Tixagevimab–cilgavimab for treatment of patients hospitalised with COVID-19: a randomised, double-blind, phase 3 trial
et al., The Lancet Respiratory Medicine, doi:10.1016/S2213-2600(22)00215-6, ACTIV-3/TICO, NCT04501978, Jul 2022
42nd treatment shown to reduce risk in
May 2022, now with p = 0.0066 from 19 studies, recognized in 33 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
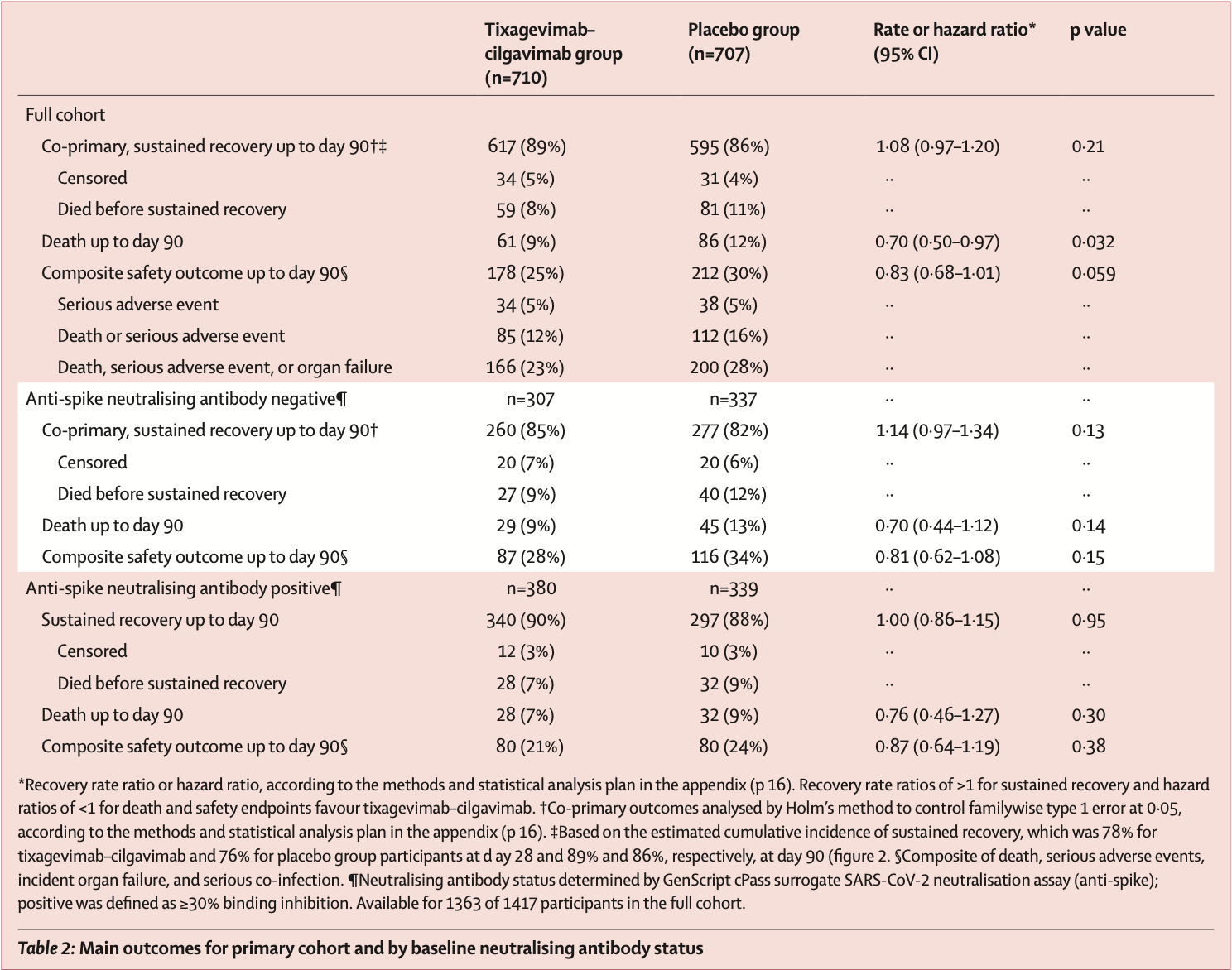
RCT with 710 hospitalized patients treated with tixagevimab/cilgavimab, and 707 placebo patients, showing lower mortality with treatment. Long-term results are reported in Mourad et al.
Efficacy is variant dependent. In Vitro research suggests a lack of efficacy for omicron BA.2.75.2, BA.4.6, BQ.1.12, BA.5, BA.2.75, XBB3,4, XBB.1.54, ХВВ.1.9.14, XBB.1.9.3, XBB.1.5.24, XBB.1.16, XBB.2.9, BQ.1.1.45, CL.1, and CH.1.15.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments6.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 23.0% lower, HR 0.77, p = 0.08, treatment 80 of 710 (11.3%), control 101 of 707 (14.3%), NNT 33, Cox proportional hazards, day 540.
|
|
risk of death, 30.0% lower, RR 0.70, p = 0.03, treatment 61 of 710 (8.6%), control 86 of 707 (12.2%), NNT 28, day 90.
|
|
risk of death/hospitalization, 3.0% lower, HR 0.97, p = 0.77, treatment 212 of 710 (29.9%), control 213 of 707 (30.1%), NNT 373, Cox proportional hazards, day 540.
|
|
risk of no recovery, 7.4% lower, RR 0.93, p = 0.21, treatment 710, control 707, inverted to make RR<1 favor treatment, sustained recovery, day 90, primary outcome.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Mourad et al., Long-term outcomes of passive immunotherapy for COVID-19: a pooled analysis of a large multinational platform randomized clinical trial, Clinical Microbiology and Infection, doi:10.1016/j.cmi.2025.02.002.
2.
Planas et al., Resistance of Omicron subvariants BA.2.75.2, BA.4.6 and BQ.1.1 to neutralizing antibodies, bioRxiv, doi:10.1101/2022.11.17.516888.
3.
Haars et al., Prevalence of SARS-CoV-2 Omicron Sublineages and Spike Protein Mutations Conferring Resistance against Monoclonal Antibodies in a Swedish Cohort during 2022–2023, Microorganisms, doi:10.3390/microorganisms11102417.
4.
Uraki et al., Antiviral efficacy against and replicative fitness of an XBB.1.9.1 clinical isolate, iScience, doi:10.1016/j.isci.2023.108147.
Holland et al., 8 Jul 2022, Double Blind Randomized Controlled Trial, placebo-controlled, USA, peer-reviewed, 103 authors, study period 10 February, 2021 - 30 September, 2021, average treatment delay 8.0 days, trial NCT04501978 (history) (ACTIV-3/TICO).
Contact: adit.ginde@cuanschutz.edu.
Tixagevimab-cilgavimab for treatment of patients hospitalised with COVID-19: a randomised, double-blind, phase 3 trial
doi:10.1016/S2213-2600(22)00215
Background Tixagevimab-cilgavimab is a neutralising monoclonal antibody combination hypothesised to improve outcomes for patients hospitalised with COVID-19. We aimed to compare tixagevimab-cilgavimab versus placebo, in patients receiving remdesivir and other standard care. Methods In a randomised, double-blind, phase 3, placebo-controlled trial, adults with symptoms for up to 12 days and hospitalised for COVID-19 at 81 sites in the USA, Europe, Uganda, and Singapore were randomly assigned in a 1:1 ratio to receive intravenous tixagevimab 300 mg-cilgavimab 300 mg or placebo, in addition to remdesivir and other standard care. Patients were excluded if they had acute organ failure including receipt of invasive mechanical ventilation, extracorporeal membrane oxygenation, vasopressor therapy, mechanical circulatory support, or new renal replacement therapy. The study drug was prepared by an unmasked pharmacist; study participants, site study staff, investigators, and clinical providers were masked to study assignment. The primary outcome was time to sustained recovery up to day 90, defined as 14 consecutive days at home after hospital discharge, with co-primary analyses for the full cohort and for participants who were neutralising antibody-negative at baseline. Efficacy and safety analyses were done in the modified intention-to-treat population, defined as participants who received a complete or partial infusion of tixagevimab-cilgavimab or placebo. This study is registered with ClinicalTrials.gov, NCT04501978 and the participant follow-up is ongoing.
Findings From Feb 10 to Sept 30, 2021, 1455 patients were randomly assigned and 1417 in the primary modified intention-to-treat population were infused with tixagevimab-cilgavimab (n=710) or placebo (n=707). The estimated cumulative incidence of sustained recovery was 89% for tixagevimab-cilgavimab and 86% for placebo group participants at day 90 in the full cohort (recovery rate ratio [RRR] 1•08 [95% CI 0•97-1•20]; p=0•21). Results were similar in the seronegative subgroup (RRR 1•14 [0•97-1•34]; p=0•13). Mortality was lower in the tixagevimabcilgavimab group (61 [9%]) versus placebo group (86 [12%]; hazard ratio [HR] 0•70 [95% CI 0•50-0•97]; p=0•032). The composite safety outcome occurred in 178 (25%) tixagevimab-cilgavimab and 212 (30%) placebo group participants (HR 0•83 [0•68-1•01]; p=0•059). Serious adverse events occurred in 34 (5%) participants in the tixagevimab-cilgavimab group and 38 (5%) in the placebo group. Interpretation Among patients hospitalised with COVID-19 receiving remdesivir and other standard care, tixagevimab-cilgavimab did not improve the primary outcome of time to sustained recovery but was safe and mortality was lower.
Contributors JDN, TAM, NE, and GG directly accessed and verified the underlying data. JDL, HCL, JDN, AGB, VJD, ACG, ESH, VK, and BTT were responsible for conceptualisation. All authors were responsible for the investigation and reviewing and editing the manuscript. JDN, TAM, NE, and GG were responsible for data curation. TAM, NE, and GG were responsible for formal analysis JDN, AGB, VJD, ACG, VK, BTT, and JDL were responsible for funding acquisition. JDL, HCL, JDN, AGB, VJD, ACG, ESH, VK, GM, and BTT were responsible for supervision. TLH, AAG, and RP composed the initial manuscript.
Declaration of interests TLH reports consulting fees from Lysovant, royalties for UpToDate topic authorship, and participation on a Staphylococcus Aureus Network Adaptive Platform Trial Data and Safety Monitoring Board (DSMB), outside of the submitted work. AAG reports grants from US National Institutes of Health (NIH) during the conduct of the study, grants from US Centers for Disease Control (CDC), US Department of Defense (DOD), AbbVie, and Faron Pharmaceuticals, and participation on a NIH DSMB, outside of the submitted work. RP reports grants from Gilead, ViiV, and MSD and consulting fees from Gilead, ViiV, MSD, Theratechnologies, and Eli Lilly, outside of the submitted work. TAM reports grants from National Institute of Allergies and Infectious Diseases (NIAID), NIH, and Leidos, outside of the submitted work. GG reports partial salary support from NIH through the University of Minnesota..
References
Abani, Abbas, Abbas, Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial, Lancet
Beigel, Tomashek, Dodd, Remdesivir for the treatment of Covid-19 --final report, N Engl J Med
Bruel, Hadjadj, Maes, Serum neutralization of SARS-CoV-2 Omicron sublineages BA.1 and BA.2 in patients receiving monoclonal antibodies, Nat Med
Cameroni, Bowen, Rosen, Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift, Nature
Cohen, Nirula, Mulligan, Effect of Bamlanivimab vs Placebo on incidence of COVID-19 among residents and staff of skilled nursing and assisted living facilities: a randomized clinical trial, JAMA
Dejnirattisai, Zhou, Supasa, Antibody evasion by the P.1 strain of SARS-CoV-2, Cell
Dong, Zost, Greaney, Genetic and structural basis for SARS-CoV-2 variant neutralization by a two-antibody cocktail, Nat Microbiol
Dougan, Nirula, Azizad, Bamlanivimab plus etesevimab in mild or moderate Covid-19, N Engl J Med
Fine, Gray, A proportional hazards model for the subdistribution of a competing risk, J Am Stat Assoc
Gray, A class of K-sample tests for comparing the cumulative incidence of a competing risk, Ann Stat
Gupta, Gonzalez-Rojas, Juarez, Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab, N Engl J Med
Holm, A simple sequentially rejective multiple test procedure, Scand J Stat
Iketani, Liu, Guo, Antibody evasion properties of SARS-CoV-2 Omicron sublineages, Nature
Junqueira, Crespo, Ranjbar, Fc-R-mediated SARS-CoV-2 infection of monocytes activates inflammation, Nature
Liu, Ginn, Dejnirattisai, Reduced neutralization of SARS-CoV-2 B.1.617 by vaccine and convalescent serum, Cell
Loo, Mctamney, Arends, The SARS-CoV-2 monoclonal antibody combination, AZD7442, is protective in nonhuman primates and has an extended half-life in humans, Sci Transl Med, doi:10.1126/scitranslmed.abl8124
Lundgren, Grund, Barkauskas, A neutralizing monoclonal antibody for hospitalized patients with Covid-19, N Engl J Med
Lundgren, Grund, Barkauskas, Responses to a neutralizing monoclonal antibody for hospitalized patients with covid-19 according to baseline antibody and antigen levels: a randomized controlled trial, Ann Intern Med
Murray, Babiker, Baker, Design and implementation of an international, multi-arm, multi-stage platform master protocol for trials of novel SARS-CoV-2 antiviral agents: Therapeutics for Inpatients with COVID-19 (TICO/ACTIV-3), Clin Trials
O'brien, Forleo-Neto, Musser, Subcutaneous REGEN-COV antibody combination to prevent Covid-19
Oganesyan, Damschroder, Woods, Cook, Wu et al., Structural characterization of a human Fc fragment engineered for extended serum half-life, Mol Immunol
Oganesyan, Gao, Shirinian, Wu, Dall et al., Structural characterization of a human Fc fragment engineered for lack of effector functions, Acta Crystallogr D Biol Crystallogr
Pocock, Ariti, Collier, Wang, The win ratio: a new approach to the analysis of composite endpoints in clinical trials based on clinical priorities, Eur Heart J
Rockett, Basile, Maddocks, Resistance mutations in SARS-CoV-2 delta variant after sotrovimab use, N Engl J Med
Somersan-Karayaka, Mylonakis, Menon, Casirivimab and imdevimab for the treatment of hospitalized patients with COVID-19, doi:10.1101/2021.11.05.21265656
Takashita, Kinoshita, Yamayoshi, Efficacy of antibodies and antiviral drugs against Covid-19 omicron variant, N Engl J Med
Vanblargan, Errico, Halfmann, An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies, Nat Med
Weinreich, Sivapalasingam, Norton, REGEN-COV Antibody combination and outcomes in outpatients with Covid-19, N Engl J Med
Zhou, Dejnirattisai, Supasa, Evidence of escape of SARS-CoV-2 variant B.1.351 from natural and vaccine-induced sera, Cell
Zhou, Latouche, Rocha, Fine, Competing risks regression for stratified data, Biometrics
DOI record:
{
"DOI": "10.1016/s2213-2600(22)00215-6",
"ISSN": [
"2213-2600"
],
"URL": "http://dx.doi.org/10.1016/S2213-2600(22)00215-6",
"alternative-id": [
"S2213260022002156"
],
"container-title": "The Lancet Respiratory Medicine",
"container-title-short": "The Lancet Respiratory Medicine",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
7,
8
]
],
"date-time": "2022-07-08T23:49:32Z",
"timestamp": 1657324172000
},
"deposited": {
"date-parts": [
[
2022,
7,
8
]
],
"date-time": "2022-07-08T23:49:49Z",
"timestamp": 1657324189000
},
"indexed": {
"date-parts": [
[
2022,
7,
9
]
],
"date-time": "2022-07-09T00:13:09Z",
"timestamp": 1657325589108
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
7
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
7,
1
]
],
"date-time": "2022-07-01T00:00:00Z",
"timestamp": 1656633600000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2213260022002156?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2213260022002156?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022,
7
]
]
},
"published-print": {
"date-parts": [
[
2022,
7
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1056/NEJMoa2108163",
"article-title": "REGEN-COV Antibody combination and outcomes in outpatients with Covid-19",
"author": "Weinreich",
"doi-asserted-by": "crossref",
"first-page": "e81",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(22)00215-6_bib1",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2107934",
"article-title": "Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab",
"author": "Gupta",
"doi-asserted-by": "crossref",
"first-page": "1941",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(22)00215-6_bib2",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2102685",
"article-title": "Bamlanivimab plus etesevimab in mild or moderate Covid-19",
"author": "Dougan",
"doi-asserted-by": "crossref",
"first-page": "1382",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(22)00215-6_bib3",
"volume": "385",
"year": "2021"
},
{
"key": "10.1016/S2213-2600(22)00215-6_bib4",
"series-title": "Letter of authorization for emergency use of EVUSHELD (tixagevimab and cilgavimab)",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2109682",
"article-title": "Subcutaneous REGEN-COV antibody combination to prevent Covid-19",
"author": "O'Brien",
"doi-asserted-by": "crossref",
"first-page": "1184",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(22)00215-6_bib5",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1001/jama.2021.8828",
"article-title": "Effect of Bamlanivimab vs Placebo on incidence of COVID-19 among residents and staff of skilled nursing and assisted living facilities: a randomized clinical trial",
"author": "Cohen",
"doi-asserted-by": "crossref",
"first-page": "46",
"journal-title": "JAMA",
"key": "10.1016/S2213-2600(22)00215-6_bib6",
"volume": "326",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(22)00163-5",
"article-title": "Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial",
"author": "Abani",
"doi-asserted-by": "crossref",
"first-page": "665",
"journal-title": "Lancet",
"key": "10.1016/S2213-2600(22)00215-6_bib7",
"volume": "399",
"year": "2022"
},
{
"DOI": "10.7326/M21-3507",
"article-title": "Responses to a neutralizing monoclonal antibody for hospitalized patients with covid-19 according to baseline antibody and antigen levels: a randomized controlled trial",
"author": "Lundgren",
"doi-asserted-by": "crossref",
"first-page": "234",
"journal-title": "Ann Intern Med",
"key": "10.1016/S2213-2600(22)00215-6_bib8",
"volume": "175",
"year": "2022"
},
{
"DOI": "10.1016/S1473-3099(21)00751-9",
"article-title": "Efficacy and safety of two neutralising monoclonal antibody therapies, sotrovimab and BRII-196 plus BRII-198, for adults hospitalised with COVID-19 (TICO): a randomised controlled trial",
"doi-asserted-by": "crossref",
"first-page": "622",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/S2213-2600(22)00215-6_bib9",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1038/s41591-021-01678-y",
"article-title": "An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies",
"author": "VanBlargan",
"doi-asserted-by": "crossref",
"first-page": "490",
"journal-title": "Nat Med",
"key": "10.1016/S2213-2600(22)00215-6_bib10",
"volume": "28",
"year": "2022"
},
{
"DOI": "10.1056/NEJMc2119407",
"article-title": "Efficacy of antibodies and antiviral drugs against Covid-19 omicron variant",
"author": "Takashita",
"doi-asserted-by": "crossref",
"first-page": "995",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(22)00215-6_bib11",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1038/s41586-022-04594-4",
"article-title": "Antibody evasion properties of SARS-CoV-2 Omicron sublineages",
"author": "Iketani",
"doi-asserted-by": "crossref",
"first-page": "553",
"journal-title": "Nature",
"key": "10.1016/S2213-2600(22)00215-6_bib13",
"volume": "604",
"year": "2022"
},
{
"DOI": "10.1038/s41586-021-04386-2",
"article-title": "Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift",
"author": "Cameroni",
"doi-asserted-by": "crossref",
"first-page": "664",
"journal-title": "Nature",
"key": "10.1016/S2213-2600(22)00215-6_bib14",
"volume": "602",
"year": "2022"
},
{
"DOI": "10.1056/NEJMc2120219",
"article-title": "Resistance mutations in SARS-CoV-2 delta variant after sotrovimab use",
"author": "Rockett",
"doi-asserted-by": "crossref",
"first-page": "1477",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(22)00215-6_bib15",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1038/s41564-021-00972-2",
"article-title": "Genetic and structural basis for SARS-CoV-2 variant neutralization by a two-antibody cocktail",
"author": "Dong",
"doi-asserted-by": "crossref",
"first-page": "1233",
"journal-title": "Nat Microbiol",
"key": "10.1016/S2213-2600(22)00215-6_bib16",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1126/scitranslmed.abl8124",
"article-title": "The SARS-CoV-2 monoclonal antibody combination, AZD7442, is protective in nonhuman primates and has an extended half-life in humans",
"author": "Loo",
"doi-asserted-by": "crossref",
"journal-title": "Sci Transl Med",
"key": "10.1016/S2213-2600(22)00215-6_bib17",
"year": "2022"
},
{
"DOI": "10.1016/j.molimm.2009.01.026",
"article-title": "Structural characterization of a human Fc fragment engineered for extended serum half-life",
"author": "Oganesyan",
"doi-asserted-by": "crossref",
"first-page": "1750",
"journal-title": "Mol Immunol",
"key": "10.1016/S2213-2600(22)00215-6_bib18",
"volume": "46",
"year": "2009"
},
{
"DOI": "10.1107/S0907444908007877",
"article-title": "Structural characterization of a human Fc fragment engineered for lack of effector functions",
"author": "Oganesyan",
"doi-asserted-by": "crossref",
"first-page": "700",
"journal-title": "Acta Crystallogr D Biol Crystallogr",
"key": "10.1016/S2213-2600(22)00215-6_bib19",
"volume": "64",
"year": "2008"
},
{
"DOI": "10.1177/17407745211049829",
"article-title": "Design and implementation of an international, multi-arm, multi-stage platform master protocol for trials of novel SARS-CoV-2 antiviral agents: Therapeutics for Inpatients with COVID-19 (TICO/ACTIV-3)",
"author": "Murray",
"doi-asserted-by": "crossref",
"first-page": "52",
"journal-title": "Clin Trials",
"key": "10.1016/S2213-2600(22)00215-6_bib20",
"volume": "19",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2033130",
"article-title": "A neutralizing monoclonal antibody for hospitalized patients with Covid-19",
"author": "Lundgren",
"doi-asserted-by": "crossref",
"first-page": "905",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(22)00215-6_bib21",
"volume": "384",
"year": "2021"
},
{
"article-title": "A simple sequentially rejective multiple test procedure",
"author": "Holm",
"first-page": "65",
"journal-title": "Scand J Stat",
"key": "10.1016/S2213-2600(22)00215-6_bib23",
"volume": "6",
"year": "1979"
},
{
"DOI": "10.1214/aos/1176350951",
"article-title": "A class of K-sample tests for comparing the cumulative incidence of a competing risk",
"author": "Gray",
"doi-asserted-by": "crossref",
"first-page": "1141",
"journal-title": "Ann Stat",
"key": "10.1016/S2213-2600(22)00215-6_bib24",
"volume": "16",
"year": "1988"
},
{
"DOI": "10.1080/01621459.1999.10474144",
"article-title": "A proportional hazards model for the subdistribution of a competing risk",
"author": "Fine",
"doi-asserted-by": "crossref",
"first-page": "496",
"journal-title": "J Am Stat Assoc",
"key": "10.1016/S2213-2600(22)00215-6_bib25",
"volume": "94",
"year": "1999"
},
{
"DOI": "10.1111/j.1541-0420.2010.01493.x",
"article-title": "Competing risks regression for stratified data",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "661",
"journal-title": "Biometrics",
"key": "10.1016/S2213-2600(22)00215-6_bib26",
"volume": "67",
"year": "2011"
},
{
"DOI": "10.1093/eurheartj/ehr352",
"article-title": "The win ratio: a new approach to the analysis of composite endpoints in clinical trials based on clinical priorities",
"author": "Pocock",
"doi-asserted-by": "crossref",
"first-page": "176",
"journal-title": "Eur Heart J",
"key": "10.1016/S2213-2600(22)00215-6_bib27",
"volume": "33",
"year": "2012"
},
{
"article-title": "Casirivimab and imdevimab for the treatment of hospitalized patients with COVID-19",
"author": "Somersan-Karayaka",
"journal-title": "medRxiv",
"key": "10.1016/S2213-2600(22)00215-6_bib28",
"year": "2021"
},
{
"DOI": "10.1038/s41586-022-04702-4",
"article-title": "Fc-R-mediated SARS-CoV-2 infection of monocytes activates inflammation",
"author": "Junqueira",
"doi-asserted-by": "crossref",
"first-page": "576",
"journal-title": "Nature",
"key": "10.1016/S2213-2600(22)00215-6_bib29",
"volume": "606",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the treatment of Covid-19 -- final report",
"author": "Beigel",
"doi-asserted-by": "crossref",
"first-page": "1813",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(22)00215-6_bib30",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2021.02.037",
"article-title": "Evidence of escape of SARS-CoV-2 variant B.1.351 from natural and vaccine-induced sera",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "2348",
"journal-title": "Cell",
"key": "10.1016/S2213-2600(22)00215-6_bib31",
"volume": "184",
"year": "2021"
},
{
"DOI": "10.1016/j.cell.2021.03.055",
"article-title": "Antibody evasion by the P.1 strain of SARS-CoV-2",
"author": "Dejnirattisai",
"doi-asserted-by": "crossref",
"first-page": "2939",
"journal-title": "Cell",
"key": "10.1016/S2213-2600(22)00215-6_bib32",
"volume": "184",
"year": "2021"
},
{
"DOI": "10.1016/j.cell.2021.06.020",
"article-title": "Reduced neutralization of SARS-CoV-2 B.1.617 by vaccine and convalescent serum",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "4220",
"journal-title": "Cell",
"key": "10.1016/S2213-2600(22)00215-6_bib33",
"volume": "184",
"year": "2021"
},
{
"DOI": "10.1038/s41591-022-01792-5",
"article-title": "Serum neutralization of SARS-CoV-2 Omicron sublineages BA.1 and BA.2 in patients receiving monoclonal antibodies",
"author": "Bruel",
"doi-asserted-by": "crossref",
"first-page": "1297",
"journal-title": "Nat Med",
"key": "10.1016/S2213-2600(22)00215-6_bib34",
"volume": "28",
"year": "2022"
}
],
"reference-count": 32,
"references-count": 32,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2213260022002156"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pulmonary and Respiratory Medicine"
],
"subtitle": [],
"title": "Tixagevimab–cilgavimab for treatment of patients hospitalised with COVID-19: a randomised, double-blind, phase 3 trial",
"type": "journal-article"
}
