Vitamin D Status and Immune Response in Hospitalized Patients with Moderate and Severe COVID-19
et al., Pharmaceuticals, doi:10.3390/ph15030305, Mar 2022
Vitamin D for COVID-19
8th treatment shown to reduce risk in
October 2020, now with p < 0.00000000001 from 136 studies, recognized in 18 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 331 hospitalized patients in Russia, showing lower risk of severe cases with higher vitamin D levels.
This is the 124th of 228 COVID-19 sufficiency studies for vitamin D, which collectively show higher levels reduce risk with p<0.0000000001.
|
risk of severe case, 22.5% lower, OR 0.78, p = 0.01, cutoff 11.4ng/mL, adjusted per study, inverted to make OR<1 favor high D levels (≥11.4ng/mL), multivariable, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Karonova et al., 2 Mar 2022, retrospective, Russia, peer-reviewed, 11 authors, study period 30 November, 2020 - 20 March, 2021.
Vitamin D Status and Immune Response in Hospitalized Patients with Moderate and Severe COVID-19
Pharmaceuticals, doi:10.3390/ph15030305
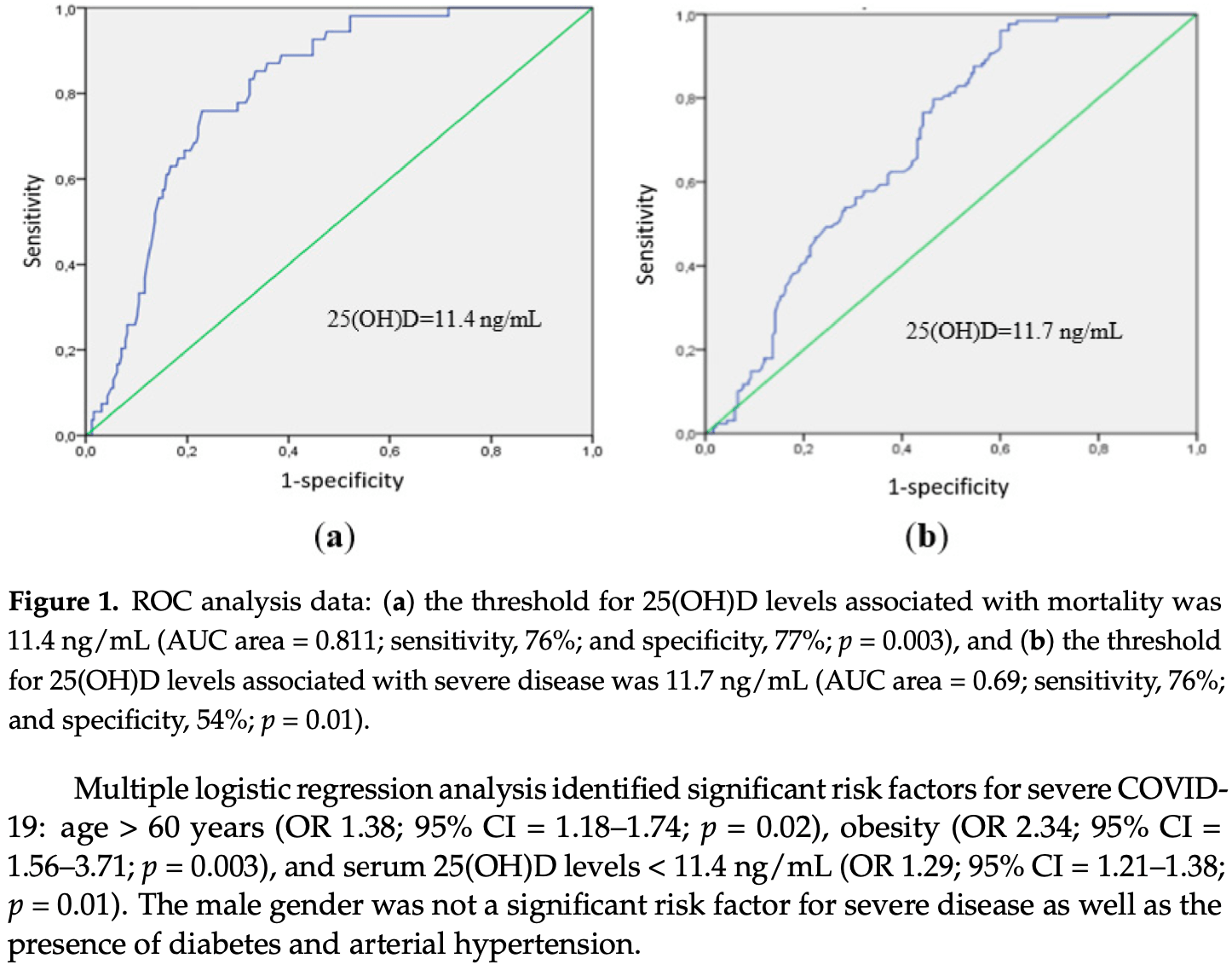
A low 25-hydroxyvitamin D (25(OH)D) level is considered as an independent risk factor for COVID-19 severity. However, the association between vitamin D status and outcomes in COVID-19 is controversial. In the present study we investigate the association between the serum 25(OH)D level, immune response, and clinical disease course in patients with COVID-19. A total of 311 patients hospitalized with COVID-19 were enrolled. For patients with a vitamin D deficiency/insufficiency, the prevalence of severe COVID-19 was higher than in those with a normal 25(OH)D level (p < 0.001). The threshold of 25(OH)D level associated with mortality was 11.4 ng/mL (p = 0.003, ROC analysis). The frequency of CD3+CD4+ T helper (Th) cells was decreased in patients with 25(OH)D level ≤ 11.4 ng/mL, compared to healthy controls (HCs). There were no differences in the frequency of naive, central memory (CM), effector memory (EM), and terminally differentiated effector memory Th cells in patients with COVID-19 compared to HCs. The frequency of T-follicular helpers was decreased both in patients with 25(OH)D level > 11.4 ng/mL (p < 0.001) and 25(OH)D level ≤ 11.4 ng/mL (p = 0.003) compared to HCs. Patients with 25(OH)D level > 11.4 ng/mL had an increased frequency of Th2 CM (p = 0.010) and decreased Th17 CM (p < 0.001). While the frequency of Th2 EM was significantly increased, the frequency of Th17 EM was significantly decreased in both groups compared to HCs. Thus, 25(OH)D level is an independent risk factor for the disease severity and mortality in patients with COVID-19. We demonstrate that the serum 25(OH)D level ≤ 11.4 ng/mL is associated with the stimulation of Th2 and the downregulation of Th17 cell polarization of the adaptive immunity in patients with COVID-19.
Conflicts of Interest: The authors declare no conflict of interest.
References
Alsafar, Grant, Hijazi, Uddin, Alkaabi et al., COVID-19 Disease Severity and Death in Relation to Vitamin D Status among SARS-CoV-2-Positive UAE Residents, Nutrients, doi:10.3390/nu13051714
Aranow, Vitamin D and the immune system, J. Investig. Med, doi:10.2310/JIM.0b013e31821b8755
Baeke, Korf, Overbergh, Van Etten, Verstuyf et al., Human T lymphocytes are direct targets of 1,25-dihydroxyvitamin D3 in the immune system, J. Steroid Biochem. Mol. Biol, doi:10.1016/j.jsbmb.2010.03.037
Baeke, Takiishi, Korf, Gysemans, Mathieu et al., Modulator of the immune system, Curr. Opin. Pharmacol, doi:10.1016/j.coph.2010.04.001
Barbarash, Kudryavtsev, Rutkovskaya, Golovkin, Cell Response in Patients with Implanted Biological and Mechanical Prosthetic Heart Valves, Mediat. Inflamm, doi:10.1155/2016/1937564
Boonstra, Barrat, Crain, Heath, Savelkoul et al., 1alpha,25-Dihydroxyvitamin d3 has a direct effect on I CD4(+) T cells to enhance the development of Th2 cells, J. Immunol, doi:10.4049/jimmunol.167.9.4974
Braun, Loyal, Frentsch, Wendisch, Georg et al., SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19, Nature, doi:10.1038/s41586-020-2598-9
Carpagnano, Di Lecce, Quaranta, Zito, Buonamico et al., Vitamin D deficiency as a predictor of poor prognosis in patients with acute respiratory failure due to COVID-19, J. Endocrinol. Investig, doi:10.1007/s40618-020-01370-x
Cascella, Rajnik, Aleem, Dulebohn, Di Napoli et al., Evaluation, and Treatment of Coronavirus (COVID-19)
Chiodini, Gatti, Soranna, Merlotti, Mingiano et al., Vitamin D Status and SARS-CoV-2 Infection and COVID-19 Clinical Outcomes, Front. Public Health
Chun, Liu, Modlin, Adams, Hewison, Impact of vitamin D on immune function: Lessons learned from genome-wide analysis, Front. Physiol, doi:10.3389/fphys.2014.00151
Currie, Findlay, Mchugh, Mackellar, Man et al., The human cathelicidin LL-37 has antiviral activity against respiratory syncytial virus, PLoS ONE, doi:10.1371/journal.pone.0073659
Cutolo, Paolino, Smith, Evidences for a protective role of vitamin D in COVID-19, RMD Open, doi:10.1136/rmdopen-2020-001454
Dankers, Davelaar, Van Hamburg, Van De Peppel, Colin et al., Human Memory Th17 Cell Populations Change into Anti-inflammatory Cells with Regulatory Capacity Upon Exposure to Active Vitamin D, Front. Immunol, doi:10.3389/fimmu.2019.01504
Dissanayake, De Silva, Sumanatilleke, De Silva, Gamage et al., Prognostic and therapeutic role of vitamin D in COVID-19: Systematic review and meta-analysis, J. Clin. Endocrinol. Metab, doi:10.1210/clinem/dgab892
Dupuis, Pagano, Pierdominici, Ortona, The role of vitamin D in autoimmune diseases: Could sex make the difference?, Biol. Sex Differ, doi:10.1186/s13293-021-00358-3
Gallelli, Mannino, Luciani, De Sire, Mancuso et al., Vitamin D Serum Levels in Subjects Tested for SARS-CoV-2: What Are the Differences among Acute, Healed, and Negative COVID-19 Patients? A Multicenter Real-Practice Study, Nutrients, doi:10.3390/nu13113932
Golovkin, Kalinina, Bezrukikh, Aquino, Zaikova et al., Imbalanced Immune Response of T cell and B cell Subsets in Patients with Moderate and Severe COVID-19, Viruses, doi:10.3390/v13101966
Grant, Al Anouti, Boucher, Dursun, Gezen-Ak et al., A Narrative Review of the Evidence for Variations in Serum 25-Hydroxyvitamin D Concentration Thresholds for Optimal Health, Nutrients, doi:10.3390/nu14030639
Heaney, Functional indices of vitamin D status and ramifications of vitamin D deficiency, Am. J. Clin. Nutr, doi:10.1093/ajcn/80.6.1706S
Hewison, Freeman, Hughes, Evans, Bland et al., Differential regulation of vitamin D receptor and its ligand in human monocyte-derived dendritic cells, J. Immunol, doi:10.4049/jimmunol.170.11.5382
Holick, Binkley, Bischoff-Ferrari, Gordon, Hanley et al., Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline, J. Clin. Endocrinol. Metab, doi:10.1210/jc.2011-0385
Holick, Vitamin, Deficiency, None, N. Engl. J. Med, doi:10.1056/NEJMra070553
Joshi, Pantalena, Liu, Gaffen, Liu et al., 1,25-dihydroxyvitamin D(3) ameliorates Th17 autoimmunity via transcriptional modulation of interleukin-17A, Mol. Cell. Biol, doi:10.1128/MCB.05020-11
Karonova, Andreeva, Golovatuk, Bykova, Simanenkova et al., Low 25(OH)D Level is Associated with Severe Course and Poor Prognosis in COVID-19, Nutrients, doi:10.3390/nu13093021
Khalifa, Swilam, El-Wahed, Du, El-Seedi et al., Beyond the Pandemic: COVID-19 Pandemic Changed the Face of Life, Int. J. Environ. Res. Public Health, doi:10.3390/ijerph18115645
Kota, Sabbah, Chang, Harnack, Xiang et al., Role of human beta-defensin-2 during tumor necrosis factor-alpha/NF-kappaB-mediated innate antiviral response against human respiratory syncytial virus, J. Biol. Chem, doi:10.1074/jbc.M710415200
Kudryavtsev, Kalinina, Bezrukikh, Melnik, Golovkin, The significance of phenotyping and quantification of plasma extracellular vesicles levels using high-sensitivity flow cytometry during COVID-19 treatment, Viruses, doi:10.3390/v13050767
Kudryavtsev, Serebriakova, Zhiduleva, Murtazalieva, Titov et al., CD73 Rather Than CD39 Is Mainly Involved in Controlling Purinergic Signaling in Calcified Aortic Valve Disease, Front. Genet, doi:10.3389/fgene.2019.00604
Macaya, Espejo Paeres, Valls, Fernandez-Ortiz, Gonzalez Del Castillo et al., Interaction between age and vitamin D deficiency in severe COVID-19 infection, Nutr. Hosp
Malkova, Kudlay, Kudryavtsev, Starshinova, Yablonskiy et al., Immunogenetic Predictors of Severe COVID-19, Vaccines, doi:10.3390/vaccines9030211
Meinken, Kamen, Wagner, Bals, Steinmeyer et al., Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response, Science, doi:10.1126/science.1123933
Mercola, Grant, Wagner, Evidence Regarding Vitamin D and Risk of COVID-19 and Its Severity, Nutrients, doi:10.3390/nu12113361
Morita, Schmitt, Bentebibel, Ranganathan, Bourdery et al., Human blood CXCR5(+) CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion, Immunity, doi:10.1016/j.immuni.2010.12.012
Panagiotou, Tee, Ihsan, Athar, Marchitelli et al., Low serum 25-hydroxyvitamin D (25[OH]D) levels in patients hospitalized with COVID-19 are associated with greater disease severity, Clin. Endocrinol, doi:10.1111/cen.14276
Pizzini, Aichner, Sahanic, Bohm, Egger et al., Impact of Vitamin D Deficiency on COVID-19-A Prospective Analysis from the CovILD Registry, Nutrients
Prevention, Diagnosis and Treatment of New Coronavirus Infection (COVID-19)
Rydyznski Moderbacher, Ramirez, Dan, Grifoni, Hastie et al., Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity, Cell, doi:10.1016/j.cell.2020.09.038
Sallusto, Lenig, Förster, Lipp, Lanzavecchia, Two subsets of memory T lymphocytes with distinct homing potentials and effector functions, Nature, doi:10.1038/44385
Selvaraj, Harishankar, Afsal, Vitamin, Immuno-modulation and tuberculosis treatment, Can. J. Physiol. Pharmacol, doi:10.1139/cjpp-2014-0386
Sette, Crotty, Adaptive immunity to SARS-CoV-2 and COVID-19, Cell, doi:10.1016/j.cell.2021.01.007
Sigmundsdottir, Pan, Debes, Alt, Habtezion et al., DCs metabolize sunlight-induced vitamin D to 'program' T cell attraction to the epidermal chemokine CCL27, Nat. Immunol, doi:10.1038/ni1433
Tan, Linster, Tan, Le Bert, Chia et al., Early induction of functional SARS-CoV-2-specific T cells associates with rapid viral clearance and mild disease in COVID-19 patients, Cell Rep, doi:10.1016/j.celrep.2021.108728
Tang, Zhou, Luger, Zhu, Silver et al., Calcitriol suppresses antiretinal autoimmunity through inhibitory effects on the Th17 effector response, J. Immunol, doi:10.4049/jimmunol.0801543
Todosenko, Vulf, Yurova, Khaziakhmatova, Mikhailova et al., Causal Links between Hypovitaminosis D and Dysregulation of the T Cell Connection of Immunity Associated with Obesity and Concomitant Pathologies, Biomedicines, doi:10.3390/biomedicines9121750
Urashima, Segawa, Okazaki, Kurihara, Wada et al., Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren, Am. J. Clin. Nutr, doi:10.3945/ajcn.2009.29094
Urry, Chambers, Xystrakis, Dimeloe, Richards et al., The role of 1α,25-dihydroxyvitamin D3 and cytokines in the promotion of distinct Foxp3+ and IL-10+ CD4+ T cells, Eur. J. Immunol, doi:10.1002/eji.201242370
Wang, Nestel, Bourdeau, Nagai, Wang et al., Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression, J. Immunol, doi:10.4049/jimmunol.173.5.2909
Wu, Sun, Vitamin D, vitamin D receptor, and macroautophagy in inflammation and infection, Discov. Med
DOI record:
{
"DOI": "10.3390/ph15030305",
"ISSN": [
"1424-8247"
],
"URL": "http://dx.doi.org/10.3390/ph15030305",
"abstract": "<jats:p>A low 25-hydroxyvitamin D (25(OH)D) level is considered as an independent risk factor for COVID-19 severity. However, the association between vitamin D status and outcomes in COVID-19 is controversial. In the present study we investigate the association between the serum 25(OH)D level, immune response, and clinical disease course in patients with COVID-19. A total of 311 patients hospitalized with COVID-19 were enrolled. For patients with a vitamin D deficiency/insufficiency, the prevalence of severe COVID-19 was higher than in those with a normal 25(OH)D level (p < 0.001). The threshold of 25(OH)D level associated with mortality was 11.4 ng/mL (p = 0.003, ROC analysis). The frequency of CD3+CD4+ T helper (Th) cells was decreased in patients with 25(OH)D level ≤ 11.4 ng/mL, compared to healthy controls (HCs). There were no differences in the frequency of naive, central memory (CM), effector memory (EM), and terminally differentiated effector memory Th cells in patients with COVID-19 compared to HCs. The frequency of T-follicular helpers was decreased both in patients with 25(OH)D level > 11.4 ng/mL (p < 0.001) and 25(OH)D level ≤ 11.4 ng/mL (p = 0.003) compared to HCs. Patients with 25(OH)D level > 11.4 ng/mL had an increased frequency of Th2 CM (p = 0.010) and decreased Th17 CM (p < 0.001). While the frequency of Th2 EM was significantly increased, the frequency of Th17 EM was significantly decreased in both groups compared to HCs. Thus, 25(OH)D level is an independent risk factor for the disease severity and mortality in patients with COVID-19. We demonstrate that the serum 25(OH)D level ≤ 11.4 ng/mL is associated with the stimulation of Th2 and the downregulation of Th17 cell polarization of the adaptive immunity in patients with COVID-19.</jats:p>",
"alternative-id": [
"ph15030305"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-1547-0123",
"affiliation": [],
"authenticated-orcid": false,
"family": "Karonova",
"given": "Tatiana L.",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0001-7204-7850",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kudryavtsev",
"given": "Igor V.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0651-7110",
"affiliation": [],
"authenticated-orcid": false,
"family": "Golovatyuk",
"given": "Ksenia A.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6516-7184",
"affiliation": [],
"authenticated-orcid": false,
"family": "Aquino",
"given": "Arthur D.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1916-5705",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kalinina",
"given": "Olga V.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4878-6909",
"affiliation": [],
"authenticated-orcid": false,
"family": "Chernikova",
"given": "Alena T.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0584-2330",
"affiliation": [],
"authenticated-orcid": false,
"family": "Zaikova",
"given": "Ekaterina K.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1808-1331",
"affiliation": [],
"authenticated-orcid": false,
"family": "Lebedev",
"given": "Denis A.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9342-507X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Bykova",
"given": "Ekaterina S.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7577-628X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Golovkin",
"given": "Alexey S.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2929-0980",
"affiliation": [],
"authenticated-orcid": false,
"family": "Shlyakhto",
"given": "Evgeny V.",
"sequence": "additional"
}
],
"container-title": "Pharmaceuticals",
"container-title-short": "Pharmaceuticals",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
3,
3
]
],
"date-time": "2022-03-03T03:53:25Z",
"timestamp": 1646279605000
},
"deposited": {
"date-parts": [
[
2022,
3,
3
]
],
"date-time": "2022-03-03T04:33:35Z",
"timestamp": 1646282015000
},
"indexed": {
"date-parts": [
[
2024,
4,
2
]
],
"date-time": "2024-04-02T15:11:53Z",
"timestamp": 1712070713868
},
"is-referenced-by-count": 12,
"issue": "3",
"issued": {
"date-parts": [
[
2022,
3,
2
]
]
},
"journal-issue": {
"issue": "3",
"published-online": {
"date-parts": [
[
2022,
3
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
3,
2
]
],
"date-time": "2022-03-02T00:00:00Z",
"timestamp": 1646179200000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/1424-8247/15/3/305/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "305",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2022,
3,
2
]
]
},
"published-online": {
"date-parts": [
[
2022,
3,
2
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.3390/ijerph18115645",
"doi-asserted-by": "publisher",
"key": "ref1"
},
{
"article-title": "Features, Evaluation, and Treatment of Coronavirus (COVID-19)",
"author": "Cascella",
"key": "ref2",
"series-title": "StatPearls [Internet]",
"year": "2022"
},
{
"DOI": "10.1139/cjpp-2014-0386",
"doi-asserted-by": "publisher",
"key": "ref3"
},
{
"DOI": "10.1126/science.1123933",
"doi-asserted-by": "publisher",
"key": "ref4"
},
{
"DOI": "10.4049/jimmunol.173.5.2909",
"doi-asserted-by": "publisher",
"key": "ref5"
},
{
"DOI": "10.3945/ajcn.2009.29094",
"doi-asserted-by": "publisher",
"key": "ref6"
},
{
"DOI": "10.3390/nu12113361",
"doi-asserted-by": "publisher",
"key": "ref7"
},
{
"DOI": "10.1111/cen.14276",
"doi-asserted-by": "publisher",
"key": "ref8"
},
{
"DOI": "10.1007/s40618-020-01370-x",
"doi-asserted-by": "publisher",
"key": "ref9"
},
{
"DOI": "10.3390/nu12092775",
"doi-asserted-by": "publisher",
"key": "ref10"
},
{
"DOI": "10.20960/nh.03193",
"doi-asserted-by": "publisher",
"key": "ref11"
},
{
"DOI": "10.3390/nu13093021",
"doi-asserted-by": "publisher",
"key": "ref12"
},
{
"DOI": "10.1210/clinem/dgab892",
"doi-asserted-by": "publisher",
"key": "ref13"
},
{
"DOI": "10.1056/NEJMra070553",
"doi-asserted-by": "publisher",
"key": "ref14"
},
{
"DOI": "10.1093/ajcn/80.6.1706S",
"doi-asserted-by": "publisher",
"key": "ref15"
},
{
"DOI": "10.1016/j.jsbmb.2010.03.037",
"doi-asserted-by": "publisher",
"key": "ref16"
},
{
"DOI": "10.4049/jimmunol.170.11.5382",
"doi-asserted-by": "publisher",
"key": "ref17"
},
{
"DOI": "10.1371/journal.pone.0073659",
"doi-asserted-by": "publisher",
"key": "ref18"
},
{
"DOI": "10.1074/jbc.M710415200",
"doi-asserted-by": "publisher",
"key": "ref19"
},
{
"DOI": "10.1371/journal.pone.0138152",
"doi-asserted-by": "publisher",
"key": "ref20"
},
{
"DOI": "10.1136/rmdopen-2020-001454",
"doi-asserted-by": "publisher",
"key": "ref21"
},
{
"DOI": "10.4049/jimmunol.167.9.4974",
"doi-asserted-by": "publisher",
"key": "ref22"
},
{
"DOI": "10.2310/JIM.0b013e31821b8755",
"doi-asserted-by": "publisher",
"key": "ref23"
},
{
"DOI": "10.3390/nu14030639",
"doi-asserted-by": "publisher",
"key": "ref24"
},
{
"DOI": "10.1016/j.immuni.2010.12.012",
"doi-asserted-by": "publisher",
"key": "ref25"
},
{
"DOI": "10.3389/fpubh.2021.736665",
"doi-asserted-by": "publisher",
"key": "ref26"
},
{
"DOI": "10.3390/nu13051714",
"doi-asserted-by": "publisher",
"key": "ref27"
},
{
"DOI": "10.3390/v13101966",
"doi-asserted-by": "publisher",
"key": "ref28"
},
{
"DOI": "10.3390/v13050767",
"doi-asserted-by": "publisher",
"key": "ref29"
},
{
"DOI": "10.3390/vaccines9030211",
"doi-asserted-by": "publisher",
"key": "ref30"
},
{
"DOI": "10.3390/nu13113932",
"doi-asserted-by": "publisher",
"key": "ref31"
},
{
"DOI": "10.1186/s13293-021-00358-3",
"doi-asserted-by": "publisher",
"key": "ref32"
},
{
"DOI": "10.1016/j.coph.2010.04.001",
"doi-asserted-by": "publisher",
"key": "ref33"
},
{
"DOI": "10.3389/fphys.2014.00151",
"doi-asserted-by": "publisher",
"key": "ref34"
},
{
"DOI": "10.1128/MCB.05020-11",
"doi-asserted-by": "publisher",
"key": "ref35"
},
{
"DOI": "10.4049/jimmunol.0801543",
"doi-asserted-by": "publisher",
"key": "ref36"
},
{
"DOI": "10.3390/biomedicines9121750",
"doi-asserted-by": "publisher",
"key": "ref37"
},
{
"DOI": "10.1002/eji.201242370",
"doi-asserted-by": "publisher",
"key": "ref38"
},
{
"DOI": "10.1038/ni1433",
"doi-asserted-by": "publisher",
"key": "ref39"
},
{
"DOI": "10.3389/fimmu.2019.01504",
"doi-asserted-by": "publisher",
"key": "ref40"
},
{
"DOI": "10.1016/j.cell.2021.01.007",
"doi-asserted-by": "publisher",
"key": "ref41"
},
{
"DOI": "10.1016/j.cell.2020.09.038",
"doi-asserted-by": "publisher",
"key": "ref42"
},
{
"DOI": "10.1016/j.celrep.2021.108728",
"doi-asserted-by": "publisher",
"key": "ref43"
},
{
"DOI": "10.1038/s41586-020-2598-9",
"doi-asserted-by": "publisher",
"key": "ref44"
},
{
"key": "ref45",
"unstructured": "Prevention, Diagnosis and Treatment of New Coronavirus Infection (COVID-19)http://www.consultant.ru/law/hotdocs/65400.html/"
},
{
"DOI": "10.1210/jc.2011-0385",
"doi-asserted-by": "publisher",
"key": "ref46"
},
{
"DOI": "10.1038/44385",
"doi-asserted-by": "publisher",
"key": "ref47"
},
{
"DOI": "10.1155/2016/1937564",
"doi-asserted-by": "publisher",
"key": "ref48"
},
{
"DOI": "10.3389/fgene.2019.00604",
"doi-asserted-by": "publisher",
"key": "ref49"
}
],
"reference-count": 49,
"references-count": 49,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/1424-8247/15/3/305"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Drug Discovery",
"Pharmaceutical Science",
"Molecular Medicine"
],
"subtitle": [],
"title": "Vitamin D Status and Immune Response in Hospitalized Patients with Moderate and Severe COVID-19",
"type": "journal-article",
"volume": "15"
}
