Convalescent Plasma as Adjunctive Therapy for Hospitalized Patients with COVID-19: The Co-CLARITY Trial
et al., Acta Medica Philippina, doi:10.47895/amp.vi0.4903, Co-CLARITY, NCT04567173, Feb 2024
Early terminated RCT 44 hospitalized COVID-19 patients showing no significant differences with convalescent plasma treatment.
|
risk of death, 400.0% higher, RR 5.00, p = 0.49, treatment 2 of 22 (9.1%), control 0 of 22 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm).
|
|
risk of ICU admission, 100% higher, RR 2.00, p = 1.00, treatment 2 of 22 (9.1%), control 1 of 22 (4.5%).
|
|
hospitalization time, 7.1% higher, relative time 1.07, p = 0.70, treatment 22, control 22.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Gauiran et al., 15 Feb 2024, Randomized Controlled Trial, Philippines, peer-reviewed, median age 60.0, 26 authors, study period 28 September, 2020 - 31 May, 2021, average treatment delay 8.0 days, trial NCT04567173 (history) (Co-CLARITY).
Contact: dvgauiran@up.edu.ph.
Convalescent Plasma as Adjunctive Therapy for Hospitalized Patients with COVID-19: The Co-CLARITY Trial
Acta Medica Philippina, doi:10.47895/amp.vi0.4903
Background and Objective. Convalescent plasma therapy (CPT) may reduce the risk of disease progression among patients with COVID-19. This study was undertaken to evaluate the efficacy and safety of CPT in preventing ICU admission among hospitalized COVID-19 patients.
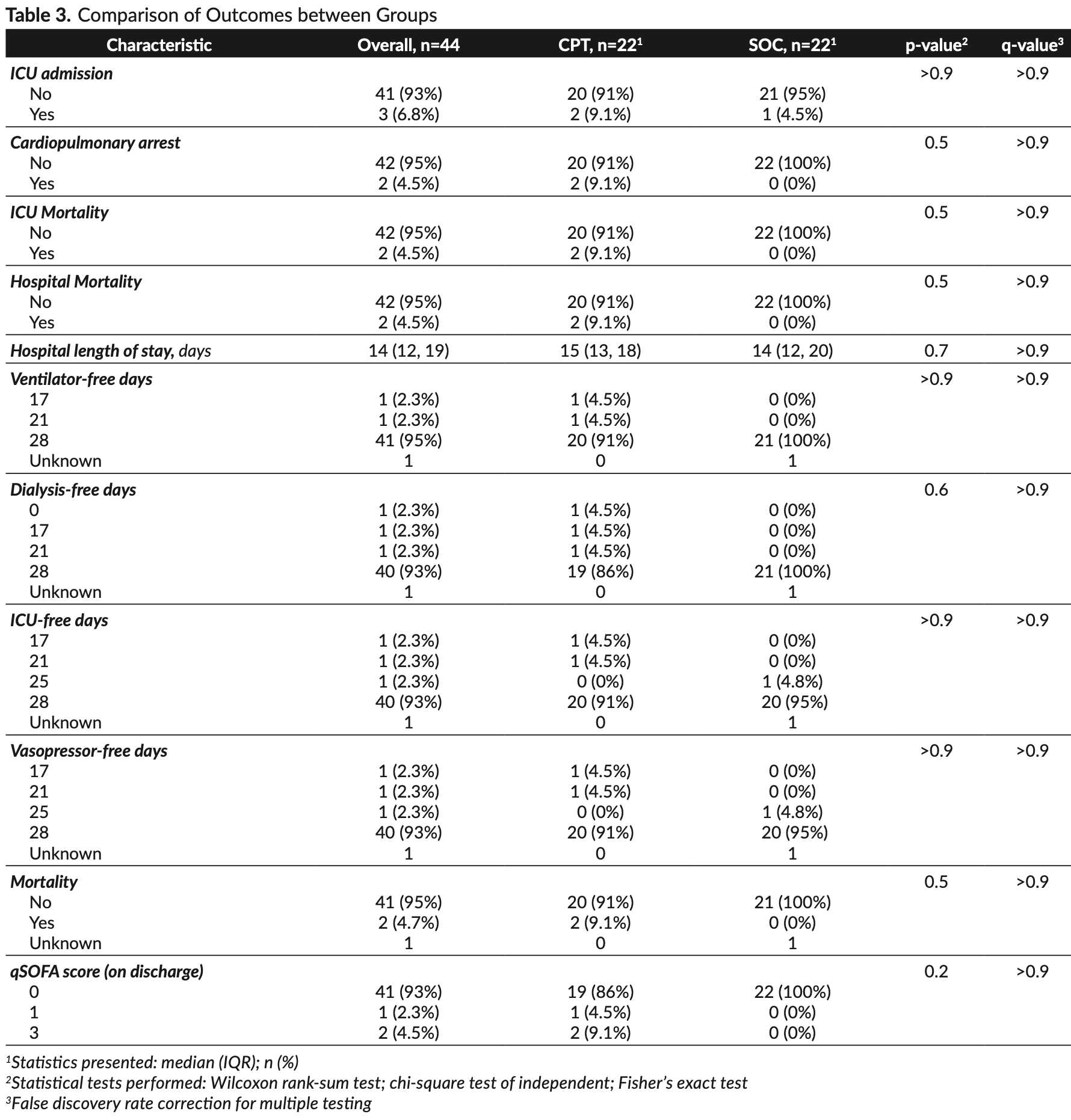
Methods. In this open-label randomized controlled trial, we randomly assigned hospitalized adult patients with COVID-19 in a 1:1 ratio to receive convalescent plasma as an adjunct to standard of care or standard of care alone. The primary endpoint was ICU admission within first 28 days of enrolment. Primary safety endpoints include rapid deterioration of respiratory or clinical status within four hours of convalescent plasma transfusion and cumulative incidence of serious adverse events during the study period including transfusion-related acute lung injury (TRALI), transfusion-associated circulatory overload (TACO), severe allergic reactions, and transfusion-related infections. Results. A total of 22 patients were assigned to receive convalescent plasma as an adjunct to standard of care and 22 to receive standard of care alone. The median time from onset of COVID-19 symptoms to study enrolment was eight days (IQR, 4 to 10). Two patients (9.1%) in the CPT group and one patient (4.5%) in the control group were admitted to the ICU. The primary outcome measure, ICU admission, was not different between the two groups (q-value >0.9). No patient who received convalescent plasma had rapid deterioration of respiratory/clinical status within four hours of transfusion and none developed TRALI, TACO, anaphylaxis, severe allergic reactions, or transfusion-related infections. There was also no significant difference in the secondary outcomes of 28-day mortality (two patients in the CPT group and none in the control group, q-value >0.90), dialysis-free days, vasopressor-free days, and ICU-free days.
Statement of Authorship DTVG, TED, MACA and FMMC contributed in the conceptualization of work, acquisition and analysis of data, drafting and revising of manuscript, and final approval of the version to be published. CDD contributed in the conceptualization of work, acquisition and analysis of data, and final approval of the version to be published. SCM, RNA, AKHQ, JACL, CFNC, ALME, RANK, FMH, LBB, GJCJ, IMSE, MCMS, AFGM, AVM, JDV, JMCJ, PYT, JAL, MMA and MALM contributed in the conceptualization of work, acquisition of data, and final approval of the version to be published. SEAS contributed in the acquisition and analysis of data, and final approval of the version to be published.
Author Disclosure All authors declared no conflicts of interest.
APPENDIx
Study Participant and Convalescent Plasma Donor Recruitment For the last quarter of 2020, there was also a decreasing number of COVID-19 hospitalizations in UP-PGH. By March 2021, there was a surge in number of COVID-19 admissions, however, these patients were already too toxic to be eligible for the trial. The UP-PGH is home to a lot of ongoing clinical trials (e.g., Solidarity, favipiravir, VCO, tocilizumab, etc.) and non-intervention studies. With the limited number of COVID-19 patients in UP-PGH, these could account for the decreasing number of patients allocated/ referred to our clinical trial. All patients admitted in UP-PGH are invited to join these trials and most of them will consent to join. However, once..
References
Abate, Ali, Mantfardo, Basu, Rate of intensive care unit admission and outcomes among patients with coronavirus: a systematic review and meta-analysis, PLoS One, doi:10.1371/journal.pone.0235653
Agarwal, Mukherjee, Kumar, Chatterjee, Bhatnagar et al., Convalescent plasma in the management of moderate COVID-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial), BMJ
Alqahtani, Abdulrahman, Almadani, Alali, Zamrooni et al., Randomized controlled trial of convalescent plasma therapy against standard therapy in patients with severe COVID-19 disease, Sci Rep, doi:10.1038/s41598-021-89444-5
Avendaño-Sola, Ramos-Martinez, Muñez-Rubio, Ruiz-Antorán, De Molina et al., Convalescent plasma for COVID-19: a multicenter, randomized clinical trial, medRxiv
Axfors, Janiaud, Schmitt, Van't Hooft, Smith et al., Association between convalescent plasma treatment and mortality in COVID-19: a collaborative systemic review and meta-analysis of randomized clinical trials, BMC Infect Dis, doi:10.1186/s12879-021-06829-7
Bajpai, Kumar, Maheshwari, Chhabra, Kale et al., COVID-19 patients: a pilot randomized controlled trial
Casadevall, Pirofski, The convalescent sera option for containing COVID-19, J Clin Invest, doi:10.1172/JCI138003
Conopio, Banquirigo, Gargaritano, Vista, Uson, Convalescent plasma therapy in Filipino patients with confirmed COVID-19 infection in a tertiary hospital in Cebu City: a retrospective cohort single center study, Philipp J Intern Med
Garraud, Heshmati, Pozzetto, Lefrere, Girot et al., Plasma therapy against infectious pathogens, as of yesterday, today and tomorrow, Transfus Clin Biol, doi:10.1016/j.tracli.2015.12.003
Gharbharan, Jordans, Geurtsvankessel, Hollander, Karim et al., Convalescent Plasma for COVID-19. A randomized clinical trial, medRxiv
Harris, Research Electronic Data Capture (REDCap) -planning, collecting and managing data for clinical and managing data for clinical and translational research, BMC Bioinformatics, doi:10.1186/1471-2105-13-S12-A15
Harris, Taylor, Minor, Elliott, Fernandez et al., The REDCap consortium: Building an international community of software partners, J Biomed Inform, doi:10.1016/j.jbi.2019.103208
Harris, Taylor, Thielke, Payne, Gonzalez et al., Research electronic data capture (REDCap) -a metadata-driven methodology and workflow process for providing translational research informatics support, J Biomed Inform, doi:10.1016/j.jbi.2008.08.010
Horby, Lim, Emberson, Mafham, Bell et al., Dexamethasone in hospitalized patients with COVID-19, N Engl J Med, doi:10.1056/NEJMoa2021436
Hung, To, Lee, Lee, Chan et al., Convalescent plasma treatment reduced mortality in patients with severe pandemic infuenza A (H1N1) 2009 virus infection, Clin Infect Dis, doi:10.1093/cid/ciq106
Joyner, Wright, Fairweather, Senefeld, Bruno et al., Early safety indicators of COVID-19 convalescent plasma in 5,000 patients, doi:10.1101/2020.05.12.20099879
Klassen, Senefeld, Johnson, Carter, Wiggins et al., The effect of convalescent plasma therapy on COVID-19 patient mortality: systematic review and meta-analysis, Mayo Clin Proc
Li, Zhang, Hu, Tong, Zheng et al., Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial, JAMA, doi:10.1001/jama.2020.10044
Libster, Marc, Wappner, Coviello, Bianchi et al., Early high-titer plasma therapy to prevent severe COVID-19 in older adults, N Engl J Med, doi:10.1056/NEJMoa2033700
Mesina, Julian, Relos, Torres, Comia et al., Use of convalescent plasma therapy with Best Available Treatment (BAT) among hospitalized COVID-19 patients: a multicenter study, J Blood Lymph
Mesina, Mangahas, Gatchalian, Ariola-Ramos, Torres, Use of convalescent plasma therapy among hospitalized Coronavirus Disease 2019 (COVID-19) patients: a single-center experience, Philipp J Intern Med
Quero, Pajares, Evasan, Clinical profiles and outcomes of COVID-19 patients receiving convalescent plasma under compassionate use in the Philippine General Hospital
Ray, Paul, Bandopadhyay, 'rozario, Sarif et al., Clinical and immunological benefits of convalescent plasma therapy in severe COVID-19: insights from a single center open label randomised control trial, medRxiv
Rezagholizadeh, Khiali, Sarbaksh, Entezari-Maleki, Remdesivir for treatment of COVID-19; an updated systematic review and metaanalysis, Eur J Pharmacol, doi:10.1016/j.ejphar.2021.173926
Sahr, Ansumana, Massaquoi, Idriss, Sesay et al., Evaluation of convalescent whole blood for treating Ebola Virus Disease in Freetown, Sierra Leone, J Infect, doi:10.1016/j.jinf.2016.11.009
Simonovich, Pratx, Scibona, Beruto, Vallone et al., A randomized trial of convalescent plasma in COVID-19 severe pneumonia, N Engl J Med, doi:10.1056/NEJMoa2031304
Soo, Cheng, Wong, Hui, Lee et al., Retrospective comparison of convalescent plasma with continuing high-dose methylprednisolone treatment in SARS patients, Clin Microbiol Infect, doi:10.1111/j.1469-0691.2004.00956.x
Wang, Hu, Chang, Zhu, Liu et al., Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China, JAMA, doi:10.1001/jama.2020.1585
Wei, Lin, Wei, Chen, He et al., Tocilizumab treatment for COVID-19 patients: a systematic review and metaanalysis, Infect Dis Poverty, doi:10.1186/s40249-021-00857-w
DOI record:
{
"DOI": "10.47895/amp.vi0.4903",
"ISSN": [
"0001-6071",
"2094-9278"
],
"URL": "http://dx.doi.org/10.47895/amp.vi0.4903",
"container-title": "Acta Medica Philippina",
"container-title-short": "Acta Med Philipp",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
2,
15
]
],
"date-time": "2023-02-15T09:43:05Z",
"timestamp": 1676454185000
},
"deposited": {
"date-parts": [
[
2023,
2,
15
]
],
"date-time": "2023-02-15T09:43:07Z",
"timestamp": 1676454187000
},
"indexed": {
"date-parts": [
[
2023,
2,
16
]
],
"date-time": "2023-02-16T05:53:48Z",
"timestamp": 1676526828227
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023
]
]
},
"member": "27557",
"original-title": [],
"prefix": "10.47895",
"published": {
"date-parts": [
[
2023
]
]
},
"published-online": {
"date-parts": [
[
2023
]
]
},
"publisher": "University of the Philippines Manila",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://actamedicaphilippina.upm.edu.ph/index.php/acta/article/view/4903"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Convalescent Plasma as Adjunctive Therapy for Hospitalized Patients with COVID-19: The Co-CLARITY Trial",
"type": "journal-article"
}