Potential Clinical Benefits of Quercetin in the Early Stage of COVID-19: Results of a Second, Pilot, Randomized, Controlled and Open-Label Clinical Trial
et al., International Journal of General Medicine, doi:10.2147/IJGM.S318949, NCT04861298, Jun 2021
Quercetin for COVID-19
27th treatment shown to reduce risk in
July 2021, now with p = 0.002 from 12 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
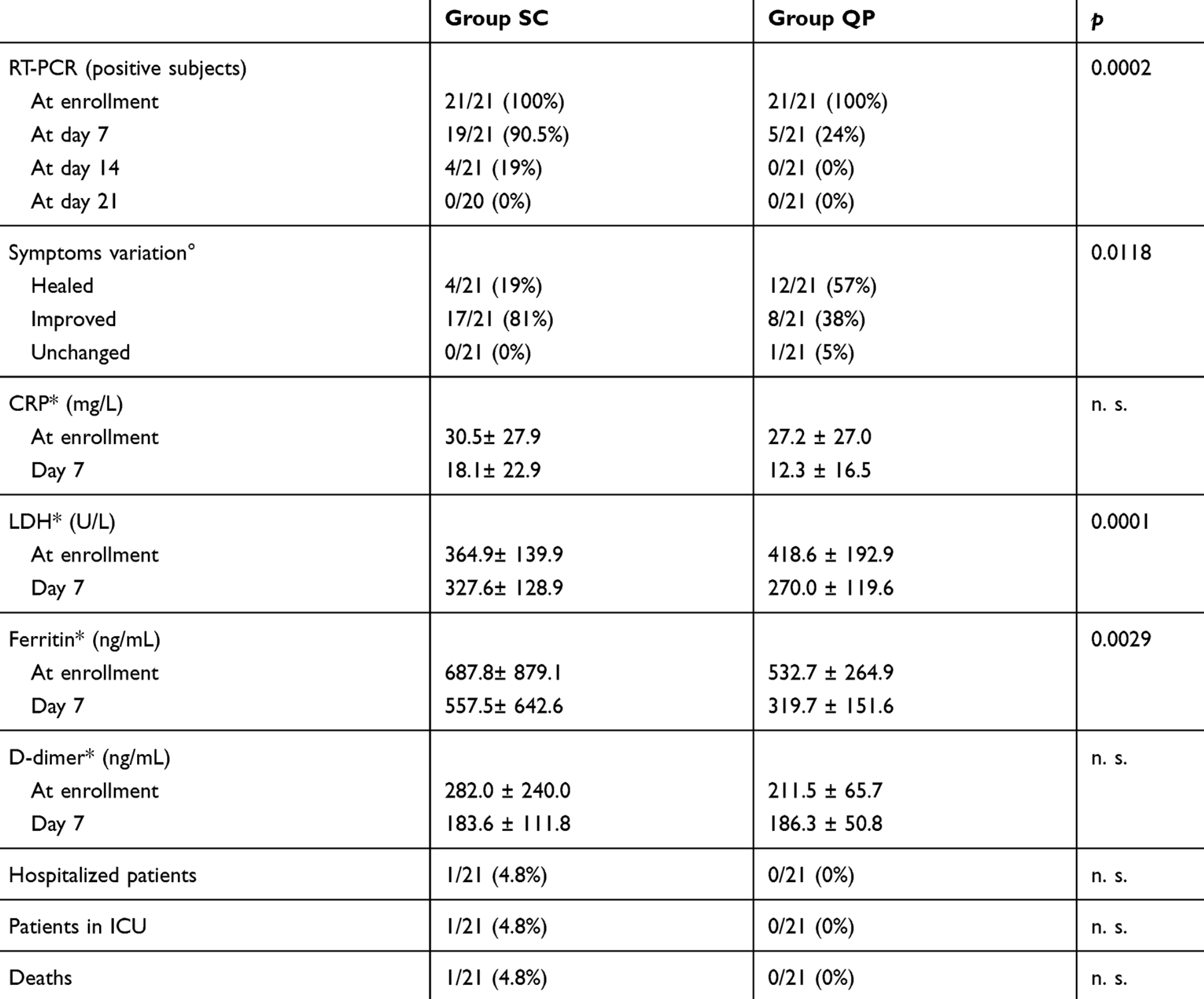
RCT 42 outpatients in Pakistan, 21 treated with quercetin phytosome, showing faster viral clearance and lower symptom severity with treatment. Patients in the treatment group were younger (43 vs. 56).
Bioavailability. Quercetin has low bioavailability and studies typically use advanced formulations to improve bioavailability which may be required to reach therapeutic concentrations.
This study is excluded in meta-analysis:
randomization resulted in significant baseline differences that were not adjusted for.
|
risk of death, 66.7% lower, RR 0.33, p = 1.00, treatment 0 of 21 (0.0%), control 1 of 21 (4.8%), NNT 21, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of ICU admission, 66.7% lower, RR 0.33, p = 1.00, treatment 0 of 21 (0.0%), control 1 of 21 (4.8%), NNT 21, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of hospitalization, 66.7% lower, RR 0.33, p = 1.00, treatment 0 of 21 (0.0%), control 1 of 21 (4.8%), NNT 21, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of no viral clearance, 73.7% lower, RR 0.26, p < 0.001, treatment 5 of 21 (23.8%), control 19 of 21 (90.5%), NNT 1.5, day 7.
|
|
risk of no viral clearance, 88.9% lower, RR 0.11, p = 0.11, treatment 0 of 21 (0.0%), control 4 of 21 (19.0%), NNT 5.2, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), day 14.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Di Pierro et al., 24 Jun 2021, Randomized Controlled Trial, Pakistan, peer-reviewed, 12 authors, study period December 2020 - March 2021, trial NCT04861298 (history).
Potential Clinical Benefits of Quercetin in the Early Stage of COVID-19: Results of a Second, Pilot, Randomized, Controlled and Open-Label Clinical Trial
International Journal of General Medicine, doi:10.2147/ijgm.s318949
Background: The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the cause of the ongoing global pandemic known as COVID-19. Based on the potential antiviral role of quercetin, and on its described anti-blood clotting, anti-inflammatory and antioxidant properties, we hypothesize that subjects with mild COVID-19 treated with Quercetin Phytosome ® (QP), a novel bioavailable form of quercetin, may have a shorter time to virus clearance, a milder symptomatology, and higher probabilities of a benign earlier resolution of the disease. Methods: In our 2-week, randomized, open-label, and controlled clinical study, we have enrolled 42 COVID-19 outpatients. Twenty-one have been treated with the standard of care (SC), and 21 with QP as add-on supplementation to the SC. Our main aims were to check virus clearance and symptoms.
Results: The interim results reveal that after 1 week of treatment, 16 patients of the QP group were tested negative for SARS-CoV-2 and 12 patients had all their symptoms diminished; in the SC group, 2 patients were tested SARS-CoV-2 negative and 4 patients had their symptoms partially improved. By 2 weeks, the remaining 5 patients of the QP group tested negative for SARS-CoV-2, whereas in the SC group out of 19 remaining patients, 17 tested negatives by week 2, one tested negative by week 3 and one patient, still positive, expired by day 20. Concerning blood parameters, the add on therapy with QP, reduced LDH (−35.5%), Ferritin (−40%), CRP (−54.8%) and D-dimer (−11.9%). Conclusion: QP statistically shortens the timing of molecular test conversion from positive to negative, reducing at the same time symptoms severity and negative predictors of COVID-19.
Disclosure FDP belongs to the Scientific Board of Pharmextracta. AB is a Pharmextracta consultant. PA, ST and AR belong to the Scientific Board of Indena. ST & PA are employees of Indena SpA, producer of Quercetin Phytosome (ingredient used in the trial). AR reports a patent WO2019016146A1 pending. The other authors do not claim possible conflicts of interest.
International Journal of General Medicine
Dovepress
DovePress International Journal of General Medicine 2021:14
References
Argenziano, Bruce, Slater, Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series, BMJ, doi:10.1136/bmj.m1996
Bae, Kim, Kim, Quercetin enhances susceptibility to NK cell-mediated lysis of tumor cells through induction of NKG2D ligands and suppression of HSP70, J Immunother, doi:10.1097/CJI.0b013e3181d32f22
Biancatelli, Berrill, Catravas, Marik, Quercetin and vitamin C: an experimental, synergistic therapy for the prevention and treatment of SARS-CoV-2 related disease (COVID-19), Front Immunol, doi:10.3389/fimmu.2020.01451
Brito, Lima, Cordeiro, Da, Nizer, Effectiveness of supplementation with quercetin-type flavonols for treatment of viral lower respiratory tract infections: systematic review and meta-analysis of preclinical studies, Phytother Res, doi:10.1002/ptr.7122
Bulut, Kato, Epidemiology of COVID-19, Turk J Med Sci, doi:10.3906/sag-2004-172
Chang, Ng, Sun, Lactoferrin as potential preventative and adjunct treatment for COVID-19, Int J Antimicrob Agents, doi:10.1016/j.ijantimicag.2020.106118
Chen, Zheng, Liu, Yan, Xu et al., Plasma CRP level is positively associated with the severity of COVID-19, Ann Clin Microbiol Antimicrob, doi:10.1186/s12941-020-00362-2
Derosa, Maffioli, Angelo, Pierro, A role for quercetin in coronavirus disease 2019 (COVID-19), Phytother Res, doi:10.1002/ptr.6887
Diniz, Souza, Duarte, Sousa, Mechanistic aspects and therapeutic potential of quercetin against COVID-19-associated acute kidney injury, Molecules, doi:10.3390/molecules25235772
Farina, Ramirez, Lorenzo, COVID-19: pharmacology and kinetics of viral clearance, Pharmacol Res, doi:10.1016/j.phrs.2020.105114
Gao, Liu, Wang, Liu, Xu et al., Preparation of a chemically stable quercetin formulation using nanosuspension technology, Int J Pharm, doi:10.1016/j.ijpharm.2010.11.009
Guan, Zhang, Fu, Clinical and inflammatory features-based machine learning model for fatal risk prediction of hospitalized COVID-19 patients: results from a retrospective cohort study, Ann Med, doi:10.1080/07853890.2020.1868564
Holford, Carr, Jovic, Vitamin C-an adjunctive therapy for respiratory infection, sepsis and COVID-19, Nutrients, doi:10.3390/nu12123760
Hu, Huang, Yin, The cytokine storm and COVID-19, J Med Virol, doi:10.1002/jmv.26232
Jamilloux, Henry, Belot, Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions, Autoimmun Rev, doi:10.1016/j.autrev.2020.102567
Jean, Lee, Hsueh, Treatment options for COVID-19: the reality and challenges, J Microbiol Immunol Infect, doi:10.1016/j.jmii.2020.03.034
Li, Yao, Han, Quercetin, inflammation and immunity, Nutrients, doi:10.3390/nu8030167
Mccullough, Kelly, Ruocco, Pathophysiological basis and rationale for early outpatient treatment of SARS-CoV-2 (COVID-19) infection, Am J Med, doi:10.1016/j.amjmed.2020.07.003
Mohammadi-Sartang, Mazloom, Sherafatmanesh, Ghorbani, Firoozi, Effects of supplementation with quercetin on plasma C-reactive protein concentrations: a systematic review and meta-analysis of randomized controlled trials, Eur J Clin Nutr, doi:10.1038/ejcn.2017.55
Moradian, Gouravani, Salehi, Cytokine release syndrome: inhibition of pro-inflammatory cytokines as a solution for reducing COVID-19 mortality, Eur Cytokine Netw, doi:10.1684/ecn.2020.0451
Pierro, Derosa, Maffioli, Possible therapeutic effects of adjuvant quercetin supplementation against early stage COVID-19 infection: a prospective, randomized, controlled, and open-label study, Int J Gen Med, doi:10.2147/IJGM.S318720
Pierro, Khan, Bertuccioli, Quercetin Phytosome ® as a potential candidate for managing COVID-19, Minerva Gastroenterol Dietol, doi:10.23736/S1121-421X.20.02771-3
Riva, Ronchi, Petrangolini, Bosisio, Allegrini, Improved Oral Absorption of quercetin from quercetin phytosome ® , a new delivery system based on food grade lecithin, Eur J Drug Metab Pharmacokinet, doi:10.1007/s13318-018-0517-3
Shanmugaraj, Siriwattananon, Wangkanont, Phoolcharoen, Perspectives on monoclonal antibody therapy as potential therapeutic intervention for Coronavirus disease-19 (COVID-19), Asian Pac J Allergy Immunol, doi:10.12932/AP-200220-0773
Sharma, Sultan, Ding, Triggle, A Review of the progress and challenges of developing a vaccine for COVID-19, Front Immunol, doi:10.3389/fimmu.2020.585354
Singh, Pritam, Pandey, Yadav, Microstructure, pathophysiology, and potential therapeutics of COVID-19: a comprehensive review, J Med Virol, doi:10.1002/jmv.26254
Suter, Consolaro, Pedroni, A simple, home-therapy algorithm to prevent hospitalization for covid-19 patients: a retrospective observational matched-cohort study, Journal of General Medicine, doi:10.1101/2021.03.25.21254296
Tanaka, Furuta, Asano, Kobayashi, Modulation of Th1/Th2 cytokine balance by quercetin in vitro, Medicines, doi:10.3390/medicines7080046
Taslidere, Dogan, Elbe, Vardi, Cetin et al., Quercetin protection against ciprofloxacin induced liver damage in rats, Biotech Histochem, doi:10.3109/10520295.2015.1085093
Toniati, Piva, Cattalini, Tocilizumab for the treatment of severe COVID-19 pneumonia with hyper-inflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy, Autoimmun Rev, doi:10.1016/j.autrev.2020.102568
Ullah, Munir, Badshah, Important flavonoids and their role as a therapeutic agent, Molecules, doi:10.3390/molecules25225243
Vicidomini, Roviello, Roviello, Molecular basis of the therapeutical potential of clove (Syzygium aromaticum L.) and clues to its anti-COVID-19 utility, Molecules, doi:10.3390/molecules26071880
Wang, Sun, Mao, The biological activities, chemical stability, metabolism, and delivery systems of quercetin: a review, Trends Food Sci Technol, doi:10.1016/j.tifs.2016.07.004
Yang, Barnard, Emert, Clinical characteristics of patients with coronavirus disease 2019 (COVID-19) receiving emergency medical services in King County, Washington, JAMA Netw Open, doi:10.1001/jamanetworkopen.2020.14549
Zhou, Huntington, Zhang, Natural Killer cell activation, reduced ACE2, TMPRSS2, cytokines G-CSF, M-CSF and SARS-CoV-2-S pseudovirus infectivity by MEK inhibitor treatment of human cells, bioRxiv, doi:10.1101/2020.08.02.230839
DOI record:
{
"DOI": "10.2147/ijgm.s318949",
"ISSN": [
"1178-7074"
],
"URL": "http://dx.doi.org/10.2147/IJGM.S318949",
"author": [
{
"ORCID": "http://orcid.org/0000-0001-6654-8675",
"affiliation": [],
"authenticated-orcid": true,
"family": "Di Pierro",
"given": "Francesco",
"sequence": "first"
},
{
"affiliation": [],
"family": "Iqtadar",
"given": "Somia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Khan",
"given": "Amjad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ullah Mumtaz",
"given": "Sami",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Masud Chaudhry",
"given": "Mohsin",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3922-9115",
"affiliation": [],
"authenticated-orcid": true,
"family": "Bertuccioli",
"given": "Alexander",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3573-4760",
"affiliation": [],
"authenticated-orcid": true,
"family": "Derosa",
"given": "Giuseppe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Maffioli",
"given": "Pamela",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Togni",
"given": "Stefano",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2819-943X",
"affiliation": [],
"authenticated-orcid": true,
"family": "Riva",
"given": "Antonella",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4380-9577",
"affiliation": [],
"authenticated-orcid": true,
"family": "Allegrini",
"given": "Pietro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Khan",
"given": "Saeed",
"sequence": "additional"
}
],
"container-title": "International Journal of General Medicine",
"container-title-short": "IJGM",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
6,
23
]
],
"date-time": "2021-06-23T21:34:35Z",
"timestamp": 1624484075000
},
"deposited": {
"date-parts": [
[
2021,
6,
23
]
],
"date-time": "2021-06-23T21:34:37Z",
"timestamp": 1624484077000
},
"indexed": {
"date-parts": [
[
2024,
5,
1
]
],
"date-time": "2024-05-01T20:13:22Z",
"timestamp": 1714594402733
},
"is-referenced-by-count": 111,
"issued": {
"date-parts": [
[
2021,
6
]
]
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc/3.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
6,
1
]
],
"date-time": "2021-06-01T00:00:00Z",
"timestamp": 1622505600000
}
}
],
"link": [
{
"URL": "https://www.dovepress.com/getfile.php?fileID=70920",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.dovepress.com/getfile.php?fileID=70920",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "301",
"original-title": [],
"page": "2807-2816",
"prefix": "10.2147",
"published": {
"date-parts": [
[
2021,
6
]
]
},
"published-online": {
"date-parts": [
[
2021,
6
]
]
},
"publisher": "Informa UK Limited",
"reference": [
{
"DOI": "10.3906/sag-2004-172",
"author": "Bulut",
"doi-asserted-by": "publisher",
"first-page": "563",
"journal-title": "Turk J Med Sci",
"key": "ref1",
"volume": "50",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2020.585354",
"author": "Sharma",
"doi-asserted-by": "publisher",
"first-page": "585354",
"journal-title": "Front Immunol",
"key": "ref2",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1002/jmv.26254",
"author": "Singh",
"doi-asserted-by": "publisher",
"first-page": "275",
"journal-title": "J Med Virol",
"key": "ref3",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.1016/j.jmii.2020.03.034",
"author": "Jean",
"doi-asserted-by": "publisher",
"first-page": "436",
"journal-title": "J Microbiol Immunol Infect",
"key": "ref4",
"volume": "53",
"year": "2020"
},
{
"DOI": "10.12932/AP-200220-0773",
"author": "Shanmugaraj",
"doi-asserted-by": "publisher",
"first-page": "10",
"journal-title": "Asian Pac J Allergy Immunol",
"key": "ref5",
"volume": "38",
"year": "2020"
},
{
"DOI": "10.1016/j.autrev.2020.102568",
"author": "Toniati",
"doi-asserted-by": "publisher",
"first-page": "102568",
"journal-title": "Autoimmun Rev",
"key": "ref6",
"volume": "19",
"year": "2020"
},
{
"DOI": "10.1101/2021.03.25.21254296",
"author": "Suter",
"doi-asserted-by": "publisher",
"journal-title": "medRxiv Preprint",
"key": "ref7"
},
{
"DOI": "10.1002/jmv.26232",
"author": "Hu",
"doi-asserted-by": "publisher",
"first-page": "250",
"journal-title": "J Med Virol",
"key": "ref8",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.1016/j.autrev.2020.102567",
"author": "Jamilloux",
"doi-asserted-by": "publisher",
"first-page": "102567",
"journal-title": "Autoimmun Rev",
"key": "ref9",
"volume": "19",
"year": "2020"
},
{
"DOI": "10.1002/ptr.6887",
"author": "Derosa",
"doi-asserted-by": "publisher",
"first-page": "1230",
"journal-title": "Phytother Res",
"key": "ref10",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1016/j.fitote.2015.09.018",
"author": "D’Andrea",
"doi-asserted-by": "publisher",
"first-page": "256",
"journal-title": "Fitoterapia",
"key": "ref11",
"volume": "106",
"year": "2015"
},
{
"DOI": "10.3109/10520295.2015.1085093",
"author": "Taslidere",
"doi-asserted-by": "publisher",
"first-page": "116",
"journal-title": "Biotech Histochem",
"key": "ref12",
"volume": "91",
"year": "2016"
},
{
"DOI": "10.3390/molecules25225243",
"author": "Ullah",
"doi-asserted-by": "publisher",
"first-page": "5243",
"journal-title": "Molecules",
"key": "ref13",
"volume": "25",
"year": "2020"
},
{
"DOI": "10.3390/nu8030167",
"author": "Li",
"doi-asserted-by": "publisher",
"first-page": "167",
"journal-title": "Nutrients",
"key": "ref14",
"volume": "8",
"year": "2016"
},
{
"DOI": "10.1684/ecn.2020.0451",
"author": "Moradian",
"doi-asserted-by": "publisher",
"first-page": "81",
"journal-title": "Eur Cytokine Netw",
"key": "ref15",
"volume": "31",
"year": "2020"
},
{
"DOI": "10.1186/s12941-020-00362-2",
"author": "Chen",
"doi-asserted-by": "publisher",
"first-page": "18",
"journal-title": "Ann Clin Microbiol Antimicrob",
"key": "ref16",
"volume": "19",
"year": "2020"
},
{
"DOI": "10.1038/ejcn.2017.55",
"author": "Mohammadi-Sartang",
"doi-asserted-by": "publisher",
"first-page": "1033",
"journal-title": "Eur J Clin Nutr",
"key": "ref17",
"volume": "71",
"year": "2017"
},
{
"DOI": "10.3390/medicines7080046",
"author": "Tanaka",
"doi-asserted-by": "publisher",
"first-page": "46",
"journal-title": "Medicines (Basel)",
"key": "ref18",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1097/CJI.0b013e3181d32f22",
"author": "Bae",
"doi-asserted-by": "publisher",
"first-page": "391",
"journal-title": "J Immunother",
"key": "ref19",
"volume": "33",
"year": "2010"
},
{
"DOI": "10.1101/2020.08.02.230839",
"author": "Zhou",
"doi-asserted-by": "publisher",
"journal-title": "bioRxiv [Preprint]",
"key": "ref20",
"volume": "2020",
"year": "2020"
},
{
"DOI": "10.1002/ptr.7122",
"author": "Brito",
"doi-asserted-by": "publisher",
"journal-title": "Phytother Res",
"key": "ref21",
"year": "2021"
},
{
"DOI": "10.1016/j.ijpharm.2010.11.009",
"author": "Gao",
"doi-asserted-by": "publisher",
"first-page": "231",
"journal-title": "Int J Pharm",
"key": "ref22",
"volume": "404",
"year": "2011"
},
{
"DOI": "10.1016/j.tifs.2016.07.004",
"author": "Wang",
"doi-asserted-by": "publisher",
"first-page": "21",
"journal-title": "Trends Food Sci Technol",
"key": "ref23",
"volume": "56",
"year": "2016"
},
{
"DOI": "10.1007/s13318-018-0517-3",
"author": "Riva",
"doi-asserted-by": "publisher",
"first-page": "169",
"journal-title": "Eur J Drug Metab Pharmacokinet",
"key": "ref24",
"volume": "44",
"year": "2019"
},
{
"DOI": "10.23736/S1121-421X.20.02771-3",
"author": "Di Pierro",
"doi-asserted-by": "publisher",
"journal-title": "Minerva Gastroenterol Dietol",
"key": "ref25",
"year": "2020"
},
{
"DOI": "10.1001/jamanetworkopen.2020.14549",
"author": "Yang",
"doi-asserted-by": "publisher",
"first-page": "7",
"journal-title": "JAMA Netw Open",
"key": "ref26",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.1136/bmj.m1996",
"author": "Argenziano",
"doi-asserted-by": "publisher",
"first-page": "m1996",
"journal-title": "BMJ",
"key": "ref27",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.1016/j.amjmed.2020.07.003",
"author": "McCullough",
"doi-asserted-by": "publisher",
"first-page": "16",
"journal-title": "Am J Med",
"key": "ref28",
"volume": "134",
"year": "2021"
},
{
"DOI": "10.3390/nu12123760",
"author": "Holford",
"doi-asserted-by": "publisher",
"first-page": "3760",
"journal-title": "Nutrients",
"key": "ref29",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1016/j.ijantimicag.2020.106118",
"author": "Chang",
"doi-asserted-by": "publisher",
"first-page": "106118",
"journal-title": "Int J Antimicrob Agents",
"key": "ref30",
"volume": "56",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2020.01451",
"author": "Colunga Biancatelli",
"doi-asserted-by": "publisher",
"first-page": "1451",
"journal-title": "Front Immunol",
"key": "ref31",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.3390/molecules25235772",
"author": "Diniz",
"doi-asserted-by": "publisher",
"first-page": "5772",
"journal-title": "Molecules",
"key": "ref32",
"volume": "25",
"year": "2020"
},
{
"DOI": "10.1016/j.phrs.2020.105114",
"author": "Farina",
"doi-asserted-by": "publisher",
"first-page": "105114",
"journal-title": "Pharmacol Res",
"key": "ref33",
"volume": "161",
"year": "2020"
},
{
"DOI": "10.2147/IJGM.S318720",
"author": "Di Pierro",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Int J Gen Med",
"key": "ref34",
"volume": "14",
"year": "2021"
},
{
"DOI": "10.1080/07853890.2020.1868564",
"author": "Guan",
"doi-asserted-by": "publisher",
"first-page": "257",
"journal-title": "Ann Med",
"key": "ref35",
"volume": "53",
"year": "2021"
},
{
"DOI": "10.3390/molecules26071880",
"author": "Vicidomini",
"doi-asserted-by": "publisher",
"first-page": "1880",
"journal-title": "Molecules",
"key": "ref36",
"volume": "26",
"year": "2021"
}
],
"reference-count": 36,
"references-count": 36,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.dovepress.com/potential-clinical-benefits-of-quercetin-in-the-early-stage-of-covid-1-peer-reviewed-fulltext-article-IJGM"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Potential Clinical Benefits of Quercetin in the Early Stage of COVID-19: Results of a Second, Pilot, Randomized, Controlled and Open-Label Clinical Trial",
"type": "journal-article",
"volume": "Volume 14"
}
