A single-center retrospective cohort study of Covid-19 medications: Remdesivir, Favipiravir, Methylprednisolone, Dexamethasone, and Interferon β1a and their combinations
et al., medRxiv, doi:10.1101/2021.03.05.21251351, Mar 2021
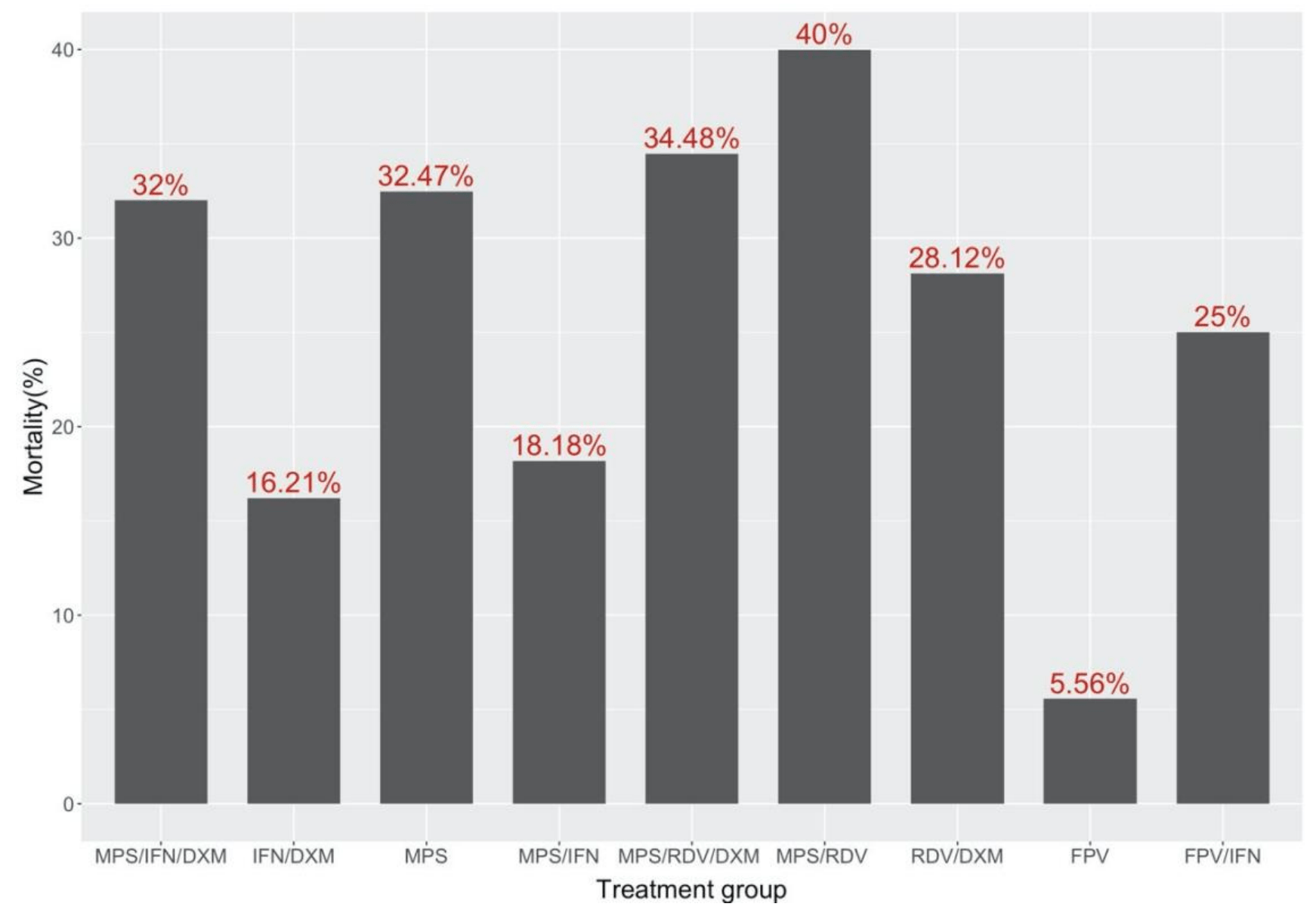
Retrospective 324 hospitalized patients in Iran reporting on the use remdesivir, favipiravir, methylprednisolone, dexamethasone, and Interferon β1a and their combinations. There is no control group in this study.
Study covers favipiravir, dexamethasone, and remdesivir.
Fateh et al., 8 Mar 2021, retrospective, Iran, preprint, 7 authors.
A single-center retrospective cohort study of Covid-19 medications: Remdesivir, Favipiravir, Methylprednisolone, Dexamethasone, and Interferon β1a and their combinations
doi:10.1101/2021.03.05.21251351
Many drugs have been suggested to be used for Covid-19. A suitable and efficient choice of drug would make the course of Covid-19 easier. we have investigated the efficacy of different treatment regimen in reducing hospitalization period (HP) and mortality of 324 confirmed Covid-19 patients. Received drugs included single therapy or combinations of Methylprednisolone, Remdesivir, Favipiravir, Interferon β1a, and Dexamethasone. HP and mortality were compared between different treatment groups to evaluate efficacy of each drug. HP and mortality were also calculated for patients in each treatment group based on their underlying diseases and age. we suggest that using IFN-β1a, RDV and corticosteroids might not have a significant effect on the HP or mortality of the Covid-19 patients as it was thought before.
References
Angus, Effect of Hydrocortisone on Mortality and Organ Support in Patients with Severe COVID-19: The REMAP-CAP COVID-19 Corticosteroid Domain Randomized Clinical Trial, JAMA -J. Am. Med. Assoc
Bani-Sadr, Corticosteroid therapy for patients with COVID-19 pneumonia: a before-after study, Int. J. Antimicrob. Agents
Beigel, Remdesivir for the Treatment of Covid-19 -Final Report, N. Engl. J. Med, doi:10.1056/nejmoa2007764
Cai, Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study, Engineering, doi:10.1016/j.eng.2020.03.007
Chen, Favipiravir versus Arbidol for COVID-19: A Randomized Clinical Trial, doi:10.1101/2020.03.17.20037432
Clementi, Ferrarese, Criscuolo, Diotti, Castelli et al., Interferon-β 1a inhibits SARS-CoV-2 in vitro when administered after virus infection Nicola
Core, R: A Language and Environment for Statistical Computing
Dastan, Subcutaneous administration of interferon beta-1a for COVID-19: A noncontrolled prospective trial, Int. Immunopharmacol
Davoudi-Monfared, A randomized clinical trial of the efficacy and safety of interferon β-1a in treatment of severe COVID-19, Antimicrob. Agents Chemother
Dong, Du, Gardner, An interactive web-based dashboard to track COVID-19 in real-time, Lancet Infect. Dis
Fadel, Early Short Course Corticosteroids in Hospitalized Patients with COVID-19
Fragkou, Review of trials currently testing treatment and prevention of COVID-19, Clin. Microbiol. Infect
Furuta, Komeno, Nakamura, Polymerase Activity (%) 100 µ mol / L Favipiravir Favipiravir-RMP Control, Proc Jpn Acad Ser B Phys Biol Sci
Gangopadhyay, Title Page Title : The role of corticosteroids in the management of critically ill patients with coronavirus disease
Goldman, Remdesivir for 5 or 10 Days in Patients with Severe Covid-19, N. Engl. J. Med
Hasan, Mortality in COVID-19 patients with acute respiratory distress syndrome and corticosteroids use: a systematic review and meta-analysis, Expert Rev. Respir. Med
Hu, Clinical Use of Short-Course and Low-Dose Corticosteroids in Patients With Non-severe COVID-19 During Pneumonia Progression, Front. Public Heal
Karlsen, A systematic review of trial registry entries for randomized clinical trials investigating COVID-19 medical prevention and treatment, PLoS One
Lee, Efficacy of Corticosteroids in Patients with SARS, MERS and COVID-19: A Systematic Review and Meta-Analysis, J. Clin. Med
Li, Efficacy Evaluation of Early, Low-Dose, Short-Term Corticosteroids in Adults Hospitalized with Non-Severe COVID-19 Pneumonia: A Retrospective Cohort Study, Infect. Dis. Ther, doi:10.1007/s40121-020-00332-3
Liu, Clinical characteristics and corticosteroids application of different clinical types in patients with coronavirus disease, Sci. Rep
Lou, Clinical Outcomes and Plasma Concentrations of Baloxavir Marboxil and Favipiravir in COVID-19 Patients: An Exploratory Randomized, Controlled Trial, Eur. J. Pharm. Sci, doi:10.1016/j.ejps.2020.105631
Payandemehr, Interferon beta-1a as a Candidate for COVID-19 Treatment;An Open-Label Single-Arm Clinical Trial, Adv. J. Emerg. Med
Ruiz-Irastorza, Second week methylprednisolone pulses improve prognosis in patients with severe coronavirus disease 2019 pneumonia: An observational comparative study using routine care data, PLoS One
Siegel, Discovery and Synthesis of a Phosphoramidate Prodrug of a Pyrrolo[2,1-f][triazin-4-amino] Adenine C-Nucleoside (GS-5734) for the Treatment of Ebola and Emerging Viruses, J. Med. Chem
Spinner, Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients with Moderate COVID-19: A Randomized Clinical Trial, JAMA -J. Am. Med. Assoc
Sterne, Association between Administration of Systemic Corticosteroids and Mortality among Critically Ill Patients with COVID-19: A Meta-analysis, JAMA -J. Am. Med. Assoc
Thoguluva Chandrasekar, Systematic review and meta-analysis of effectiveness of treatment options against SARS-CoV-2 infection, J. Med. Virol, doi:10.1002/jmv.26302
Tlayjeh, Efficacy of Corticosteroids in COVID-19 Patients: A Systematic Review and Meta-Analysis
Usfoodanddrugadministration, Coronavirus (COVID-19) update: FDA issues Emergency Use Authorization for potential COVID-19 treatment
Wang, Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res
Wang, Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial, Lancet
Wu, Systemic corticosteroids show no benefit in severe and critical COVID-19 patients in Wuhan, China: A retrospective cohort study, doi:10.1101/2020.05.11.20097709
Xu, Arbidol/IFN-α2b therapy for patients with coronavirus disease 2019: a retrospective multicenter cohort study, Microbes Infect
Yin, Wunderink, Mers, SARS and other coronaviruses as causes of pneumonia, Respirology
Zha, Corticosteroid treatment of patients with coronavirus disease 2019 (COVID-19), Med. J. Aust
DOI record:
{
"DOI": "10.1101/2021.03.05.21251351",
"URL": "http://dx.doi.org/10.1101/2021.03.05.21251351",
"abstract": "<jats:title>Abstract</jats:title><jats:p>Many drugs have been suggested to be used for Covid-19. A suitable and efficient choice of drug would make the course of Covid-19 easier. we have investigated the efficacy of different treatment regimen in reducing hospitalization period (HP) and mortality of 324 confirmed Covid-19 patients. Received drugs included single therapy or combinations of Methylprednisolone, Remdesivir, Favipiravir, Interferon β1a, and Dexamethasone. HP and mortality were compared between different treatment groups to evaluate efficacy of each drug. HP and mortality were also calculated for patients in each treatment group based on their underlying diseases and age. we suggest that using IFN-β1a, RDV and corticosteroids might not have a significant effect on the HP or mortality of the Covid-19 patients as it was thought before.</jats:p>",
"accepted": {
"date-parts": [
[
2021,
3,
8
]
]
},
"author": [
{
"affiliation": [],
"family": "Fateh",
"given": "Sahand Tehrani",
"sequence": "first"
},
{
"affiliation": [],
"family": "Fateh",
"given": "Sepand Tehrani",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Salehi",
"given": "Esmaeil",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rezai",
"given": "Nima",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Haririan",
"given": "Nazanin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Asgari",
"given": "Abdollah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Salehi-Najafabadi",
"given": "Amir",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
3,
8
]
],
"date-time": "2021-03-08T17:45:19Z",
"timestamp": 1615225519000
},
"deposited": {
"date-parts": [
[
2021,
3,
10
]
],
"date-time": "2021-03-10T18:10:41Z",
"timestamp": 1615399841000
},
"group-title": "Infectious Diseases (except HIV/AIDS)",
"indexed": {
"date-parts": [
[
2022,
4,
2
]
],
"date-time": "2022-04-02T05:54:28Z",
"timestamp": 1648878868157
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2021,
3,
8
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2021.03.05.21251351",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2021,
3,
8
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2021,
3,
8
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference": [
{
"DOI": "10.1016/S1473-3099(20)30120-1",
"doi-asserted-by": "publisher",
"key": "2021031010100751000_2021.03.05.21251351v1.1"
},
{
"DOI": "10.1016/j.cmi.2020.05.019",
"article-title": "Review of trials currently testing treatment and prevention of COVID-19",
"doi-asserted-by": "crossref",
"first-page": "988",
"journal-title": "Clin. Microbiol. Infect",
"key": "2021031010100751000_2021.03.05.21251351v1.2",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1371/journal.pone.0232391",
"doi-asserted-by": "publisher",
"key": "2021031010100751000_2021.03.05.21251351v1.3"
},
{
"DOI": "10.1002/jmv.26302",
"doi-asserted-by": "publisher",
"key": "2021031010100751000_2021.03.05.21251351v1.4"
},
{
"DOI": "10.1016/j.micinf.2020.05.012",
"article-title": "Arbidol/IFN-α2b therapy for patients with coronavirus disease 2019: a retrospective multicenter cohort study",
"doi-asserted-by": "crossref",
"first-page": "200",
"journal-title": "Microbes Infect",
"key": "2021031010100751000_2021.03.05.21251351v1.5",
"volume": "22",
"year": "2020"
},
{
"DOI": "10.1101/2020.03.17.20037432",
"doi-asserted-by": "publisher",
"key": "2021031010100751000_2021.03.05.21251351v1.6"
},
{
"DOI": "10.1016/j.eng.2020.03.007",
"doi-asserted-by": "publisher",
"key": "2021031010100751000_2021.03.05.21251351v1.7"
},
{
"DOI": "10.1101/2020.05.04.20074609",
"doi-asserted-by": "crossref",
"key": "2021031010100751000_2021.03.05.21251351v1.8",
"unstructured": "Fadel Raef, A. M. Early Short Course Corticosteroids in Hospitalized Patients with COVID-19. (2020)."
},
{
"DOI": "10.1101/2020.05.11.20097709",
"doi-asserted-by": "publisher",
"key": "2021031010100751000_2021.03.05.21251351v1.9"
},
{
"key": "2021031010100751000_2021.03.05.21251351v1.10",
"unstructured": "R Core Team. R: A Language and Environment for Statistical Computing. (2020)."
},
{
"key": "2021031010100751000_2021.03.05.21251351v1.11",
"unstructured": "RStudio Team. RStudio: Integrated Development Environment for R. (2020)."
},
{
"DOI": "10.1021/acs.jmedchem.6b01594",
"doi-asserted-by": "publisher",
"key": "2021031010100751000_2021.03.05.21251351v1.12"
},
{
"key": "2021031010100751000_2021.03.05.21251351v1.13",
"unstructured": "USFoodandDrugAdministration. Coronavirus (COVID-19) update: FDA issues Emergency Use Authorization for potential COVID-19 treatment. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-emergency-use-authorization-potential-covid-19-treatment (2020)."
},
{
"DOI": "10.1056/nejmoa2007764",
"doi-asserted-by": "publisher",
"key": "2021031010100751000_2021.03.05.21251351v1.14"
},
{
"DOI": "10.1016/S0140-6736(20)31022-9",
"doi-asserted-by": "publisher",
"key": "2021031010100751000_2021.03.05.21251351v1.15"
},
{
"DOI": "10.1001/jama.2020.16349",
"article-title": "Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients with Moderate COVID-19: A Randomized Clinical Trial",
"doi-asserted-by": "crossref",
"first-page": "1048",
"journal-title": "JAMA - J. Am. Med. Assoc",
"key": "2021031010100751000_2021.03.05.21251351v1.16",
"volume": "324",
"year": "2020"
},
{
"article-title": "Remdesivir for 5 or 10 Days in Patients with Severe Covid-19",
"first-page": "2019",
"journal-title": "N. Engl. J. Med",
"key": "2021031010100751000_2021.03.05.21251351v1.17",
"volume": "2019",
"year": "2020"
},
{
"DOI": "10.2183/pjab.93.027",
"doi-asserted-by": "publisher",
"key": "2021031010100751000_2021.03.05.21251351v1.18"
},
{
"DOI": "10.1016/j.ejps.2020.105631",
"doi-asserted-by": "publisher",
"key": "2021031010100751000_2021.03.05.21251351v1.19"
},
{
"DOI": "10.1111/resp.13196",
"doi-asserted-by": "publisher",
"key": "2021031010100751000_2021.03.05.21251351v1.20"
},
{
"first-page": "1",
"journal-title": "Interferon-β 1a inhibits SARS-CoV-2 in vitro when administered after virus infection Nicola",
"key": "2021031010100751000_2021.03.05.21251351v1.21",
"volume": "6",
"year": "2018"
},
{
"DOI": "10.1128/AAC.01061-20",
"doi-asserted-by": "crossref",
"key": "2021031010100751000_2021.03.05.21251351v1.22",
"unstructured": "Davoudi-Monfared, E. et al. A randomized clinical trial of the efficacy and safety of interferon β-1a in treatment of severe COVID-19. Antimicrob. Agents Chemother. 64, (2020)."
},
{
"article-title": "Interferon beta-1a as a Candidate for COVID-19 Treatment;An Open-Label Single-Arm Clinical Trial",
"first-page": "1",
"journal-title": "Adv. J. Emerg. Med",
"key": "2021031010100751000_2021.03.05.21251351v1.23",
"volume": "4",
"year": "2020"
},
{
"DOI": "10.1016/j.intimp.2020.106688",
"article-title": "Subcutaneous administration of interferon beta-1a for COVID-19: A non-controlled prospective trial",
"doi-asserted-by": "crossref",
"first-page": "106688",
"journal-title": "Int. Immunopharmacol",
"key": "2021031010100751000_2021.03.05.21251351v1.24",
"volume": "85",
"year": "2020"
},
{
"DOI": "10.1016/j.ijantimicag.2020.106077",
"doi-asserted-by": "crossref",
"key": "2021031010100751000_2021.03.05.21251351v1.25",
"unstructured": "Bani-Sadr, F. et al. Corticosteroid therapy for patients with COVID-19 pneumonia: a before-after study. Int. J. Antimicrob. Agents 56, (2020)."
},
{
"DOI": "10.1080/17476348.2020.1804365",
"article-title": "Mortality in COVID-19 patients with acute respiratory distress syndrome and corticosteroids use: a systematic review and meta-analysis",
"doi-asserted-by": "crossref",
"first-page": "1149",
"journal-title": "Expert Rev. Respir. Med",
"key": "2021031010100751000_2021.03.05.21251351v1.26",
"volume": "14",
"year": "2020"
},
{
"DOI": "10.3390/jcm9082392",
"article-title": "Efficacy of Corticosteroids in Patients with SARS, MERS and COVID-19: A Systematic Review and Meta-Analysis",
"doi-asserted-by": "crossref",
"first-page": "2392",
"journal-title": "J. Clin. Med",
"key": "2021031010100751000_2021.03.05.21251351v1.27",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.17023",
"article-title": "Association between Administration of Systemic Corticosteroids and Mortality among Critically Ill Patients with COVID-19: A Meta-analysis",
"doi-asserted-by": "crossref",
"first-page": "1330",
"journal-title": "JAMA - J. Am. Med. Assoc",
"key": "2021031010100751000_2021.03.05.21251351v1.28",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.17022",
"article-title": "Effect of Hydrocortisone on Mortality and Organ Support in Patients with Severe COVID-19: The REMAP-CAP COVID-19 Corticosteroid Domain Randomized Clinical Trial",
"doi-asserted-by": "crossref",
"first-page": "1317",
"journal-title": "JAMA - J. Am. Med. Assoc",
"key": "2021031010100751000_2021.03.05.21251351v1.29",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.3389/fpubh.2020.00355",
"doi-asserted-by": "crossref",
"key": "2021031010100751000_2021.03.05.21251351v1.30",
"unstructured": "Hu, Z. et al. Clinical Use of Short-Course and Low-Dose Corticosteroids in Patients With Non-severe COVID-19 During Pneumonia Progression. Front. Public Heal. 8, (2020)."
},
{
"DOI": "10.1038/s41422-020-0282-0",
"doi-asserted-by": "publisher",
"key": "2021031010100751000_2021.03.05.21251351v1.31"
},
{
"DOI": "10.5694/mja2.50577",
"article-title": "Corticosteroid treatment of patients with coronavirus disease 2019 (COVID-19)",
"doi-asserted-by": "crossref",
"first-page": "416",
"journal-title": "Med. J. Aust",
"key": "2021031010100751000_2021.03.05.21251351v1.32",
"volume": "212",
"year": "2020"
},
{
"DOI": "10.1101/2020.04.17.20069773",
"doi-asserted-by": "crossref",
"key": "2021031010100751000_2021.03.05.21251351v1.33",
"unstructured": "Gangopadhyay, K. K. et al. Title Page Title : The role of corticosteroids in the management of critically ill patients with coronavirus disease 2019 (COVID-19): A meta-analysis Author details : Abstract Objective : Data Sources : Data extraction : Data synthesis: (2020)."
},
{
"DOI": "10.1101/2020.08.13.20174201",
"doi-asserted-by": "crossref",
"key": "2021031010100751000_2021.03.05.21251351v1.34",
"unstructured": "Tlayjeh, H. et al. Efficacy of Corticosteroids in COVID-19 Patients: A Systematic Review and Meta-Analysis. medRxiv (2020)."
},
{
"DOI": "10.1007/s40121-020-00332-3",
"doi-asserted-by": "publisher",
"key": "2021031010100751000_2021.03.05.21251351v1.35"
},
{
"DOI": "10.1371/journal.pone.0232391",
"doi-asserted-by": "publisher",
"key": "2021031010100751000_2021.03.05.21251351v1.36"
},
{
"DOI": "10.1038/s41598-020-59121-0",
"doi-asserted-by": "publisher",
"key": "2021031010100751000_2021.03.05.21251351v1.37"
}
],
"reference-count": 37,
"references-count": 37,
"relation": {},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2021.03.05.21251351"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"subtype": "preprint",
"title": "A single-center retrospective cohort study of Covid-19 medications: Remdesivir, Favipiravir, Methylprednisolone, Dexamethasone, and Interferon β1a and their combinations",
"type": "posted-content"
}
fateh