Effectiveness of Regdanvimab Treatment in High-Risk COVID-19 Patients to Prevent Progression to Severe Disease
et al., Frontiers in Immunology, doi:10.3389/fimmu.2021.772320, Nov 2021
39th treatment shown to reduce risk in
March 2022, now with p = 0.00049 from 12 studies, recognized in 27 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 778 mild COVID-19 patients showing significantly lower progression to severe disease with regdanvimab treatment.
Confounding by treatment propensity. This study analyzes a population
where only a fraction of eligible patients received the treatment. Patients
receiving treatment may be more likely to follow other recommendations, more
likely to receive additional care, and more likely to use additional
treatments that are not tracked in the data (e.g., nasal/oral hygiene1,2, vitamin D3, etc.) — either because the physician
recommending regdanvimab also recommended them, or
because the patient seeking out regdanvimab is more
likely to be familiar with the efficacy of additional treatments and more
likely to take the time to use them.
Therefore, these kind of studies may
overestimate efficacy.
Efficacy is variant dependent. In Vitro research suggests a lack of efficacy for omicron BA.2, BA.4, BA.54, ХВВ.1.9.1, XBB.1.9.3, XBB.1.5.24, XBB.1.16, XBB.2.9, BQ.1.1.45, CL.1, and CH.1.15.
|
risk of death, 58.9% lower, RR 0.41, p = 1.00, treatment 0 of 234 (0.0%), control 1 of 544 (0.2%), NNT 544, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
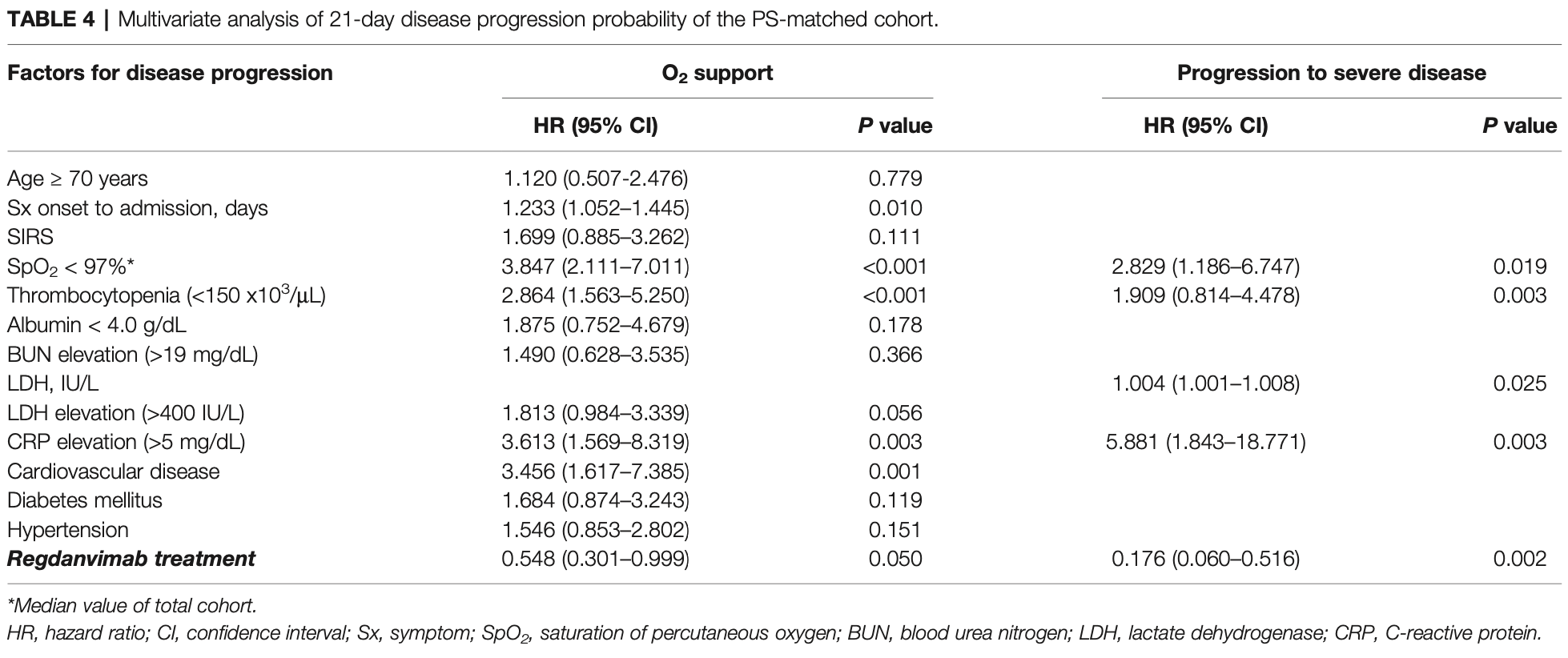
risk of severe case, 82.4% lower, HR 0.18, p = 0.002, treatment 234, control 234, adjusted per study, propensity score matching, multivariable.
|
|
risk of oxygen therapy, 45.2% lower, HR 0.55, p = 0.05, treatment 234, control 234, adjusted per study, propensity score matching, multivariable.
|
|
time to discharge, 8.3% lower, relative time 0.92, p = 0.001, treatment 234, control 544.
|
|
risk of no hospital discharge, 76.8% lower, RR 0.23, p < 0.001, treatment 5 of 234 (2.1%), control 50 of 544 (9.2%), NNT 14.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Lee et al., 23 Nov 2021, retrospective, South Korea, peer-reviewed, 10 authors, study period 26 November, 2020 - 28 February, 2021.
Contact: sshhissh@gmail.com, krpeck@skku.edu.
Effectiveness of Regdanvimab Treatment in High-Risk COVID-19 Patients to Prevent Progression to Severe Disease
Frontiers in Immunology, doi:10.3389/fimmu.2021.772320
Objective: To evaluate clinical effectiveness of regdanvimab, a monoclonal antibody agent for treating coronavirus 2019 . Methods: A retrospective cohort study was conducted at two general hospitals during the study period of December 2020 to May 2021. Mild COVID-19 patients with risk factors for disease progression admitted to the hospitals within seven days of symptom onset were enrolled and followed until discharge or referral. Multivariate analyses for disease progression were conducted in the total and propensity score (PS)-matched cohorts. Results: A total of 778 mild COVID-19 patients were included and classified as the regdanvimab (n = 234) and supportive care (n = 544) groups. Significantly fewer patients required O 2 supplementation via nasal prong in the regdanvimab group (8.1%) than in the supportive care group (18.4%, P < 0.001). The decreased risk for O 2 support by regdanvimab treatment was noticed in the multivariate analysis of the total cohort (HR 0.570, 95% CI 0.343-0.946, P = 0.030), but it was not statistically significant in the PSmatched cohort (P = 0.057). Progression to severe disease was also significantly lower in the regdanvimab group (2.1%) than in the supportive care group (9.6%, P < 0.001). The significantly reduced risk for progression to severe disease by regdanvimab treatment was observed in the analysis of both the total cohort (HR 0.262, 95% CI 0.103-0.667, P = 0.005) and PS-matched cohort (HR 0.176, 95% CI 0.060-0.516, P = 0.002). Potential risk factors for progression were investigated in the supportive care group and SpO 2 < 97% and CRP elevation >1.5 mg/dL were common risk factors for O 2 support and progression to severe disease. Among the patients with any of these factors, regdanvimab treatment was associated with decreased risk for progression to severe disease with slightly lower HR (HR 0.202, 95% CI 0.062-0.657, P = 0.008) than that of the total cohort.
ETHICS STATEMENT The studies involving human participants were reviewed and approved by Samsung Medical Center. Written informed consent for participation was not required for this study in acc ordance with the national l egis lation and the institutional requirements.
AUTHOR CONTRIBUTIONS JiL, JeL, J-HK, SS, and KP contributed to the conceptualization. JiL, JeL, J-HK, MH, HK, SC, YDL, JS, and SS, and KP contributed to the investigation. J-HK and SS contributed to the statistical analysis. KP contributed to the supervision. J-HK, SS, and KP contributed to the writing, review, and editing. All authors contributed to the article and approved the submitted version.
SUPPLEMENTARY MATERIAL The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.772320/ full#supplementary-material Conflict of Interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Publisher's Note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. Copyright © 2021 Lee, Lee, Ko, Hyun, Kim, Cho, Lee, Song, Shin and Peck. This is..
References
Chen, Nirula, Heller, Gottlieb, Boscia et al., SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients With Covid-19, N Engl J Med, doi:10.1056/NEJMoa2029849
Dougan, Nirula, Azizad, Mocherla, Gottlieb et al., Bamlanivimab Plus Etesevimab in Mild or Moderate Covid-19, N Engl J Med, doi:10.1056/NEJMoa2102685
Ema, EMA Review of Regdanvimab for COVID-19 to Support National Decisions on Early Use
Group, A Neutralizing Monoclonal Antibody for Hospitalized Patients With Covid-19, N Engl J Med, doi:10.1056/NEJMoa2033130
Kdca, Regular Situation Report for COVID-19
Kim, Kim, Huh, Choi, Kim et al., Korean Society of Infectious Diseases/National Evidence-Based Healthcare Collaborating Agency Recommendations for Anti-SARS-CoV-2 Monoclonal Antibody Treatment of Patients With COVID-19, Infect Chemother, doi:10.3947/ic.2021.0304
Kim, Ryu, Lee, Kim, Seo et al., A Therapeutic Neutralizing Antibody Targeting Receptor Binding Domain of SARS-CoV-2 Spike Protein, Nat Commun, doi:10.1038/s41467-020-20602-5
Mfds, Approval of Regdanvimab for the Treatment of COVID-19
Moon, Lee, Park, Yun, Lee et al., Clinical Characteristics and Mortality Predictors of COVID-19 Patients Hospitalized at Nationally-Designated Treatment Hospitals, J Korean Med Sci, doi:10.3346/jkms.2020.35.e328
Peck, Early Diagnosis and Rapid Isolation: Response to COVID-19 Outbreak in Korea, Clin Microbiol Infect, doi:10.1016/j.cmi.2020.04.025
Rosenbaum, Db, Reducing Bias in Observational Studies Using Subclassification on the Propensity Score, J Am Stat Assoc, doi:10.1080/01621459.1984.10478078
Suh, Lee, Park, Clinical Characteristics of COVID-19: Risk Factors for Early Oxygen Requirement After Hospitalization, J Korean Med Sci, doi:10.3346/jkms.2021.36.e139
Sung, Kim, Heo, Seo, Ys et al., Clinical Course and Outcomes of 3,060 Patients With Coronavirus Disease 2019 in Korea, January-May 2020, J Korean Med Sci, doi:10.3346/jkms.2020.35.e280
Verderese, Stepanova, Lam, Racila, Kolacevski et al., Neutralizing Monoclonal Antibody Treatment Reduces Hospitalization for Mild and Moderate COVID-19: A Real-World Experience, Clin Infect Dis, doi:10.1093/cid/ciab579
Weinreich, Sivapalasingam, Norton, Ali, Gao et al., REGEN-COV Antibody Combination and Outcomes in Outpatients With Covid-19, N Engl J Med, doi:10.1056/NEJMoa2108163
Weinreich, Sivapalasingam, Norton, Ali, Gao et al., REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients With Covid-19, N Engl J Med, doi:10.1056/NEJMoa2035002
Who, WHO Coronavirus (COVID-19) Dashboard
Wolff, Nee, Hickey, Marschollek, Risk Factors for Covid-19 Severity and Fatality: A Structured Literature Review, Infection, doi:10.1007/s15010-020-01509-1
Yoon, Lee, Kim, Peck, A Systematic Narrative Review of Comprehensive Preparedness Strategies of Healthcare Resources for a Large Resurgence of COVID-19 Nationally, With Local or Regional Epidemics: Present Era and Beyond, J Korean Med Sci, doi:10.3346/jkms.2020.35.e387
DOI record:
{
"DOI": "10.3389/fimmu.2021.772320",
"ISSN": [
"1664-3224"
],
"URL": "http://dx.doi.org/10.3389/fimmu.2021.772320",
"abstract": "<jats:sec><jats:title>Objective</jats:title><jats:p>To evaluate clinical effectiveness of regdanvimab, a monoclonal antibody agent for treating coronavirus 2019 (COVID-19).</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>A retrospective cohort study was conducted at two general hospitals during the study period of December 2020 to May 2021. Mild COVID-19 patients with risk factors for disease progression admitted to the hospitals within seven days of symptom onset were enrolled and followed until discharge or referral. Multivariate analyses for disease progression were conducted in the total and propensity score (PS)-matched cohorts.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>A total of 778 mild COVID-19 patients were included and classified as the regdanvimab (n = 234) and supportive care (n = 544) groups. Significantly fewer patients required O<jats:sub>2</jats:sub> supplementation <jats:italic>via</jats:italic> nasal prong in the regdanvimab group (8.1%) than in the supportive care group (18.4%, <jats:italic>P</jats:italic> &lt; 0.001). The decreased risk for O<jats:sub>2</jats:sub> support by regdanvimab treatment was noticed in the multivariate analysis of the total cohort (HR 0.570, 95% CI 0.343–0.946, <jats:italic>P</jats:italic> = 0.030), but it was not statistically significant in the PS-matched cohort (<jats:italic>P</jats:italic> = 0.057). Progression to severe disease was also significantly lower in the regdanvimab group (2.1%) than in the supportive care group (9.6%, <jats:italic>P</jats:italic> &lt; 0.001). The significantly reduced risk for progression to severe disease by regdanvimab treatment was observed in the analysis of both the total cohort (HR 0.262, 95% CI 0.103–0.667, <jats:italic>P</jats:italic> = 0.005) and PS-matched cohort (HR 0.176, 95% CI 0.060–0.516, <jats:italic>P</jats:italic> = 0.002). Potential risk factors for progression were investigated in the supportive care group and SpO<jats:sub>2</jats:sub> &lt; 97% and CRP elevation &gt;1.5 mg/dL were common risk factors for O<jats:sub>2</jats:sub> support and progression to severe disease. Among the patients with any of these factors, regdanvimab treatment was associated with decreased risk for progression to severe disease with slightly lower HR (HR 0.202, 95% CI 0.062–0.657, <jats:italic>P</jats:italic> = 0.008) than that of the total cohort.</jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p>Regdanvimab treatment was associated with a decreased risk of progression to severe disease.</jats:p></jats:sec>",
"alternative-id": [
"10.3389/fimmu.2021.772320"
],
"author": [
{
"affiliation": [],
"family": "Lee",
"given": "Ji Yeon",
"sequence": "first"
},
{
"affiliation": [],
"family": "Lee",
"given": "Jee Young",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ko",
"given": "Jae-Hoon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hyun",
"given": "Miri",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kim",
"given": "Hyun Ah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cho",
"given": "Seongcheol",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lee",
"given": "Yong Dae",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Song",
"given": "Junghoon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shin",
"given": "Seunghwan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Peck",
"given": "Kyong Ran",
"sequence": "additional"
}
],
"container-title": "Frontiers in Immunology",
"container-title-short": "Front. Immunol.",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"frontiersin.org"
]
},
"created": {
"date-parts": [
[
2021,
12,
1
]
],
"date-time": "2021-12-01T03:00:49Z",
"timestamp": 1638327649000
},
"deposited": {
"date-parts": [
[
2021,
12,
1
]
],
"date-time": "2021-12-01T03:00:52Z",
"timestamp": 1638327652000
},
"funder": [
{
"DOI": "10.13039/100018688",
"doi-asserted-by": "publisher",
"name": "Korea Disease Control and Prevention Agency"
}
],
"indexed": {
"date-parts": [
[
2024,
3,
9
]
],
"date-time": "2024-03-09T17:40:47Z",
"timestamp": 1710006047886
},
"is-referenced-by-count": 27,
"issued": {
"date-parts": [
[
2021,
11,
23
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
11,
23
]
],
"date-time": "2021-11-23T00:00:00Z",
"timestamp": 1637625600000
}
}
],
"link": [
{
"URL": "https://www.frontiersin.org/articles/10.3389/fimmu.2021.772320/full",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1965",
"original-title": [],
"prefix": "10.3389",
"published": {
"date-parts": [
[
2021,
11,
23
]
]
},
"published-online": {
"date-parts": [
[
2021,
11,
23
]
]
},
"publisher": "Frontiers Media SA",
"reference": [
{
"key": "B1",
"unstructured": "WHO Coronavirus (COVID-19) Dashboard2021"
},
{
"DOI": "10.1056/NEJMoa2033130",
"article-title": "A Neutralizing Monoclonal Antibody for Hospitalized Patients With Covid-19",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "B2",
"volume": "384",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2035002",
"article-title": "REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients With Covid-19",
"author": "Weinreich",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "B3",
"volume": "384",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciab579",
"article-title": "Neutralizing Monoclonal Antibody Treatment Reduces Hospitalization for Mild and Moderate COVID-19: A Real-World Experience",
"author": "Verderese",
"doi-asserted-by": "publisher",
"journal-title": "Clin Infect Dis",
"key": "B4",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2029849",
"article-title": "SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients With Covid-19",
"author": "Chen",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "B5",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1038/s41467-020-20602-5",
"article-title": "A Therapeutic Neutralizing Antibody Targeting Receptor Binding Domain of SARS-CoV-2 Spike Protein",
"author": "Kim",
"doi-asserted-by": "publisher",
"first-page": "288",
"journal-title": "Nat Commun",
"key": "B6",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2108163",
"article-title": "REGEN-COV Antibody Combination and Outcomes in Outpatients With Covid-19",
"author": "Weinreich",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "B7",
"year": "2021"
},
{
"key": "B8",
"unstructured": "Fact Sheet For Healthcare Providers Emergency Use Authorization (EUA) Of Sotrovimab2021"
},
{
"DOI": "10.1056/NEJMoa2102685",
"article-title": "Bamlanivimab Plus Etesevimab in Mild or Moderate Covid-19",
"author": "Dougan",
"doi-asserted-by": "publisher",
"journal-title": "N Engl J Med",
"key": "B9",
"volume": "385",
"year": "2021"
},
{
"key": "B10",
"unstructured": "COVID-19 Treatment Guidelines, Anti-SARS-CoV-2 Monoclonal Antibodies2021"
},
{
"key": "B11",
"unstructured": "Approval of Regdanvimab for the Treatment of COVID-19, February 5, 20212021"
},
{
"key": "B12",
"unstructured": "EMA Review of Regdanvimab for COVID-19 to Support National Decisions on Early Use2021"
},
{
"DOI": "10.3947/ic.2021.0304",
"article-title": "Korean Society of Infectious Diseases/National Evidence-Based Healthcare Collaborating Agency Recommendations for Anti-SARS-CoV-2 Monoclonal Antibody Treatment of Patients With COVID-19",
"author": "Kim",
"doi-asserted-by": "publisher",
"first-page": "395",
"journal-title": "Infect Chemother",
"key": "B13",
"volume": "53",
"year": "2021"
},
{
"key": "B14",
"unstructured": "Celltrion’s Covid-19 Drug Regdanvimab Meets Phase III Endpoints2021"
},
{
"DOI": "10.3346/jkms.2020.35.e280",
"article-title": "Clinical Course and Outcomes of 3,060 Patients With Coronavirus Disease 2019 in Korea, January–May 2020",
"author": "Sung",
"doi-asserted-by": "publisher",
"journal-title": "J Korean Med Sci",
"key": "B15",
"volume": "35",
"year": "2020"
},
{
"DOI": "10.1080/01621459.1984.10478078",
"article-title": "Reducing Bias in Observational Studies Using Subclassification on the Propensity Score",
"author": "Rosenbaum",
"doi-asserted-by": "publisher",
"journal-title": "J Am Stat Assoc",
"key": "B16",
"volume": "79",
"year": "1984"
},
{
"DOI": "10.3346/jkms.2021.36.e139",
"article-title": "Clinical Characteristics of COVID-19: Risk Factors for Early Oxygen Requirement After Hospitalization",
"author": "Suh",
"doi-asserted-by": "publisher",
"journal-title": "J Korean Med Sci",
"key": "B17",
"volume": "36",
"year": "2021"
},
{
"DOI": "10.1007/s15010-020-01509-1",
"article-title": "Risk Factors for Covid-19 Severity and Fatality: A Structured Literature Review",
"author": "Wolff",
"doi-asserted-by": "publisher",
"first-page": "15",
"journal-title": "Infection",
"key": "B18",
"volume": "49",
"year": "2021"
},
{
"DOI": "10.3346/jkms.2020.35.e328",
"article-title": "Clinical Characteristics and Mortality Predictors of COVID-19 Patients Hospitalized at Nationally-Designated Treatment Hospitals",
"author": "Moon",
"doi-asserted-by": "publisher",
"first-page": "e328",
"journal-title": "J Korean Med Sci",
"key": "B19",
"volume": "35",
"year": "2020"
},
{
"key": "B20",
"unstructured": "Fact Sheet For Health Care Providers Emergency Use Authorization Of REGEN-COV"
},
{
"DOI": "10.3346/jkms.2020.35.e387",
"article-title": "A Systematic Narrative Review of Comprehensive Preparedness Strategies of Healthcare Resources for a Large Resurgence of COVID-19 Nationally, With Local or Regional Epidemics: Present Era and Beyond",
"author": "Yoon",
"doi-asserted-by": "publisher",
"first-page": "e387",
"journal-title": "J Korean Med Sci",
"key": "B21",
"volume": "35",
"year": "2020"
},
{
"DOI": "10.1016/j.cmi.2020.04.025",
"article-title": "Early Diagnosis and Rapid Isolation: Response to COVID-19 Outbreak in Korea",
"author": "Peck",
"doi-asserted-by": "publisher",
"journal-title": "Clin Microbiol Infect",
"key": "B22",
"volume": "26",
"year": "2020"
},
{
"key": "B23",
"unstructured": "Regular Situation Report for COVID-19, June 1, 20212021"
}
],
"reference-count": 23,
"references-count": 23,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.frontiersin.org/articles/10.3389/fimmu.2021.772320/full"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Immunology",
"Immunology and Allergy"
],
"subtitle": [],
"title": "Effectiveness of Regdanvimab Treatment in High-Risk COVID-19 Patients to Prevent Progression to Severe Disease",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.3389/crossmark-policy",
"volume": "12"
}