Sci Res Essays",
"key": "bibr27-2515690X211026193",
"volume": "4",
"year": "2009"
},
{
"DOI": "10.1371/journal.pone.0109180",
"doi-asserted-by": "publisher",
"key": "bibr28-2515690X211026193"
},
{
"DOI": "10.1096/fj.202000502",
"doi-asserted-by": "publisher",
"key": "bibr29-2515690X211026193"
},
{
"DOI": "10.3892/mmr.2016.5492",
"doi-asserted-by": "publisher",
"key": "bibr30-2515690X211026193"
},
{
"DOI": "10.1093/crocol/otaa026",
"doi-asserted-by": "publisher",
"key": "bibr31-2515690X211026193"
},
{
"DOI": "10.1021/jf5014633",
"doi-asserted-by": "publisher",
"key": "bibr32-2515690X211026193"
},
{
"author": "Smith M",
"journal-title": "Chemrxiv",
"key": "bibr33-2515690X211026193"
},
{
"DOI": "10.3390/molecules21040450",
"doi-asserted-by": "publisher",
"key": "bibr34-2515690X211026193"
},
{
"DOI": "10.1016/j.cyto.2019.01.001",
"doi-asserted-by": "publisher",
"key": "bibr35-2515690X211026193"
},
{
"DOI": "10.1016/j.biopha.2017.03.026",
"doi-asserted-by": "publisher",
"key": "bibr36-2515690X211026193"
},
{
"DOI": "10.1128/AAC.00307-16",
"doi-asserted-by": "publisher",
"key": "bibr37-2515690X211026193"
},
{
"DOI": "10.3390/v8010006",
"doi-asserted-by": "publisher",
"key": "bibr38-2515690X211026193"
},
{
"DOI": "10.1128/JVI.78.20.11334-11339.2004",
"doi-asserted-by": "publisher",
"key": "bibr39-2515690X211026193"
},
{
"DOI": "10.1002/ptr.6430",
"doi-asserted-by": "publisher",
"key": "bibr40-2515690X211026193"
},
{
"DOI": "10.5468/ogs.2019.62.4.242",
"doi-asserted-by": "publisher",
"key": "bibr41-2515690X211026193"
},
{
"DOI": "10.3390/molecules22020209",
"doi-asserted-by": "publisher",
"key": "bibr42-2515690X211026193"
},
{
"DOI": "10.2174/0929867323666160909153707",
"doi-asserted-by": "publisher",
"key": "bibr43-2515690X211026193"
},
{
"DOI": "10.1016/j.ejmech.2018.06.053",
"doi-asserted-by": "publisher",
"key": "bibr44-2515690X211026193"
},
{
"DOI": "10.1111/1750-3841.14317",
"doi-asserted-by": "publisher",
"key": "bibr45-2515690X211026193"
},
{
"DOI": "10.1016/S0161-4754(99)70005-9",
"doi-asserted-by": "publisher",
"key": "bibr46-2515690X211026193"
},
{
"author": "Hemilä H",
"first-page": "CD000980",
"issue": "1",
"journal-title": "Cochrane Database Syst Rev",
"key": "bibr47-2515690X211026193",
"year": "2013"
},
{
"DOI": "10.3310/hta23020",
"doi-asserted-by": "publisher",
"key": "bibr48-2515690X211026193"
},
{
"DOI": "10.3390/nu12072097",
"doi-asserted-by": "publisher",
"key": "bibr49-2515690X211026193"
},
{
"DOI": "10.1001/jamanetworkopen.2020.19722",
"doi-asserted-by": "publisher",
"key": "bibr50-2515690X211026193"
},
{
"DOI": "10.1002/iub.1976",
"doi-asserted-by": "publisher",
"key": "bibr51-2515690X211026193"
},
{
"DOI": "10.3390/nu10111614",
"doi-asserted-by": "publisher",
"key": "bibr52-2515690X211026193"
},
{
"DOI": "10.1177/156482650402500203",
"doi-asserted-by": "publisher",
"key": "bibr53-2515690X211026193"
},
{
"author": "Sugeng MW",
"first-page": "2",
"journal-title": "Biochem Physiol",
"key": "bibr54-2515690X211026193",
"volume": "5",
"year": "2015"
},
{
"DOI": "10.1016/0272-0590(92)90181-G",
"doi-asserted-by": "publisher",
"key": "bibr56-2515690X211026193"
},
{
"DOI": "10.1007/s00787-010-0087-7",
"author": "Szumilas M",
"doi-asserted-by": "crossref",
"first-page": "227",
"issue": "3",
"journal-title": "J Can Acad Child Adolesc Psychiatry",
"key": "bibr57-2515690X211026193",
"volume": "19",
"year": "2010"
},
{
"author": "Chen Y",
"first-page": "667",
"issue": "5",
"journal-title": "Zhonghua Liu Xing Bing Xue Za Zhi",
"key": "bibr59-2515690X211026193",
"volume": "41",
"year": "2020"
},
{
"DOI": "10.1001/jamainternmed.2020.2020",
"doi-asserted-by": "publisher",
"key": "bibr60-2515690X211026193"
},
{
"DOI": "10.1126/scitranslmed.abc1126",
"doi-asserted-by": "publisher",
"key": "bibr61-2515690X211026193"
},
{
"DOI": "10.1001/jama.2020.12458",
"doi-asserted-by": "publisher",
"key": "bibr63-2515690X211026193"
},
{
"DOI": "10.1016/j.ejphar.2020.173530",
"doi-asserted-by": "publisher",
"key": "bibr64-2515690X211026193"
},
{
"author": "Skalny AV",
"first-page": "17",
"issue": "1",
"journal-title": "Int J Mol Med.",
"key": "bibr65-2515690X211026193",
"volume": "46",
"year": "2020"
},
{
"DOI": "10.1620/tjem.251.175",
"doi-asserted-by": "publisher",
"key": "bibr66-2515690X211026193"
},
{
"author": "Pal A",
"first-page": "1",
"journal-title": "Biol Trace Elem Res",
"key": "bibr67-2515690X211026193",
"year": "2020"
},
{
"DOI": "10.1099/jmm.0.001250",
"doi-asserted-by": "publisher",
"key": "bibr68-2515690X211026193"
},
{
"author": "Hewings-Martin Y",
"journal-title": "Medical News Today (Newsletter)",
"key": "bibr69-2515690X211026193"
},
{
"author": "Osterholm M",
"journal-title": "VuMedi Presentation",
"key": "bibr70-2515690X211026193"
},
{
"DOI": "10.1016/j.jaim.2020.04.002",
"doi-asserted-by": "publisher",
"key": "bibr71-2515690X211026193"
},
{
"DOI": "10.1016/j.jaim.2021.02.002",
"doi-asserted-by": "publisher",
"key": "bibr72-2515690X211026193"
},
{
"DOI": "10.1016/j.jaim.2021.02.008",
"doi-asserted-by": "publisher",
"key": "bibr73-2515690X211026193"
},
{
"DOI": "10.1089/acm.2020.0129",
"doi-asserted-by": "publisher",
"key": "bibr74-2515690X211026193"
},
{
"DOI": "10.1016/j.phymed.2020.153337",
"doi-asserted-by": "publisher",
"key": "bibr75-2515690X211026193"
},
{
"DOI": "10.1186/s13020-020-00419-6",
"doi-asserted-by": "publisher",
"key": "bibr76-2515690X211026193"
},
{
"DOI": "10.1016/j.clim.2020.108409",
"doi-asserted-by": "publisher",
"key": "bibr77-2515690X211026193"
}
],
"reference-count": 72,
"references-count": 72,
"relation": {},
"resource": {
"primary": {
"URL": "http://journals.sagepub.com/doi/10.1177/2515690X211026193"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Complementary and alternative medicine"
],
"subtitle": [],
"title": "20-Week Study of Clinical Outcomes of Over-the-Counter COVID-19 Prophylaxis and Treatment",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1177/sage-journals-update-policy",
"volume": "26"
}
margolin
