Preliminary Evidence of Good Safety Profile and Outcomes of Early Treatment With Tixagevimab/Cilgavimab Compared to Previously Employed Monoclonal Antibodies for COVID-19 in Immunocompromised Patients
et al., Preprints, doi:10.20944/preprints202301.0359.v1, Jan 2023
42nd treatment shown to reduce risk in
May 2022, now with p = 0.0066 from 19 studies, recognized in 33 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective immunocompromised patients, showing no significant difference between tixagevimab/cilgavimab and other mAbs.
Efficacy is variant dependent. In Vitro research suggests a lack of efficacy for omicron BA.2.75.2, BA.4.6, BQ.1.11, BA.5, BA.2.75, XBB2,3, XBB.1.53, ХВВ.1.9.13, XBB.1.9.3, XBB.1.5.24, XBB.1.16, XBB.2.9, BQ.1.1.45, CL.1, and CH.1.14.
This study is excluded in the after exclusion results of meta-analysis:
study compares against another treatment showing significant efficacy.
|
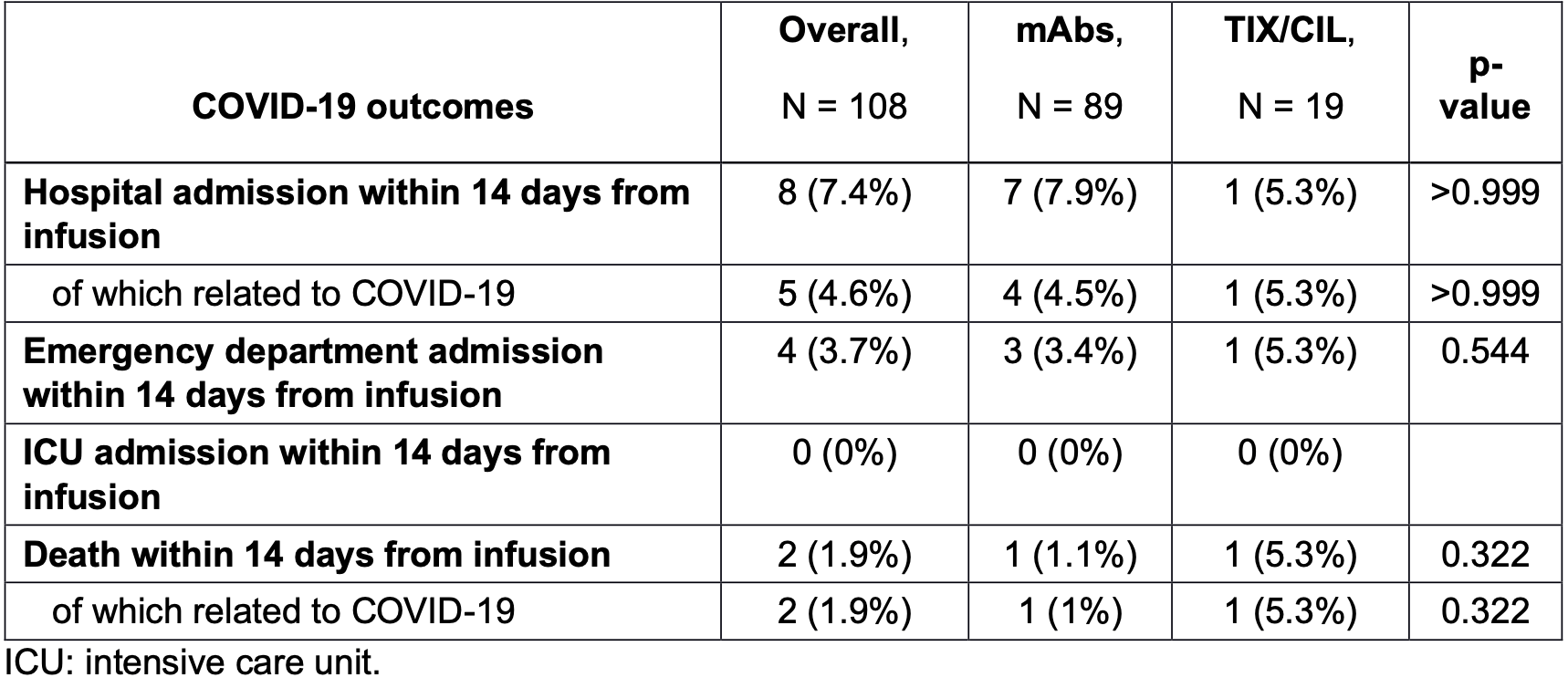
risk of death, 368.4% higher, RR 4.68, p = 0.32, treatment 1 of 19 (5.3%), control 1 of 89 (1.1%), day 14.
|
|
risk of hospitalization, 33.1% lower, RR 0.67, p = 1.00, treatment 1 of 19 (5.3%), control 7 of 89 (7.9%), NNT 38, day 14.
|
|
risk of no viral clearance, 23.7% higher, RR 1.24, p = 0.30, treatment 14 of 19 (73.7%), control 53 of 89 (59.6%), day 14.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Planas et al., Resistance of Omicron subvariants BA.2.75.2, BA.4.6 and BQ.1.1 to neutralizing antibodies, bioRxiv, doi:10.1101/2022.11.17.516888.
2.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
Lombardi et al., 19 Jan 2023, retrospective, Italy, preprint, 21 authors, study period 28 August, 2022 - 15 October, 2022, this trial compares with another treatment - results may be better when compared to placebo.
Contact: andrea.lombardi@unimi.it.
Preliminary Evidence of Good Safety Profile and Outcomes of Early Treatment With Tixagevimab/Cilgavimab Compared to Previously Employed Monoclonal Antibodies for COVID-19 in Immunocompromised Patients
doi:10.20944/preprints202301.0359.v1
The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content.
Authors contributions AL, SV, AG, and AB conceived the study. GV, SB, EP, CA, NI, BM, CG, MT, AT, MF, AGV, LCM, FD, GC, RC, MC and AM enrolled the patients and collected clinical data. AL and SV performed the statistical analysis. AL, GV and SV wrote the first draft of the manuscript. All the other authors reviewed the final version of the manuscript.
Conflict of interests AL Gilead Sciences Inc. and Insmed Italia. AB Quiagen, Pfizer, Nordic Pharma, ViiV, SOBI, and Gilead Sciences. FD Kedrion, Gilead Sciences, Biotest, and Novartis. AM Gilead Sciences, Menarini, and Nordic Pharma. SB Infectopharma. MC Takeda and Kedrion. All the other authors have nothing to declare.
References
Aifa, AIFA Evusheld trattamento 2022
Arora, Kempf, Nehlmeier, Schulz, Jäck et al., Omicron sublineage BQ.1.1 resistance to monoclonal antibodies, Lancet Infect Dis, doi:10.1016/S1473-3099(22)00733-2
Biscarini, Villa, Genovese, Tomasello, Tonizzo et al., Safety Profile and Outcomes of Early COVID-19 Treatments in Immunocompromised Patients: A Single-Centre Cohort Study, Biomedicines, doi:10.3390/biomedicines10082002
Ecdc, ECDC VOCs monitoring
Holland, Ginde, Paredes, Murray, Engen et al., Tixagevimabcilgavimab for treatment of patients hospitalised with COVID-19: a randomised, doubleblind, phase 3 trial, Lancet Respir Med, doi:10.1016/S2213-2600(22)00215-6
Jurdi, Morena, Cote, Bethea, Azzi et al., Tixagevimab/cilgavimab preexposure prophylaxis is associated with lower breakthrough infection risk in vaccinated solid organ transplant recipients during the omicron wave, Am J Transplant, doi:10.1111/ajt.17128
Levin, Ustianowski, Wit, Launay, Avila et al., Intramuscular AZD7442 (Tixagevimab-Cilgavimab) for Prevention of Covid-19, N Engl J Med, doi:10.1056/NEJMoa2116620
Loo, Mctamney, Arends, Abram, Aksyuk et al., The SARS-CoV-2 monoclonal antibody combination, AZD7442, is protective in nonhuman primates and has an extended half-life in humans, Sci Transl Med, doi:10.1126/scitranslmed.abl8124
Mcconnell, Harte, Walsh, Murphy, Barry, Comparative effectiveness of neutralising monoclonal antibodies in high risk COVID-19 patients: a Bayesian network meta-analysis, Sci Rep, doi:10.1038/s41598-022-22431-6
Montgomery, Hobbs, Padilla, Arbetter, Templeton et al., Efficacy and safety of intramuscular administration of tixagevimab-cilgavimab for early outpatient treatment of COVID-19 (TACKLE): a phase 3, randomised, double-blind, placebo-controlled trial, Lancet Respir Med, doi:10.1016/S2213-2600(22)00180-1
Nguyen, Flahault, Chavarot, Melenotte, Cheminant et al., Preexposure prophylaxis with tixagevimab and cilgavimab (Evusheld) for COVID-19 among 1112 severely immunocompromised patients, Clin Microbiol Infect, doi:10.1016/j.cmi.2022.07.015
DOI record:
{
"DOI": "10.20944/preprints202301.0359.v1",
"URL": "http://dx.doi.org/10.20944/preprints202301.0359.v1",
"abstract": "<jats:p>Objectives: Monoclonal antibodies (mAbs) have proven to be a valuable tool against COVID-19, mostly among subjects with risk factors for progression to severe illness. Tixagevimab/cilgavimab (TIX/CIL), a combination of two Fc-modified human monoclonal antibodies, has been recently approved to be employed as early treatment. Methods: Two groups of immunocompromised patients exposed to different early treatments (i.e., TIX/CIL vs. other mAbs [casirivimab/imdevimab, bamlanivimab/etesevimab, sotrovimab]) were compared in terms of clinical outcomes (hospitalization and mortality within 14 days from administration) and time to the negativity of nasal swabs. We used either Pearson&rsquo;s chi-square or Fisher&rsquo;s exact test for categorical variables, whereas the Wilcoxon rank&ndash;sum test was employed for continuous ones. Kaplan&ndash;Meier curves were produced to compare the time to nasopharyngeal swab negativity. Results: Early treatment with TIX/CIL was administered to 19 immunocompromised patients, while 89 patients received other mAbs. Most of them were solid organ transplant recipients or suffering from hematologic or solid malignancies. Overall, no significant difference was observed between the two groups in terms of clinical outcomes. In the TIX/CIL group, one patient (1/19, 5.3%), who was admitted to the emergency room within the first 14 days from treatment and was hospitalised due to COVID-19 progression, died. Regarding the time to nasal swab negativity, no significant difference (p=0.088) emerged. Conclusions: Early treatment of SARS-CoV-2 infection with TIX/CIL shows favourable outcomes in a small group of immunocompromised patients, reporting no significant difference when compared to similar patients treated with other mAbs.</jats:p>",
"accepted": {
"date-parts": [
[
2023,
1,
17
]
]
},
"author": [
{
"ORCID": "http://orcid.org/0000-0002-0383-9579",
"affiliation": [],
"authenticated-orcid": false,
"family": "Lombardi",
"given": "Andrea",
"sequence": "first"
},
{
"affiliation": [],
"family": "Viero",
"given": "Giulia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Villa",
"given": "Simone",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Biscarini",
"given": "Simona",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1386-6180",
"affiliation": [],
"authenticated-orcid": false,
"family": "Palomba",
"given": "Emanuele",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Azzara'",
"given": "Cecilia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Iannotti",
"given": "Nathalie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mariani",
"given": "Bianca",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Genovese",
"given": "Camilla",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tomasello",
"given": "Mara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tonizzo",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fava",
"given": "Marco",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Valzano",
"given": "Antonia Grazia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Morlacchi",
"given": "Letizia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Donato",
"given": "Maria Francesca",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Castellano",
"given": "Giuseppe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cassin",
"given": "Ramona",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0717-9096",
"affiliation": [],
"authenticated-orcid": false,
"family": "Carrabba",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Muscatello",
"given": "Antonio",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6587-4794",
"affiliation": [],
"authenticated-orcid": false,
"family": "Gori",
"given": "Andrea",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bandera",
"given": "Alessandra",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
1,
20
]
],
"date-time": "2023-01-20T03:00:59Z",
"timestamp": 1674183659000
},
"deposited": {
"date-parts": [
[
2023,
1,
20
]
],
"date-time": "2023-01-20T03:01:13Z",
"timestamp": 1674183673000
},
"group-title": "MEDICINE & PHARMACOLOGY",
"indexed": {
"date-parts": [
[
2023,
1,
21
]
],
"date-time": "2023-01-21T06:05:00Z",
"timestamp": 1674281100445
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
1,
19
]
]
},
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
1,
19
]
],
"date-time": "2023-01-19T00:00:00Z",
"timestamp": 1674086400000
}
}
],
"member": "1968",
"original-title": [],
"posted": {
"date-parts": [
[
2023,
1,
19
]
]
},
"prefix": "10.20944",
"published": {
"date-parts": [
[
2023,
1,
19
]
]
},
"publisher": "MDPI AG",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.preprints.org/manuscript/202301.0359/v1"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "Preliminary Evidence of Good Safety Profile and Outcomes of Early Treatment With Tixagevimab/Cilgavimab Compared to Previously Employed Monoclonal Antibodies for COVID-19 in Immunocompromised Patients",
"type": "posted-content"
}
