Quercetin as a possible complementary agent for early-stage COVID-19: Concluding results of a randomized clinical trial
et al., Frontiers in Pharmacology, doi:10.3389/fphar.2022.1096853, NCT04861298, Jan 2023
Quercetin for COVID-19
36th treatment shown to reduce risk in
January 2022, now with p = 0.0018 from 9 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 100 outpatients in Pakistan, 50 treated with quercetin phytosome, showing faster viral clearance and improved recovery with treatment.
Patients in the treatment group were significantly younger (41 vs. 54). Authors report performing a covariance analysis but do not provide any data.
Table 1 reports the standard deviation for age as exactly 2.03 in both the control group and the quercetin group, which is possible but relatively unlikely. The text states the overall mean age was 47.6 +/- 15.7 years. However, mathematically pooling two groups of n=50 with means of 54.1 and 41.1 and standard deviations of 2.03 yields a combined standard deviation of approximately 6.83, not 15.7. The text states the modal age group was between 30-40 years, comprising 23% of total cases. However, given the reported means and assuming a normal distribution, virtually 0% of the patients would fall into the 30-40 age bracket. It is likely that one or both of the 2.03 standard deviations is a typo/incorrect.
The text claims that by week two, 98% in the quercetin group tested negative for SARS-CoV-2. However, Figure 3 shows 2 patients (4%) positive at day 14 in the quercetin group, meaning only 96% tested negative.
Bioavailability. Quercetin has low bioavailability and studies typically use advanced formulations to improve bioavailability which may be required to reach therapeutic concentrations.
This is the 6th of 9 COVID-19 RCTs for quercetin, which collectively show efficacy with p=0.0018.
This study is excluded in the after exclusion results of meta-analysis:
randomization resulted in significant baseline differences that were not adjusted for.
|
risk of death, 66.7% lower, RR 0.33, p = 1.00, treatment 0 of 50 (0.0%), control 1 of 50 (2.0%), NNT 50, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of ICU admission, 66.7% lower, RR 0.33, p = 1.00, treatment 0 of 50 (0.0%), control 1 of 50 (2.0%), NNT 50, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of hospitalization, 66.7% lower, RR 0.33, p = 1.00, treatment 0 of 50 (0.0%), control 1 of 50 (2.0%), NNT 50, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of no recovery, 36.8% lower, RR 0.63, p = 0.007, treatment 24 of 50 (48.0%), control 38 of 50 (76.0%), NNT 3.6, day 7.
|
|
risk of no viral clearance, 57.9% lower, RR 0.42, p < 0.001, treatment 16 of 50 (32.0%), control 38 of 50 (76.0%), NNT 2.3, mid-recovery, day 7.
|
|
risk of no viral clearance, 50.0% higher, RR 1.50, p = 1.00, treatment 3 of 50 (6.0%), control 2 of 50 (4.0%), day 14.
|
|
risk of no viral clearance, 66.7% lower, RR 0.33, p = 1.00, treatment 0 of 50 (0.0%), control 1 of 50 (2.0%), NNT 50, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), day 21.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Di Pierro et al., 13 Jan 2023, Randomized Controlled Trial, Pakistan, peer-reviewed, mean age 47.6, 13 authors, study period December 2020 - September 2021, trial NCT04861298 (history).
Contact: f.dipierro@vellejaresearch.com, amjadkhan@lumhs.edu.pk.
Quercetin as a possible complementary agent for early-stage COVID-19: Concluding results of a randomized clinical trial
Frontiers in Pharmacology, doi:10.3389/fphar.2022.1096853
Background: Quercetin, a natural polyphenol with demonstrated broadspectrum antiviral, anti-inflammatory, and antioxidant properties, has been proposed as an adjuvant for early-stage coronavirus disease 2019 (COVID-19) infection. Objective: To explore the possible therapeutic effect of quercetin in outpatients with early-stage mild to moderate symptoms of COVID-19.
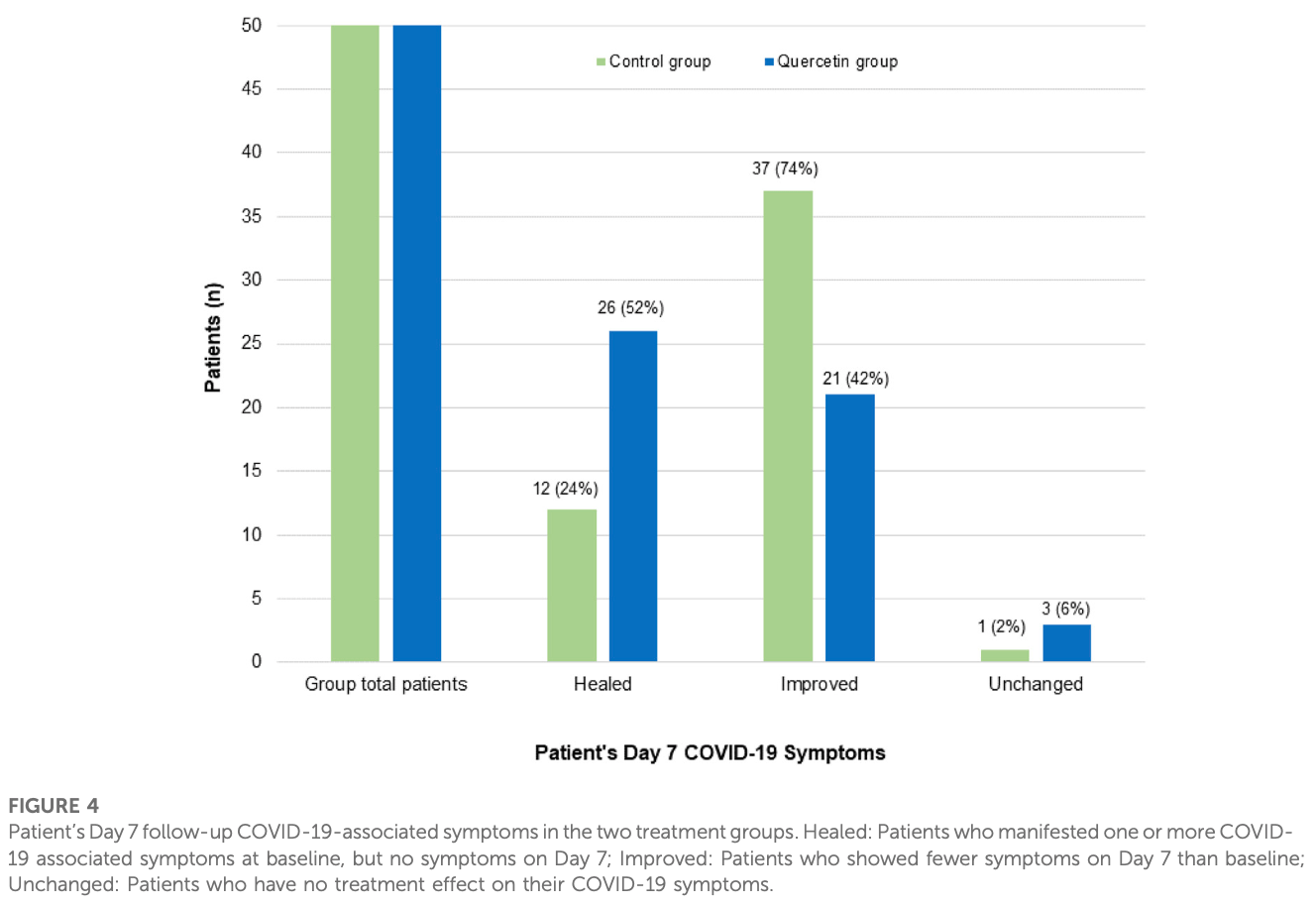
Methods: This was an open-label randomized controlled clinical trial conducted at the department of medicine, King Edward Medical University, Lahore, PK. Patients were randomized to receive either standard of care (SC) plus an oral quercetin supplement (500 mg Quercetin Phytosome ® , 1st week, TDS: 2nd week, BDS) (n = 50, quercetin group) or SC alone (n = 50, control group). Results: After one week of treatment, patients in the quercetin group showed a speedy recovery from COVID-19 as compared to the control group, i.e., 34 patients (vs. 12 in the control group) tested negative for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (p = 0.0004), and 26 patients (vs. 12 in the control group) had their COVID-19-associated acute symptoms resolved (p = 0.0051). Patients in the quercetin group also showed a significant fall in the serum lactate dehydrogenase (LDH) mean values i.e., from 406.56 ± 183.92 to 257.74 ± 110.73 U/L, p = 0.0001. Quercetin was well-tolerated by all the 50 patients, and no side effects were reported.
Ethics statement The study was approved by the Institutional Review Board, King Edward Medical University, Lahore, Pakistan via Ref. No. 192 /RC/KEMU. The patients/participants provided their written informed consent to participate in this study.
Author contributions
Conflict of interest FDP belongs to the Scientific Board of Pharmextracta. AB is a Pharmextracta scientific advisor. ST, PA, and AR belong to the Scientific Board of Indena. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abian, Ortega-Alarcon, Jimenez-Alesanco, Ceballos-Laita, Vega et al., Structural stability of SARS-CoV-2 3CL pro and identification of quercetin as an inhibitor by experimental screening, Int. J. Biol. Macromol, doi:10.1016/j.ijbiomac.2020.07.235
Bahun, Jukic, Oblak, Kranjc, Bajc et al., None
Biancatelli, Berrill, Catravas, Marik, None
Chakraborty, Sharma, Bhattacharya, Agoramoorthy, Lee, None
Chen, Li, Luo, Liu, Xu et al., Binding interaction of quercetin-3-beta-galactoside and its synthetic derivatives with SARS-CoV 3CL(pro): Structure-activity relationship studies reveal salient pharmacophore features, Bioorg Med. Chem, doi:10.1016/j.bmc.2006.09.014
Chiang, Chiang, Liu, Lin, In vitro antiviral activities of Caesalpinia pulcherrima and its related flavonoids, J. Antimicrob. Chemother, doi:10.1093/jac/dkg291
Derosa, Maffioli, D'angelo, Di Pierro, A role for quercetin in coronavirus disease 2019 (COVID-19), Phytother. Res, doi:10.1002/ptr.6887
Di Petrillo, Orrù, Fais, Fantini, Quercetin and its derivates as antiviral potentials: A comprehensive review, Phytother. Res, doi:10.1002/ptr.7309
Di Pierro, Derosa, Maffioli, Bertuccioli, Togni et al., Possible therapeutic effects of adjuvant quercetin supplementation against early-stage COVID-19 infection: A prospective, randomized, controlled, and open-label study, Am. J. Emerg. Med, doi:10.1016/j.ajem.2020.05.073
Imran, Thabet, Alaqel, Alzahrani, Abida et al., The therapeutic and prophylactic potential of quercetin against COVID-19: An outlook on the clinical studies, inventive compositions, and patent literature, Antioxidants, doi:10.3390/antiox11050876
Kalil, Patterson, Mehta, Tomashek, Wolfe et al., Baricitinib plus remdesivir for hospitalized adults with covid-19, N. Engl. J. Med, doi:10.1056/NEJMoa2031994
Kandeil, Mostafa, Kutkat, Moatasim, Al-Karmalawy et al., Bioactive polyphenolic compounds showing strong antiviral activities against severe acute respiratory syndrome coronavirus 2, Pathogens, doi:10.3390/pathogens10060758
Lan, Ge, Yu, Shan, Zhou et al., Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor, Nature, doi:10.1038/s41586-020-2180-5
Lee, Yu, Trimpert, Benthani, Mairhofer et al., Virus-induced senescence is a driver and therapeutic target in COVID-19, Nature, doi:10.1038/s41586-021-03995-1
Liu, Raghuvanshi, Ceylan, Bolling, Quercetin and its metabolites inhibit recombinant human angiotensin-converting enzyme 2
Onal, Arslan, Ucuncu Ergun, Topuz, Yilmaz Semerci et al., None, J. Agric. Food Chem, doi:10.1021/acs.jafc.0c05064
Ono, Nakane, Tocilizumab in patients admitted to hospital with COVID-19: A randomised, controlled, openlabel, platform trial, N. Engl. J. Med, doi:10.1016/S0140-6736(21)00676-0
Quercetin, An experimental, synergistic therapy for the prevention and treatment of SARS-CoV-2 related disease (COVID-19), Front. Immunol, doi:10.3389/fimmu.2020.01451
Rizzuti, Grande, Conforti, Jimenez-Alesanco, Ceballos-Laita et al., Rutin is a low micromolar inhibitor of SARS-CoV-2 main protease 3CLpro: Implications for drug Design of quercetin analogs, Biomedicines, doi:10.3390/biomedicines9040375
Rondanelli, Perna, Gasparri, Petrangolini, Allegrini et al., Promising effects of 3-month period of quercetin Phytosome ® supplementation in the prevention of symptomatic COVID-19 disease in healthcare workers: A pilot study, Life, doi:10.3390/life12010066
Saeedi-Boroujeni, Mahmoudian-Sani, Anti-inflammatory potential of Quercetin in COVID-19 treatment, J. Inflamm. (Lond), doi:10.1186/s12950-021-00268-6
Shohan, Nashibi, Mahmoudian-Sani, Abolnezhadian, Ghafourian et al., The therapeutic efficacy of quercetin in combination with antiviral drugs in hospitalized COVID-19 patients: A randomized controlled trial, Eur. J. Pharmacol, doi:10.1016/j.ejphar.2021.174615
Szarpak, Ruetzler, Safiejko, Hampel, Pruc et al., Lactate dehydrogenase level as a COVID-19 severity marker, Am. J. Emerg. Med, doi:10.1016/j.ajem.2020.11.025
Tőzsér, Benkő, Natural Compounds as Regulators of NLRP3
Wu, Li, Li, He, Jiang et al., Quercetin as an antiviral agent inhibits influenza A virus (iav) entry, Viruses, doi:10.3390/v8010006
Yan, Zhang, Li, Xia, Guo et al., Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2, Science, doi:10.1126/science.abb2762
Yi, Li, Yuan, Qu, Chen et al., Small molecules blocking the entry of severe acute respiratory syndrome coronavirus into host cells, J. Virol, doi:10.1128/jvi.78.20.11334-11339.2004
Zupanets, Golubovska, Tarasenko, Bezuhla, Pasichnik et al., Efficacy of quercetin in patients with pneumonia associated with coronavirus disease (COVID-19), Zaporizhia Med. J
DOI record:
{
"DOI": "10.3389/fphar.2022.1096853",
"ISSN": [
"1663-9812"
],
"URL": "http://dx.doi.org/10.3389/fphar.2022.1096853",
"abstract": "<jats:p><jats:bold>Background:</jats:bold> Quercetin, a natural polyphenol with demonstrated broad-spectrum antiviral, anti-inflammatory, and antioxidant properties, has been proposed as an adjuvant for early-stage coronavirus disease 2019 (COVID-19) infection.</jats:p><jats:p><jats:bold>Objective:</jats:bold> To explore the possible therapeutic effect of quercetin in outpatients with early-stage mild to moderate symptoms of COVID-19.</jats:p><jats:p><jats:bold>Methods:</jats:bold> This was an open-label randomized controlled clinical trial conducted at the department of medicine, King Edward Medical University, Lahore, PK. Patients were randomized to receive either standard of care (SC) plus an oral quercetin supplement (500 mg Quercetin Phytosome®, 1st week, TDS: 2nd week, BDS) (<jats:italic>n</jats:italic> = 50, quercetin group) or SC alone (<jats:italic>n</jats:italic> = 50, control group).</jats:p><jats:p><jats:bold>Results:</jats:bold> After one week of treatment, patients in the quercetin group showed a speedy recovery from COVID-19 as compared to the control group, i.e., 34 patients (vs. 12 in the control group) tested negative for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (<jats:italic>p</jats:italic> = 0.0004), and 26 patients (vs. 12 in the control group) had their COVID-19-associated acute symptoms resolved (<jats:italic>p</jats:italic> = 0.0051). Patients in the quercetin group also showed a significant fall in the serum lactate dehydrogenase (LDH) mean values i.e., from 406.56 ± 183.92 to 257.74 ± 110.73 U/L, <jats:italic>p</jats:italic> = 0.0001. Quercetin was well-tolerated by all the 50 patients, and no side effects were reported.</jats:p><jats:p><jats:bold>Conclusion:</jats:bold> Our results, suggest the possible therapeutic role of quercetin in early-stage COVID-19, including speedy clearance of SARS-CoV-2, early resolution of the acute symptoms and modulation of the host’s hyperinflammatory response.</jats:p><jats:p><jats:bold>Clinical Trial Registration:</jats:bold><jats:ext-link>clinicaltrials.gov</jats:ext-link>, identifier NCT04861298</jats:p>",
"alternative-id": [
"10.3389/fphar.2022.1096853"
],
"author": [
{
"affiliation": [],
"family": "Di Pierro",
"given": "Francesco",
"sequence": "first"
},
{
"affiliation": [],
"family": "Khan",
"given": "Amjad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Iqtadar",
"given": "Somia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mumtaz",
"given": "Sami Ullah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chaudhry",
"given": "Muhammad Nabeel Akbar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bertuccioli",
"given": "Alexander",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Derosa",
"given": "Giuseppe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Maffioli",
"given": "Pamela",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Togni",
"given": "Stefano",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Riva",
"given": "Antonella",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Allegrini",
"given": "Pietro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Recchia",
"given": "Martino",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zerbinati",
"given": "Nicola",
"sequence": "additional"
}
],
"container-title": "Frontiers in Pharmacology",
"container-title-short": "Front. Pharmacol.",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"frontiersin.org"
]
},
"created": {
"date-parts": [
[
2023,
1,
13
]
],
"date-time": "2023-01-13T05:32:47Z",
"timestamp": 1673587967000
},
"deposited": {
"date-parts": [
[
2023,
1,
13
]
],
"date-time": "2023-01-13T05:32:50Z",
"timestamp": 1673587970000
},
"indexed": {
"date-parts": [
[
2023,
1,
14
]
],
"date-time": "2023-01-14T06:17:46Z",
"timestamp": 1673677066891
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
1,
13
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
1,
13
]
],
"date-time": "2023-01-13T00:00:00Z",
"timestamp": 1673568000000
}
}
],
"link": [
{
"URL": "https://www.frontiersin.org/articles/10.3389/fphar.2022.1096853/full",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1965",
"original-title": [],
"prefix": "10.3389",
"published": {
"date-parts": [
[
2023,
1,
13
]
]
},
"published-online": {
"date-parts": [
[
2023,
1,
13
]
]
},
"publisher": "Frontiers Media SA",
"reference": [
{
"DOI": "10.1016/j.ijbiomac.2020.07.235",
"article-title": "Structural stability of SARS-CoV-2 3CL pro and identification of quercetin as an inhibitor by experimental screening",
"author": "Abian",
"doi-asserted-by": "publisher",
"first-page": "1693",
"journal-title": "Int. J. Biol. Macromol.",
"key": "B1",
"volume": "164",
"year": "2020"
},
{
"DOI": "10.1016/j.foodchem.2021.131594",
"article-title": "Inhibition of the SARS-CoV-2 3CL(pro) main protease by plant polyphenols",
"author": "Bahun",
"doi-asserted-by": "publisher",
"first-page": "131594",
"journal-title": "Food. Chem.",
"key": "B2",
"volume": "373",
"year": "2022"
},
{
"DOI": "10.3389/fphar.2021.704205",
"article-title": "The drug repurposing for COVID-19 clinical trials provide very effective therapeutic combinations: Lessons learned from major clinical studies",
"author": "Chakraborty",
"doi-asserted-by": "publisher",
"first-page": "704205",
"journal-title": "Front. Pharmacol.",
"key": "B3",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1016/j.bmc.2006.09.014",
"article-title": "Binding interaction of quercetin-3-beta-galactoside and its synthetic derivatives with SARS-CoV 3CL(pro): Structure-activity relationship studies reveal salient pharmacophore features",
"author": "Chen",
"doi-asserted-by": "publisher",
"first-page": "8295",
"journal-title": "Bioorg Med. Chem.",
"key": "B4",
"volume": "14",
"year": "2006"
},
{
"DOI": "10.1093/jac/dkg291",
"article-title": "In vitro antiviral activities of Caesalpinia pulcherrima and its related flavonoids",
"author": "Chiang",
"doi-asserted-by": "publisher",
"first-page": "194",
"journal-title": "J. Antimicrob. Chemother.",
"key": "B5",
"volume": "52",
"year": "2003"
},
{
"DOI": "10.3389/fimmu.2020.01451",
"article-title": "Quercetin and vitamin C: An experimental, synergistic therapy for the prevention and treatment of SARS-CoV-2 related disease (COVID-19)",
"author": "Colunga Biancatelli",
"doi-asserted-by": "publisher",
"first-page": "1451",
"journal-title": "Front. Immunol.",
"key": "B6",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1002/ptr.6887",
"article-title": "A role for quercetin in coronavirus disease 2019 (COVID-19)",
"author": "Derosa",
"doi-asserted-by": "publisher",
"first-page": "1230",
"journal-title": "Phytother. Res.",
"key": "B7",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1002/ptr.7309",
"article-title": "Quercetin and its derivates as antiviral potentials: A comprehensive review",
"author": "Di Petrillo",
"doi-asserted-by": "publisher",
"first-page": "266",
"journal-title": "Phytother. Res.",
"key": "B8",
"volume": "36",
"year": "2022"
},
{
"DOI": "10.2147/IJGM.S318720",
"article-title": "Possible therapeutic effects of adjuvant quercetin supplementation against early-stage COVID-19 infection: A prospective, randomized, controlled, and open-label study",
"author": "Di Pierro",
"doi-asserted-by": "publisher",
"first-page": "2359",
"journal-title": "Int. J. Gen. Med.",
"key": "B9",
"volume": "14",
"year": ""
},
{
"DOI": "10.2147/IJGM.S318949",
"article-title": "Potential clinical benefits of quercetin in the early stage of COVID-19: Results of a second, pilot, randomized, controlled and open-label clinical trial",
"author": "Di Pierro",
"doi-asserted-by": "publisher",
"first-page": "2807",
"journal-title": "Int. J. Gen. Med.",
"key": "B10",
"volume": "14",
"year": ""
},
{
"DOI": "10.1016/j.ajem.2020.05.073",
"article-title": "Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: A pooled analysis",
"author": "Henry",
"doi-asserted-by": "publisher",
"first-page": "1722",
"journal-title": "Am. J. Emerg. Med.",
"key": "B11",
"volume": "38",
"year": "2020"
},
{
"DOI": "10.3390/antiox11050876",
"article-title": "The therapeutic and prophylactic potential of quercetin against COVID-19: An outlook on the clinical studies, inventive compositions, and patent literature",
"author": "Imran",
"doi-asserted-by": "publisher",
"first-page": "876",
"journal-title": "Antioxidants (Basel)",
"key": "B12",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2031994",
"article-title": "Baricitinib plus remdesivir for hospitalized adults with covid-19",
"author": "Kalil",
"doi-asserted-by": "publisher",
"first-page": "795",
"journal-title": "N. Engl. J. Med.",
"key": "B13",
"volume": "384",
"year": "2020"
},
{
"DOI": "10.3390/pathogens10060758",
"article-title": "Bioactive polyphenolic compounds showing strong antiviral activities against severe acute respiratory syndrome coronavirus 2",
"author": "Kandeil",
"doi-asserted-by": "publisher",
"first-page": "758",
"journal-title": "Pathogens",
"key": "B14",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1038/s41586-020-2180-5",
"article-title": "Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor",
"author": "Lan",
"doi-asserted-by": "publisher",
"first-page": "215",
"journal-title": "Nature",
"key": "B15",
"volume": "581",
"year": "2020"
},
{
"DOI": "10.1038/s41586-021-03995-1",
"article-title": "Virus-induced senescence is a driver and therapeutic target in COVID-19",
"author": "Lee",
"doi-asserted-by": "publisher",
"first-page": "283",
"journal-title": "Nature",
"key": "B16",
"volume": "599",
"year": "2021"
},
{
"DOI": "10.1021/acs.jafc.0c05064",
"article-title": "Quercetin and its metabolites inhibit recombinant human angiotensin-converting enzyme 2 (ACE2) activity",
"author": "Liu",
"doi-asserted-by": "publisher",
"first-page": "13982",
"journal-title": "J. Agric. Food Chem.",
"key": "B17",
"volume": "68",
"year": "2020"
},
{
"DOI": "10.3906/biy-2104-16",
"article-title": "Treatment of COVID-19 patients with quercetin: A prospective, single center, randomized, controlled trial",
"author": "Onal",
"doi-asserted-by": "publisher",
"first-page": "518",
"journal-title": "Turk J. Biol.",
"key": "B18",
"volume": "45",
"year": "2021"
},
{
"DOI": "10.1093/oxfordjournals.jbchem.a123251",
"article-title": "Mechanisms of inhibition of various cellular DNA and RNA polymerases by several flavonoids",
"author": "Ono",
"doi-asserted-by": "publisher",
"first-page": "609",
"journal-title": "J. Biochem.",
"key": "B19",
"volume": "108",
"year": "1990"
},
{
"DOI": "10.1056/NEJMoa2021436",
"article-title": "Dexamethasone in hospitalized patients with covid-19",
"doi-asserted-by": "publisher",
"first-page": "693",
"journal-title": "N. Engl. J. Med.",
"key": "B20",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(21)00676-0",
"article-title": "Tocilizumab in patients admitted to hospital with COVID-19: A randomised, controlled, open-label, platform trial",
"doi-asserted-by": "publisher",
"first-page": "1637",
"journal-title": "Lancet",
"key": "B21",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.3390/biomedicines9040375",
"article-title": "Rutin is a low micromolar inhibitor of SARS-CoV-2 main protease 3CLpro: Implications for drug Design of quercetin analogs",
"author": "Rizzuti",
"doi-asserted-by": "publisher",
"first-page": "375",
"journal-title": "Biomedicines",
"key": "B22",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.3390/life12010066",
"article-title": "Promising effects of 3-month period of quercetin Phytosome® supplementation in the prevention of symptomatic COVID-19 disease in healthcare workers: A pilot study",
"author": "Rondanelli",
"doi-asserted-by": "publisher",
"first-page": "66",
"journal-title": "Life (Basel)",
"key": "B23",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1186/s12950-021-00268-6",
"article-title": "Anti-inflammatory potential of Quercetin in COVID-19 treatment",
"author": "Saeedi-Boroujeni",
"doi-asserted-by": "publisher",
"first-page": "3",
"journal-title": "J. Inflamm. (Lond)",
"key": "B24",
"volume": "18",
"year": "2021"
},
{
"DOI": "10.1016/j.ejphar.2021.174615",
"article-title": "The therapeutic efficacy of quercetin in combination with antiviral drugs in hospitalized COVID-19 patients: A randomized controlled trial",
"author": "Shohan",
"doi-asserted-by": "publisher",
"first-page": "174615",
"journal-title": "Eur. J. Pharmacol.",
"key": "B25",
"volume": "914",
"year": "2022"
},
{
"DOI": "10.1016/j.ajem.2020.11.025",
"article-title": "Lactate dehydrogenase level as a COVID-19 severity marker",
"author": "Szarpak",
"doi-asserted-by": "publisher",
"first-page": "638",
"journal-title": "Am. J. Emerg. Med.",
"key": "B26",
"volume": "45",
"year": "2021"
},
{
"DOI": "10.1155/2016/5460302",
"article-title": "Natural Compounds as Regulators of NLRP3 Inflammasome-Mediated IL-1β Production",
"author": "Tőzsér",
"doi-asserted-by": "publisher",
"first-page": "5460302",
"journal-title": "Mediators Inflamm.",
"key": "B32",
"year": "2016"
},
{
"key": "B27",
"unstructured": "GRAS notices: Agency response letter GRAS notice No. GRN 000341\n Us Food and Drug Administration Letter\n 2010"
},
{
"DOI": "10.3390/v8010006",
"article-title": "Quercetin as an antiviral agent inhibits influenza A virus (iav) entry",
"author": "Wu",
"doi-asserted-by": "publisher",
"first-page": "6",
"journal-title": "Viruses",
"key": "B28",
"volume": "8",
"year": "2015"
},
{
"DOI": "10.1126/science.abb2762",
"article-title": "Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2",
"author": "Yan",
"doi-asserted-by": "publisher",
"first-page": "1444",
"journal-title": "Science",
"key": "B29",
"volume": "367",
"year": "2020"
},
{
"DOI": "10.1128/jvi.78.20.11334-11339.2004",
"article-title": "Small molecules blocking the entry of severe acute respiratory syndrome coronavirus into host cells",
"author": "Yi",
"doi-asserted-by": "publisher",
"first-page": "11334",
"journal-title": "J. Virol.",
"key": "B30",
"volume": "78",
"year": "2004"
},
{
"DOI": "10.14739/2310-1210.2021.5.231714",
"article-title": "Efficacy of quercetin in patients with pneumonia associated with coronavirus disease (COVID-19)",
"author": "Zupanets",
"doi-asserted-by": "publisher",
"first-page": "636",
"journal-title": "Zaporizhia Med. J.",
"key": "B31",
"volume": "23",
"year": "2021"
}
],
"reference-count": 32,
"references-count": 32,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.frontiersin.org/articles/10.3389/fphar.2022.1096853/full"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology (medical)",
"Pharmacology"
],
"subtitle": [],
"title": "Quercetin as a possible complementary agent for early-stage COVID-19: Concluding results of a randomized clinical trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.3389/crossmark-policy",
"volume": "13"
}
