Epidemiology, outcomes, and utilization of intensive care unit resources for critically ill COVID-19 patients in Libya: A prospective multi-center cohort study
et al., PLOS ONE, doi:10.1371/journal.pone.0251085, Apr 2021
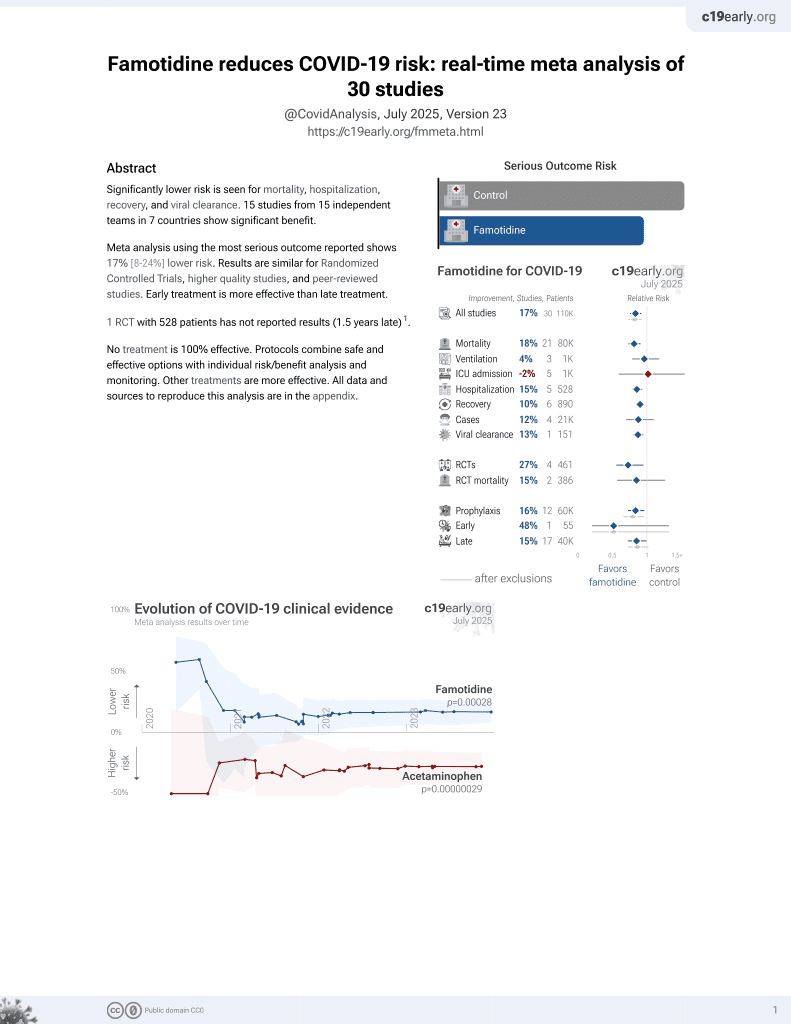
Famotidine for COVID-19
29th treatment shown to reduce risk in
October 2021, now with p = 0.00028 from 30 studies, recognized in 2 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
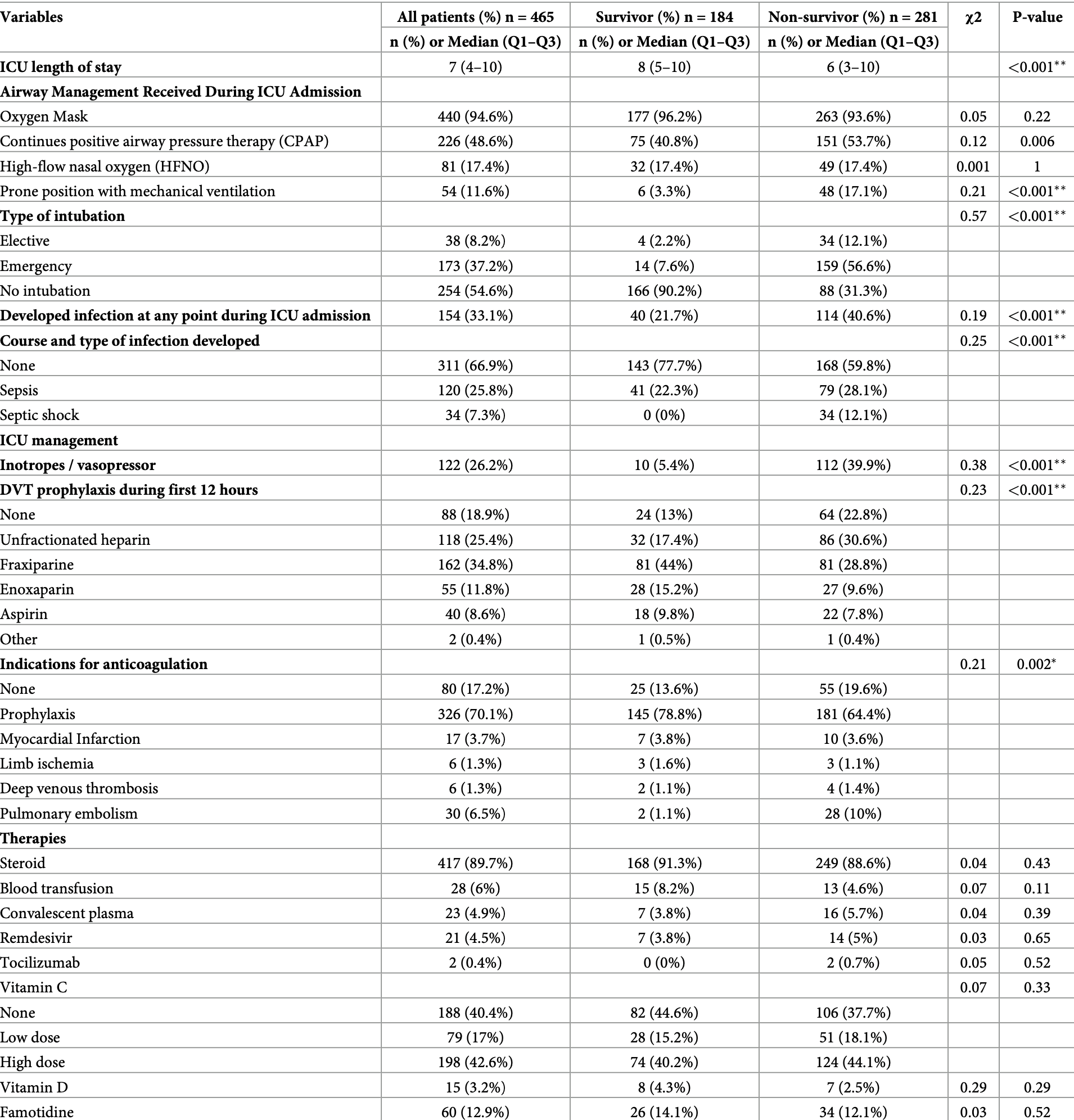
Prospective study of 465 COVID-19 ICU patients in Libya showing no significant differences with treatment.
This study is excluded in the after exclusion results of meta-analysis:
unadjusted results with no group details.
|
risk of death, 7.1% lower, RR 0.93, p = 0.57, treatment 34 of 60 (56.7%), control 247 of 405 (61.0%), NNT 23.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Elhadi et al., 30 Apr 2021, prospective, Libya, peer-reviewed, 21 authors, study period 29 May, 2020 - 30 December, 2020.
Epidemiology, outcomes, and utilization of intensive care unit resources for critically ill COVID-19 patients in Libya: A prospective multi-center cohort study
PLOS ONE, doi:10.1371/journal.pone.0251085
Background The coronavirus disease (COVID-19) pandemic has severely affected African countries, specifically the countries, such as Libya, that are in constant conflict. Clinical and laboratory information, including mortality and associated risk factors in relation to hospital settings and available resources, about critically ill patients with COVID-19 in Africa is not available. This study aimed to determine the mortality and morbidity of COVID-19 patients in intensive care units (ICU) following 60 days after ICU admission, and explore the factors that influence in-ICU mortality rate.
Methods This is a multicenter prospective observational study among COVID-19 critical care patients in 11 ICUs in Libya from May 29th to December 30th 2020. Basic demographic data, clinical characteristics, laboratory values, admission Sequential Organ Failure Assessment (SOFA) score, quick SOFA, and clinical management were analyzed.
Result We included 465 consecutive COVID-19 critically ill patients. The majority (67.1%) of the patients were older than 60 years, with a median (IQR) age of 69 (56.5-75); 240 (51.6%) were male. At 60 days of follow-up, 184 (39.6%) were discharged alive, while 281 (60.4%) died in the intensive care unit. The median (IQR) ICU length of stay was 7 days (4-10) and
Author Contributions Conceptualization: Muhammed Elhadi.
Data curation:
References
Armstrong, Kane, Cook, Outcomes from intensive care in patients with COVID-19: a systematic review and meta-analysis of observational studies, doi:10.1111/anae.15201
Auld, Caridi-Scheible, Blum, Robichaux, Kraft et al., ICU and Ventilator Mortality Among Critically Ill Adults With Coronavirus Disease 2019, Crit Care Med, doi:10.1097/CCM.0000000000004457
Babapoor-Farrokhran, Rasekhi, Gill, Babapoor, Amanullah, Arrhythmia in COVID-19, SN Compr Clin Med, doi:10.1007/s42399-020-00454-2
Borobia, Carcas, Arnalich, ´lvarez-Sala, Monserrat-Villatoro et al., A Cohort of Patients with COVID-19 in a Major Teaching Hospital in Europe, Journal of clinical medicine, doi:10.3390/jcm9061733
Cai, Chen, Wang, Luo, Liu et al., Obesity and COVID-19 Severity in a Designated Hospital in Shenzhen, China, Diabetes care, doi:10.2337/dc20-0576
Cheng, Li, Li, Liu, Yan et al., Ferritin in the coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis, Journal of clinical laboratory analysis, doi:10.1002/jcla.23618
Chew, Lee, Ghimiray, Tan, Chua, Characteristics and Outcomes of COVID-19 Patients with Respiratory Failure Admitted to a "Pandemic Ready" Intensive Care Unit-Lessons from Singapore, Annals of the Academy of Medicine
Desai, Sharma, Jadeja, Desai, Moliya, COVID-19 pandemic induced stress cardiomyopathy: A literature review, IJC Heart & Vasculature, doi:10.1016/j.ijcha.2020.100628
Desforges, Coupanec, Dubeau, Bourgouin, Lajoie et al., Human Coronaviruses and Other Respiratory Viruses: Underestimated Opportunistic Pathogens of the Central Nervous System?, Viruses, doi:10.3390/v12010014
Dong, Du, Gardner, An interactive web-based dashboard to track COVID-19 in real time, Lancet Infect Dis, doi:10.1016/S1473-3099%2820%2930120-1
Du, Lv, Zha, Zhou, Hong, Association of Body mass index (BMI) with Critical COVID-19 and in-hospital Mortality: a dose-response meta-analysis, Metabolism, doi:10.1016/j.metabol.2020.154373
Elhadi, Momen, Senussi Abdulhadi, A COVID-19 case in Libya acquired in Saudi Arabia. Travel medicine and infectious disease, doi:10.1016/j.tmaid.2020.101705
Elhadi, Msherghi, Alkeelani, Alsuyihili, Khaled et al., Concerns for low-resource countries, with under-prepared intensive care units, facing the COVID-19 pandemic, Infection, doi:10.1016/j.idh.2020.05.008
Elhadi, Msherghi, Alkeelani, Zorgani, Zaid et al., Assessment of Healthcare Workers? Levels of Preparedness and Awareness Regarding COVID-19 Infection in Low-Resource Settings. The American journal of tropical medicine and hygiene, doi:10.4269/ajtmh.20-0330
Fadel, Al-Jaghbeer, Kumar, Griffiths, Wang et al., Clinical characteristics and outcomes of critically Ill patients with COVID-19 in Northeast Ohio: low mortality and length of stay. Acute and critical care, doi:10.4266/acc.2020.00619
Fernando, Tran, Taljaard, Cheng, Rochwerg et al., Prognostic Accuracy of the Quick Sequential Organ Failure Assessment for Mortality in Patients With Suspected Infection, Annals of internal medicine, doi:10.7326/M17-2820
Ferrando, Mellado-Artigas, Gea, Arruti, Aldecoa et al., Patient characteristics, clinical course and factors associated to ICU mortality in critically ill patients infected with SARS-CoV-2 in Spain: A prospective, cohort, multicentre study, Rev Esp Anestesiol Reanim, doi:10.1016/j.redar.2020.07.003
Ferreira, Bota, Bross, Me ´lot, Vincent, Serial evaluation of the SOFA score to predict outcome in critically ill patients, Jama, doi:10.1001/jama.286.14.1754
Gandini, Criniti, Ballesio, Giglio, Galardo et al., Serum Ferritin is an independent risk factor for Acute Respiratory Distress Syndrome in COVID-19, J Infect, doi:10.1016/j.jinf.2020.09.006
Garcia, Fumeaux, Guerci, Heuberger, Montomoli et al., Prognostic factors associated with mortality risk and disease progression in 639 critically ill patients with COVID-19 in Europe: Initial report of the international RISC-19-ICU prospective observational cohort, EClinicalMedicine, doi:10.1016/j.eclinm.2020.100449
Grasselli, Pesenti, Cecconi, Critical Care Utilization for the COVID-19 Outbreak in Lombardy, Italy: Early Experience and Forecast During an Emergency Response, Jama, doi:10.1001/jama.2020.4031
Grasselli, Zangrillo, Zanella, Antonelli, Cabrini et al., Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy, doi:10.1001/jama.2020.5394
Guan, Ni, Hu, Liang, Ou et al., Clinical Characteristics of Coronavirus Disease 2019 in China, The New England journal of medicine, doi:10.1056/NEJMoa2002032
Huang, Pranata, Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis, Journal of Intensive Care, doi:10.1186/s40560-020-00453-4
Huang, Wang, Li, Ren, Zhao et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet, doi:10.1016/s0140-6736%2820%2930183-5
Investigators, An African, Multi-Centre Evaluation of Patient Care and Clinical Outcomes for Patients with COVID-19 Infection Admitted to High-Care or Intensive Care Units, The Lancet
Iroegbu, Ifenatuoha, Ijomone, Potential neurological impact of coronaviruses: implications for the novel SARS-CoV-2, Neurol Sci, doi:10.1007/s10072-020-04469-4
Jiang, Huang, Xie, Lv, Quan, The association between severe COVID-19 and low platelet count: evidence from 31 observational studies involving 7613 participants, doi:10.1111/bjh.16817
Kaeuffer, Hyaric, Fabacher, Mootien, Dervieux et al., Clinical characteristics and risk factors associated with severe COVID-19: prospective analysis of 1,045 hospitalised cases in North-Eastern France, doi:10.2807/1560-7917.Es.2020.25.48.2000895
Kocayig ˘it, Su ¨ner, Tomak, Demir, Kocayig ˘it et al., Characteristics and Outcomes of Critically Ill Patients with Covid-19 in Sakarya, Turkey: A Single Center Cohort Study, Turkish journal of medical sciences, doi:10.3906/sag-2005-57
Kuba, Imai, Rao, Jiang, Penninger, Lessons from SARS: control of acute lung failure by the SARS receptor ACE2, Journal of molecular medicine, doi:10.1007/s00109-006-0094-9
Lansbury, Lim, Baskaran, Lim, Co-infections in people with COVID-19: a systematic review and meta-analysis, J Infect, doi:10.1016/j.jinf.2020.05.046
Larsson, Brattstro ¨m O, Agvald-O ¨hman, Grip, Jalde, Strålin, Characteristics and outcomes of patients with COVID-19 admitted to ICU in a tertiary hospital in Stockholm, Sweden, Acta anaesthesiologica Scandinavica, doi:10.1111/aas.13694
Lin, Yan, Chen, He, Lin et al., COVID-19 and coagulation dysfunction in adults: A systematic review and meta-analysis, J Med Virol, doi:10.1002/jmv.26346
Lippi, Lavie, Sanchis-Gomar, Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): Evidence from a meta-analysis, Prog Cardiovasc Dis, doi:10.1016/j.pcad.2020.03.001
Lippi, Plebani, Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): A meta-analysis, Clinica Chimica Acta, doi:10.1016/j.cca.2020.03.004
Livingston, Bucher, Coronavirus Disease 2019 (COVID-19) in Italy, Jama, doi:10.1001/jama.2020.4344
Luo, Zhou, Yan, Guo, Wang et al., Clinical infectious diseases: an official publication of the Infectious Diseases Society of America, doi:10.1093/cid/ciaa641
Malik, Patel, Mehta, Patel, Kelkar et al., Biomarkers and outcomes of COVID-19 hospitalisations: systematic review and meta-analysis, BMJ evidence-based medicine, doi:10.1136/bmjebm-2020-111536
Marone, Rinaldi, Upsurge of deep venous thrombosis in patients affected by COVID-19: Preliminary data and possible explanations, J Vasc Surg Venous Lymphat Disord, doi:10.1016/j.jvsv.2020.04.004
Nadeem, Hamed, Saleh, Abduljawad, Mallat, ICU outcomes of COVID-19 critically ill patients: An international comparative study, Anaesth Crit Care Pain Med, doi:10.1016/j.accpm.2020.07.001
Nadkarni, Alderson, Collett, Maiden, Reddi et al., Impact of COVID-19 on an Australian intensive care unit: lessons learned from South Australia, doi:10.1111/imj.14963
Narula, Joseph, Katyal, Daouk, Acharya et al., Seizure and COVID-19: Association and review of potential mechanism, Neurol Psychiatry Brain Res, doi:10.1016/j.npbr.2020.10.001
Paolisso, Bergamaschi, Angelo, Donati, Giannella et al., Preliminary Experience With Low Molecular Weight Heparin Strategy in COVID-19 Patients, doi:10.3389/fphar.2020.01124
Primmaz, Terrier, Suh, Ventura, Boroli et al., Preparedness and Reorganization of Care for Coronavirus Disease 2019 Patients in a Swiss ICU: Characteristics and Outcomes of 129 Patients, Crit Care Explor, doi:10.1097/CCE.0000000000000173
Pun, Badenes, La Calle, Orun, Chen et al., Prevalence and risk factors for delirium in critically ill patients with COVID-19 (COVID-D): a multicentre cohort study, The Lancet Respiratory medicine, doi:10.1016/S2213-2600%2820%2930552-X
Richardson, Hirsch, Narasimhan, Crawford, Mcginn et al., Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area, Jama, doi:10.1001/jama.2020.6775
Roedl, Jarczak, Thasler, Bachmann, Schulte et al., Mechanical ventilation and mortality among 223 critically ill patients with coronavirus disease, Australian Critical Care, doi:10.1016/j.aucc.2020.10.009
Rostami, Mansouritorghabeh, D-dimer level in COVID-19 infection: a systematic review, Expert review of hematology, doi:10.1080/17474086.2020.1831383
Sawalha, Abozenah, Kadado, Battisha, Al-Akchar et al., Systematic review of COVID-19 related myocarditis: Insights on management and outcome, Cardiovasc Revasc Med, doi:10.1016/j.carrev.2020.08.028
Schmidt, Hajage, Demoule, Pham, Combes et al., Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Medicine, doi:10.1007/s00134-020-06294-x
Suleyman, Fadel, Malette, Hammond, Abdulla et al., Clinical Characteristics and Morbidity Associated With Coronavirus Disease 2019 in a Series of Patients in Metropolitan Detroit, JAMA Netw Open, doi:10.1001/jamanetworkopen.2020.12270
Słomka, Kowalewski, Żekanowska, Coronavirus Disease 2019 (COVID-19): A Short Review on Hematological Manifestations, Pathogens, doi:10.3390/pathogens9060493
Tabah, Ramanan, Laupland, Buetti, Cortegiani et al., Personal protective equipment and intensive care unit healthcare worker safety in the COVID-19 era (PPE-SAFE): An international survey, Journal of critical care, doi:10.1016/j.jcrc.2020.06.005
Vincent, De Mendonc ¸a, Cantraine, Moreno, Takala et al., Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on "sepsis-related problems" of the European Society of Intensive Care Medicine, Crit Care Med, doi:10.1097/00003246-199811000-00016
Von Elm, Altman, Egger, Pocock, Gotzsche et al., The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies, Journal of clinical epidemiology, doi:10.1016/j.jclinepi.2007.11.008
Wang, Hu, Hu, Zhu, Liu et al., Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China, Jama, doi:10.1001/jama.2020.1585
Wang, Lu, Li, Chen, Chen et al., Clinical Course and Outcomes of 344 Intensive Care Patients with COVID-19. American journal of respiratory and critical care medicine, doi:10.1164/rccm.202003-0736LE
Wynants, Van Calster, Collins, Riley, Heinze et al., Prediction models for diagnosis and prognosis of covid-19 infection: systematic review and critical appraisal, BMJ (Clinical research ed, doi:10.1136/bmj.m1328
Yang, Lipes, Dial, Schwartz, Laporta et al., Outcomes and clinical practice in patients with COVID-19 admitted to the intensive care unit in Montre ´al, Canada: a descriptive analysis, CMAJ open, doi:10.9778/cmajo.20200159
Yu, Xu, Fu, Zhang, Yang et al., Patients with COVID-19 in 19 ICUs in Wuhan, China: a cross-sectional study, Critical Care, doi:10.1186/s13054-020-02939-x
Zheng, Peng, Xu, Zhao, Liu et al., Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis, J Infect, doi:10.1016/j.jinf.2020.04.021
Zhou, Yu, Du, Fan, Liu et al., Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, Lancet, doi:10.1016/S0140-6736%2820%2930566-3
DOI record:
{
"DOI": "10.1371/journal.pone.0251085",
"ISSN": [
"1932-6203"
],
"URL": "http://dx.doi.org/10.1371/journal.pone.0251085",
"abstract": "<jats:sec id=\"sec001\">\n<jats:title>Background</jats:title>\n<jats:p>The coronavirus disease (COVID-19) pandemic has severely affected African countries, specifically the countries, such as Libya, that are in constant conflict. Clinical and laboratory information, including mortality and associated risk factors in relation to hospital settings and available resources, about critically ill patients with COVID-19 in Africa is not available. This study aimed to determine the mortality and morbidity of COVID-19 patients in intensive care units (ICU) following 60 days after ICU admission, and explore the factors that influence in‐ICU mortality rate.</jats:p>\n</jats:sec>\n<jats:sec id=\"sec002\">\n<jats:title>Methods</jats:title>\n<jats:p>This is a multicenter prospective observational study among COVID-19 critical care patients in 11 ICUs in Libya from May 29th to December 30th 2020. Basic demographic data, clinical characteristics, laboratory values, admission Sequential Organ Failure Assessment (SOFA) score, quick SOFA, and clinical management were analyzed.</jats:p>\n</jats:sec>\n<jats:sec id=\"sec003\">\n<jats:title>Result</jats:title>\n<jats:p>We included 465 consecutive COVID-19 critically ill patients. The majority (67.1%) of the patients were older than 60 years, with a median (IQR) age of 69 (56.5–75); 240 (51.6%) were male. At 60 days of follow-up, 184 (39.6%) were discharged alive, while 281 (60.4%) died in the intensive care unit. The median (IQR) ICU length of stay was 7 days (4–10) and non-survivors had significantly shorter stay, 6 (3–10) days. The body mass index was 27.9 (24.1–31.6) kg/m2. At admission to the intensive care unit, quick SOFA median (IQR) score was 1 (1–2), whereas total SOFA score was 6 (4–7). In univariate analysis, the following parameters were significantly associated with increased/decreased hazard of mortality: increased age, BMI, white cell count, neutrophils, procalcitonin, cardiac troponin, C-reactive protein, ferritin, fibrinogen, prothrombin, and d-dimer levels were associated with higher risk of mortality. Decreased lymphocytes, and platelet count were associated with higher risk of mortality. Quick SOFA and total SOFA scores increase, emergency intubation, inotrope use, stress myocardiopathy, acute kidney injury, arrythmia, and seizure were associated with higher mortality.</jats:p>\n</jats:sec>\n<jats:sec id=\"sec004\">\n<jats:title>Conclusion</jats:title>\n<jats:p>Our study reported the highest mortality rate (60.4%) among critically ill patients with COVID-19 60 days post-ICU admission. Several factors were found to be predictive of mortality, which may help to identify patients at risk of mortality during the ongoing COVID-19 pandemic.</jats:p>\n</jats:sec>",
"author": [
{
"ORCID": "http://orcid.org/0000-0001-6406-4212",
"affiliation": [],
"authenticated-orcid": true,
"family": "Elhadi",
"given": "Muhammed",
"sequence": "first"
},
{
"affiliation": [],
"family": "Alsoufi",
"given": "Ahmed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Abusalama",
"given": "Abdurraouf",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alkaseek",
"given": "Akram",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Abdeewi",
"given": "Saedah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yahya",
"given": "Mohammed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mohammed",
"given": "Alsnosy",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Abdelkabir",
"given": "Mohammed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Huwaysh",
"given": "Mohammed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Amkhatirah",
"given": "Emad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alshorbaji",
"given": "Kamel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Khel",
"given": "Samer",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gamra",
"given": "Marwa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alhadi",
"given": "Abdulmueti",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Abubaker",
"given": "Taha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Anaiba",
"given": "Mohamed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Elmugassabi",
"given": "Mohammed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Binnawara",
"given": "Muhannud",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Khaled",
"given": "Ala",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zaid",
"given": "Ahmed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Msherghi",
"given": "Ahmed",
"sequence": "additional"
}
],
"container-title": [
"PLOS ONE"
],
"content-domain": {
"crossmark-restriction": false,
"domain": [
"www.plosone.org"
]
},
"created": {
"date-parts": [
[
2021,
4,
30
]
],
"date-time": "2021-04-30T17:44:06Z",
"timestamp": 1619804646000
},
"deposited": {
"date-parts": [
[
2021,
4,
30
]
],
"date-time": "2021-04-30T17:44:57Z",
"timestamp": 1619804697000
},
"editor": [
{
"affiliation": [],
"family": "Zivkovic",
"given": "Aleksandar R.",
"sequence": "first"
}
],
"indexed": {
"date-parts": [
[
2022,
3,
15
]
],
"date-time": "2022-03-15T14:49:02Z",
"timestamp": 1647355742105
},
"is-referenced-by-count": 9,
"issn-type": [
{
"type": "electronic",
"value": "1932-6203"
}
],
"issue": "4",
"issued": {
"date-parts": [
[
2021,
4,
30
]
]
},
"journal-issue": {
"issue": "4",
"published-online": {
"date-parts": [
[
2021,
4,
30
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
4,
30
]
],
"date-time": "2021-04-30T00:00:00Z",
"timestamp": 1619740800000
}
}
],
"link": [
{
"URL": "https://dx.plos.org/10.1371/journal.pone.0251085",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "340",
"original-title": [],
"page": "e0251085",
"prefix": "10.1371",
"published": {
"date-parts": [
[
2021,
4,
30
]
]
},
"published-online": {
"date-parts": [
[
2021,
4,
30
]
]
},
"publisher": "Public Library of Science (PLoS)",
"reference": [
{
"DOI": "10.1016/S1473-3099(20)30120-1",
"article-title": "An interactive web-based dashboard to track COVID-19 in real time",
"author": "E Dong",
"doi-asserted-by": "crossref",
"first-page": "533",
"issue": "5",
"journal-title": "Lancet Infect Dis",
"key": "pone.0251085.ref001",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"article-title": "Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China",
"author": "C Huang",
"doi-asserted-by": "crossref",
"first-page": "497",
"issue": "10223",
"journal-title": "Lancet (London, England)",
"key": "pone.0251085.ref002",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.5394",
"article-title": "Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy",
"author": "G Grasselli",
"doi-asserted-by": "crossref",
"first-page": "1574",
"issue": "16",
"journal-title": "Jama.",
"key": "pone.0251085.ref003",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2002032",
"article-title": "Clinical Characteristics of Coronavirus Disease 2019 in China",
"author": "WJ Guan",
"doi-asserted-by": "crossref",
"first-page": "1708",
"issue": "18",
"journal-title": "The New England journal of medicine",
"key": "pone.0251085.ref004",
"volume": "382",
"year": "2020"
},
{
"article-title": "Prediction models for diagnosis and prognosis of covid-19 infection: systematic review and critical appraisal",
"author": "L Wynants",
"first-page": "m1328",
"journal-title": "BMJ (Clinical research ed)",
"key": "pone.0251085.ref005",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"article-title": "Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study",
"author": "F Zhou",
"doi-asserted-by": "crossref",
"first-page": "1054",
"issue": "10229",
"journal-title": "Lancet (London, England)",
"key": "pone.0251085.ref006",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.1585",
"article-title": "Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China",
"author": "D Wang",
"doi-asserted-by": "crossref",
"first-page": "1061",
"issue": "11",
"journal-title": "Jama.",
"key": "pone.0251085.ref007",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.4031",
"article-title": "Critical Care Utilization for the COVID-19 Outbreak in Lombardy, Italy: Early Experience and Forecast During an Emergency Response",
"author": "G Grasselli",
"doi-asserted-by": "crossref",
"first-page": "1545",
"issue": "16",
"journal-title": "Jama",
"key": "pone.0251085.ref008",
"volume": "323",
"year": "2020"
},
{
"article-title": "Coronavirus Disease 2019 (COVID-19) in Italy",
"author": "E Livingston",
"first-page": "1335",
"issue": "14",
"journal-title": "Jama. 2020",
"key": "pone.0251085.ref009",
"volume": "323"
},
{
"DOI": "10.1001/jama.2020.6775",
"article-title": "Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area",
"author": "S Richardson",
"doi-asserted-by": "crossref",
"first-page": "2052",
"issue": "20",
"journal-title": "Jama",
"key": "pone.0251085.ref010",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.15585/mmwr.mm6912e2",
"article-title": "Severe Outcomes Among Patients with Coronavirus Disease 2019 (COVID-19)—United States, February 12-March 16, 2020",
"doi-asserted-by": "crossref",
"first-page": "343",
"issue": "12",
"journal-title": "MMWR Morbidity and mortality weekly report",
"key": "pone.0251085.ref011",
"volume": "69",
"year": "2020"
},
{
"DOI": "10.1164/rccm.202003-0736LE",
"article-title": "Clinical Course and Outcomes of 344 Intensive Care Patients with COVID-19",
"author": "Y Wang",
"doi-asserted-by": "crossref",
"first-page": "1430",
"issue": "11",
"journal-title": "American journal of respiratory and critical care medicine",
"key": "pone.0251085.ref012",
"volume": "201",
"year": "2020"
},
{
"DOI": "10.3390/jcm9061733",
"article-title": "A Cohort of Patients with COVID-19 in a Major Teaching Hospital in Europe",
"author": "AM Borobia",
"doi-asserted-by": "crossref",
"issue": "6",
"journal-title": "Journal of clinical medicine",
"key": "pone.0251085.ref013",
"volume": "9",
"year": "2020"
},
{
"article-title": "Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study",
"author": "M Schmidt",
"first-page": "60",
"issue": "1",
"journal-title": "Intensive Care Medicine. 2021",
"key": "pone.0251085.ref014",
"volume": "47"
},
{
"article-title": "Outcomes from intensive care in patients with COVID-19: a systematic review and meta-analysis of observational studies",
"author": "RA Armstrong",
"first-page": "1340",
"issue": "10",
"key": "pone.0251085.ref015",
"volume": "75",
"year": "2020"
},
{
"article-title": "An African, Multi-Centre Evaluation of Patient Care and Clinical Outcomes for Patients with COVID-19 Infection Admitted to High-Care or Intensive Care Units",
"author": "TA Investigators",
"journal-title": "The Lancet",
"key": "pone.0251085.ref016",
"year": "2021"
},
{
"DOI": "10.4269/ajtmh.20-0330",
"article-title": "Assessment of Healthcare Workers? Levels of Preparedness and Awareness Regarding COVID-19 Infection in Low-Resource Settings",
"author": "M Elhadi",
"doi-asserted-by": "crossref",
"first-page": "828",
"issue": "2",
"journal-title": "The American journal of tropical medicine and hygiene",
"key": "pone.0251085.ref017",
"volume": "103",
"year": "2020"
},
{
"DOI": "10.1016/j.jcrc.2020.06.005",
"article-title": "Personal protective equipment and intensive care unit healthcare worker safety in the COVID-19 era (PPE-SAFE): An international survey",
"author": "A Tabah",
"doi-asserted-by": "crossref",
"first-page": "70",
"journal-title": "Journal of critical care",
"key": "pone.0251085.ref018",
"volume": "59",
"year": "2020"
},
{
"DOI": "10.1016/j.tmaid.2020.101705",
"article-title": "A COVID-19 case in Libya acquired in Saudi Arabia",
"author": "M Elhadi",
"doi-asserted-by": "crossref",
"first-page": "101705",
"journal-title": "Travel medicine and infectious disease",
"key": "pone.0251085.ref019",
"volume": "37",
"year": "2020"
},
{
"article-title": "Concerns for low-resource countries, with under-prepared intensive care units, facing the COVID-19 pandemic",
"author": "M Elhadi",
"journal-title": "Infection, disease & health",
"key": "pone.0251085.ref020",
"year": "2020"
},
{
"DOI": "10.1097/00003246-199811000-00016",
"article-title": "Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on \"sepsis-related problems\" of the European Society of Intensive Care Medicine",
"author": "JL Vincent",
"doi-asserted-by": "crossref",
"first-page": "1793",
"issue": "11",
"journal-title": "Crit Care Med",
"key": "pone.0251085.ref021",
"volume": "26",
"year": "1998"
},
{
"DOI": "10.1001/jama.286.14.1754",
"article-title": "Serial evaluation of the SOFA score to predict outcome in critically ill patients",
"author": "FL Ferreira",
"doi-asserted-by": "crossref",
"first-page": "1754",
"issue": "14",
"journal-title": "Jama",
"key": "pone.0251085.ref022",
"volume": "286",
"year": "2001"
},
{
"DOI": "10.7326/M17-2820",
"article-title": "Prognostic Accuracy of the Quick Sequential Organ Failure Assessment for Mortality in Patients With Suspected Infection",
"author": "SM Fernando",
"doi-asserted-by": "crossref",
"first-page": "266",
"issue": "4",
"journal-title": "Annals of internal medicine",
"key": "pone.0251085.ref023",
"volume": "168",
"year": "2018"
},
{
"DOI": "10.1016/j.jclinepi.2007.11.008",
"article-title": "statement: guidelines for reporting observational studies",
"author": "E von Elm",
"doi-asserted-by": "crossref",
"first-page": "344",
"issue": "4",
"journal-title": "Journal of clinical epidemiology",
"key": "pone.0251085.ref024",
"volume": "61",
"year": "2008"
},
{
"DOI": "10.1186/s13054-020-02939-x",
"article-title": "Patients with COVID-19 in 19 ICUs in Wuhan, China: a cross-sectional study",
"author": "Y Yu",
"doi-asserted-by": "crossref",
"first-page": "219",
"issue": "1",
"journal-title": "Critical Care",
"key": "pone.0251085.ref025",
"volume": "24",
"year": "2020"
},
{
"article-title": "Patient characteristics, clinical course and factors associated to ICU mortality in critically ill patients infected with SARS-CoV-2 in Spain: A prospective, cohort, multicentre study",
"author": "C Ferrando",
"first-page": "425",
"issue": "8",
"journal-title": "Rev Esp Anestesiol Reanim",
"key": "pone.0251085.ref026",
"volume": "67",
"year": "2020"
},
{
"article-title": "Clinical characteristics and risk factors associated with severe COVID-19: prospective analysis of 1,045 hospitalised cases in North-Eastern France, March 2020",
"author": "C Kaeuffer",
"issue": "48",
"journal-title": "Euro surveillance: bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin",
"key": "pone.0251085.ref027",
"volume": "25",
"year": "2020"
},
{
"DOI": "10.1111/aas.13694",
"article-title": "Characteristics and outcomes of patients with COVID-19 admitted to ICU in a tertiary hospital in Stockholm, Sweden",
"author": "E Larsson",
"doi-asserted-by": "crossref",
"first-page": "76",
"issue": "1",
"journal-title": "Acta anaesthesiologica Scandinavica",
"key": "pone.0251085.ref028",
"volume": "65",
"year": "2021"
},
{
"article-title": "Mechanical ventilation and mortality among 223 critically ill patients with coronavirus disease 2019: A multicentric study in Germany",
"author": "K Roedl",
"journal-title": "Australian Critical Care",
"key": "pone.0251085.ref029",
"year": "2020"
},
{
"article-title": "Prognostic factors associated with mortality risk and disease progression in 639 critically ill patients with COVID-19 in Europe: Initial report of the international RISC-19-ICU prospective observational cohort",
"author": "PD Wendel Garcia",
"first-page": "25",
"journal-title": "EClinicalMedicine.",
"key": "pone.0251085.ref030",
"year": "2020"
},
{
"article-title": "ICU and Ventilator Mortality Among Critically Ill Adults With Coronavirus Disease 2019",
"author": "SC Auld",
"first-page": "e799",
"issue": "9",
"journal-title": "Crit Care Med.",
"key": "pone.0251085.ref031",
"volume": "48",
"year": "2020"
},
{
"DOI": "10.4266/acc.2020.00619",
"article-title": "Clinical characteristics and outcomes of critically Ill patients with COVID-19 in Northeast Ohio: low mortality and length of stay",
"author": "FA Fadel",
"doi-asserted-by": "crossref",
"first-page": "242",
"issue": "4",
"journal-title": "Acute and critical care",
"key": "pone.0251085.ref032",
"volume": "35",
"year": "2020"
},
{
"DOI": "10.1001/jamanetworkopen.2020.12270",
"article-title": "Clinical Characteristics and Morbidity Associated With Coronavirus Disease 2019 in a Series of Patients in Metropolitan Detroit",
"author": "G Suleyman",
"doi-asserted-by": "crossref",
"first-page": "e2012270",
"issue": "6",
"journal-title": "JAMA Netw Open",
"key": "pone.0251085.ref033",
"volume": "3",
"year": "2020"
},
{
"author": "A Nadkarni",
"first-page": "1146",
"issue": "9",
"journal-title": "Impact of COVID-19 on an Australian intensive care unit: lessons learned from South Australia",
"key": "pone.0251085.ref034",
"volume": "50",
"year": "2020"
},
{
"DOI": "10.47102/annals-acadmedsg.2020161",
"article-title": "Characteristics and Outcomes of COVID-19 Patients with Respiratory Failure Admitted to a \"Pandemic Ready\" Intensive Care Unit—Lessons from Singapore",
"author": "SY Chew",
"doi-asserted-by": "crossref",
"first-page": "434",
"issue": "7",
"journal-title": "Annals of the Academy of Medicine, Singapore",
"key": "pone.0251085.ref035",
"volume": "49",
"year": "2020"
},
{
"article-title": "Characteristics and Outcomes of Critically Ill Patients with Covid-19 in Sakarya, Turkey: A Single Center Cohort Study",
"author": "H KocayİĞİt",
"journal-title": "Turkish journal of medical sciences",
"key": "pone.0251085.ref036",
"year": "2020"
},
{
"DOI": "10.1016/j.accpm.2020.07.001",
"article-title": "ICU outcomes of COVID-19 critically ill patients: An international comparative study",
"author": "A Nadeem",
"doi-asserted-by": "crossref",
"first-page": "487",
"issue": "4",
"journal-title": "Anaesth Crit Care Pain Med",
"key": "pone.0251085.ref037",
"volume": "39",
"year": "2020"
},
{
"article-title": "Preparedness and Reorganization of Care for Coronavirus Disease 2019 Patients in a Swiss ICU: Characteristics and Outcomes of 129 PatientsCrit Care Explor.",
"author": "S Primmaz",
"first-page": "e0173",
"issue": "8",
"key": "pone.0251085.ref038",
"volume": "2",
"year": "2020"
},
{
"DOI": "10.9778/cmajo.20200159",
"article-title": "Outcomes and clinical practice in patients with COVID-19 admitted to the intensive care unit in Montréal, Canada: a descriptive analysis",
"author": "SS Yang",
"doi-asserted-by": "crossref",
"first-page": "E788",
"issue": "4",
"journal-title": "CMAJ open.",
"key": "pone.0251085.ref039",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1016/j.jinf.2020.04.021",
"article-title": "Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis",
"author": "Z Zheng",
"doi-asserted-by": "crossref",
"first-page": "e16",
"issue": "2",
"journal-title": "J Infect.",
"key": "pone.0251085.ref040",
"volume": "81",
"year": "2020"
},
{
"DOI": "10.2337/dc20-0576",
"article-title": "Obesity and COVID-19 Severity in a Designated Hospital in Shenzhen, China",
"author": "Q Cai",
"doi-asserted-by": "crossref",
"first-page": "1392",
"issue": "7",
"journal-title": "Diabetes care.",
"key": "pone.0251085.ref041",
"volume": "43",
"year": "2020"
},
{
"article-title": "with Critical COVID-19 and in-hospital Mortality: a dose-response meta-analysis",
"author": "Y Du",
"first-page": "154373",
"journal-title": "Metabolism",
"key": "pone.0251085.ref042",
"year": "2020"
},
{
"DOI": "10.3390/pathogens9060493",
"article-title": "Coronavirus Disease 2019 (COVID-19): A Short Review on Hematological Manifestations",
"author": "A Słomka",
"doi-asserted-by": "crossref",
"first-page": "493",
"issue": "6",
"journal-title": "Pathogens.",
"key": "pone.0251085.ref043",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1186/s40560-020-00453-4",
"article-title": "Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis",
"author": "I Huang",
"doi-asserted-by": "crossref",
"first-page": "36",
"issue": "1",
"journal-title": "Journal of Intensive Care",
"key": "pone.0251085.ref044",
"volume": "8",
"year": "2020"
},
{
"author": "S-Q Jiang",
"first-page": "e29",
"issue": "1",
"journal-title": "The association between severe COVID-19 and low platelet count: evidence from 31 observational studies involving 7613 participants",
"key": "pone.0251085.ref045",
"volume": "190",
"year": "2020"
},
{
"DOI": "10.1016/j.cca.2020.03.004",
"article-title": "Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): A meta-analysis",
"author": "G Lippi",
"doi-asserted-by": "crossref",
"first-page": "190",
"journal-title": "Clinica Chimica Acta",
"key": "pone.0251085.ref046",
"volume": "505",
"year": "2020"
},
{
"DOI": "10.1016/j.pcad.2020.03.001",
"article-title": "Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): Evidence from a meta-analysis",
"author": "G Lippi",
"doi-asserted-by": "crossref",
"first-page": "390",
"issue": "3",
"journal-title": "Prog Cardiovasc Dis.",
"key": "pone.0251085.ref047",
"volume": "63",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa641",
"article-title": "Prognostic Value of C-Reactive Protein in Patients With Coronavirus 2019",
"author": "X Luo",
"doi-asserted-by": "crossref",
"first-page": "2174",
"issue": "16",
"journal-title": "Clinical infectious diseases: an official publication of the Infectious Diseases Society of America",
"key": "pone.0251085.ref048",
"volume": "71",
"year": "2020"
},
{
"DOI": "10.1002/jcla.23618",
"article-title": "Ferritin in the coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis",
"author": "L Cheng",
"doi-asserted-by": "crossref",
"first-page": "e23618",
"issue": "10",
"journal-title": "Journal of clinical laboratory analysis",
"key": "pone.0251085.ref049",
"volume": "34",
"year": "2020"
},
{
"DOI": "10.1016/j.jinf.2020.09.006",
"article-title": "Serum Ferritin is an independent risk factor for Acute Respiratory Distress Syndrome in COVID-19",
"author": "O Gandini",
"doi-asserted-by": "crossref",
"first-page": "979",
"issue": "6",
"journal-title": "J Infect",
"key": "pone.0251085.ref050",
"volume": "81",
"year": "2020"
},
{
"article-title": "Biomarkers and outcomes of COVID-19 hospitalisations: systematic review and meta-analysis",
"author": "P Malik",
"journal-title": "BMJ evidence-based medicine",
"key": "pone.0251085.ref051",
"year": "2020"
},
{
"DOI": "10.1002/jmv.26346",
"article-title": "COVID-19 and coagulation dysfunction in adults: A systematic review and meta-analysis",
"author": "J Lin",
"doi-asserted-by": "crossref",
"first-page": "934",
"issue": "2",
"journal-title": "J Med Virol",
"key": "pone.0251085.ref052",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.1080/17474086.2020.1831383",
"article-title": "D-dimer level in COVID-19 infection: a systematic review",
"author": "M Rostami",
"doi-asserted-by": "crossref",
"first-page": "1265",
"issue": "11",
"journal-title": "Expert review of hematology",
"key": "pone.0251085.ref053",
"volume": "13",
"year": "2020"
},
{
"DOI": "10.1016/j.jinf.2020.05.046",
"article-title": "Co-infections in people with COVID-19: a systematic review and meta-analysis",
"author": "L Lansbury",
"doi-asserted-by": "crossref",
"first-page": "266",
"issue": "2",
"journal-title": "J Infect.",
"key": "pone.0251085.ref054",
"volume": "81",
"year": "2020"
},
{
"DOI": "10.1016/j.jvsv.2020.04.004",
"article-title": "Upsurge of deep venous thrombosis in patients affected by COVID-19: Preliminary data and possible explanations",
"author": "EM Marone",
"doi-asserted-by": "crossref",
"first-page": "694",
"issue": "4",
"journal-title": "J Vasc Surg Venous Lymphat Disord",
"key": "pone.0251085.ref055",
"volume": "8",
"year": "2020"
},
{
"author": "P Paolisso",
"issue": "1124",
"journal-title": "Preliminary Experience With Low Molecular Weight Heparin Strategy in COVID-19 Patients",
"key": "pone.0251085.ref056",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1016/j.ijcha.2020.100628",
"article-title": "COVID-19 pandemic induced stress cardiomyopathy: A literature review",
"author": "HD Desai",
"doi-asserted-by": "crossref",
"first-page": "100628",
"journal-title": "IJC Heart & Vasculature",
"key": "pone.0251085.ref057",
"volume": "31",
"year": "2020"
},
{
"article-title": "Arrhythmia in COVID-19",
"author": "S Babapoor-Farrokhran",
"first-page": "1",
"journal-title": "SN Compr Clin Med",
"key": "pone.0251085.ref058",
"volume": "2020"
},
{
"article-title": "Systematic review of COVID-19 related myocarditis: Insights on management and outcome",
"author": "K Sawalha",
"journal-title": "Cardiovasc Revasc Med",
"key": "pone.0251085.ref059",
"year": "2020"
},
{
"article-title": "Prevalence and risk factors for delirium in critically ill patients with COVID-19 (COVID-D): a multicentre cohort study",
"author": "BT Pun",
"journal-title": "The Lancet Respiratory medicine",
"key": "pone.0251085.ref060",
"year": "2021"
},
{
"DOI": "10.1016/j.npbr.2020.10.001",
"article-title": "Seizure and COVID-19: Association and review of potential mechanism",
"author": "N Narula",
"doi-asserted-by": "crossref",
"first-page": "49",
"journal-title": "Neurol Psychiatry Brain Res",
"key": "pone.0251085.ref061",
"volume": "38",
"year": "2020"
},
{
"DOI": "10.3390/v12010014",
"article-title": "Human Coronaviruses and Other Respiratory Viruses: Underestimated Opportunistic Pathogens of the Central Nervous System?",
"author": "M Desforges",
"doi-asserted-by": "crossref",
"first-page": "14",
"issue": "1",
"journal-title": "Viruses",
"key": "pone.0251085.ref062",
"volume": "12",
"year": "2019"
},
{
"DOI": "10.1007/s00109-006-0094-9",
"article-title": "Lessons from SARS: control of acute lung failure by the SARS receptor ACE2",
"author": "K Kuba",
"doi-asserted-by": "crossref",
"first-page": "814",
"issue": "10",
"journal-title": "Journal of molecular medicine (Berlin, Germany)",
"key": "pone.0251085.ref063",
"volume": "84",
"year": "2006"
},
{
"DOI": "10.1007/s10072-020-04469-4",
"article-title": "Potential neurological impact of coronaviruses: implications for the novel SARS-CoV-2",
"author": "JD Iroegbu",
"doi-asserted-by": "crossref",
"first-page": "1329",
"issue": "6",
"journal-title": "Neurol Sci.",
"key": "pone.0251085.ref064",
"volume": "41",
"year": "2020"
}
],
"reference-count": 64,
"references-count": 64,
"relation": {},
"score": 1,
"short-container-title": [
"PLoS ONE"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Multidisciplinary"
],
"subtitle": [],
"title": [
"Epidemiology, outcomes, and utilization of intensive care unit resources for critically ill COVID-19 patients in Libya: A prospective multi-center cohort study"
],
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1371/journal.pone.corrections_policy",
"volume": "16"
}
elhadi