Advances and Challenges in Antiviral Development for Respiratory Viruses
et al., Pathogens, doi:10.3390/pathogens14010020, Dec 2024
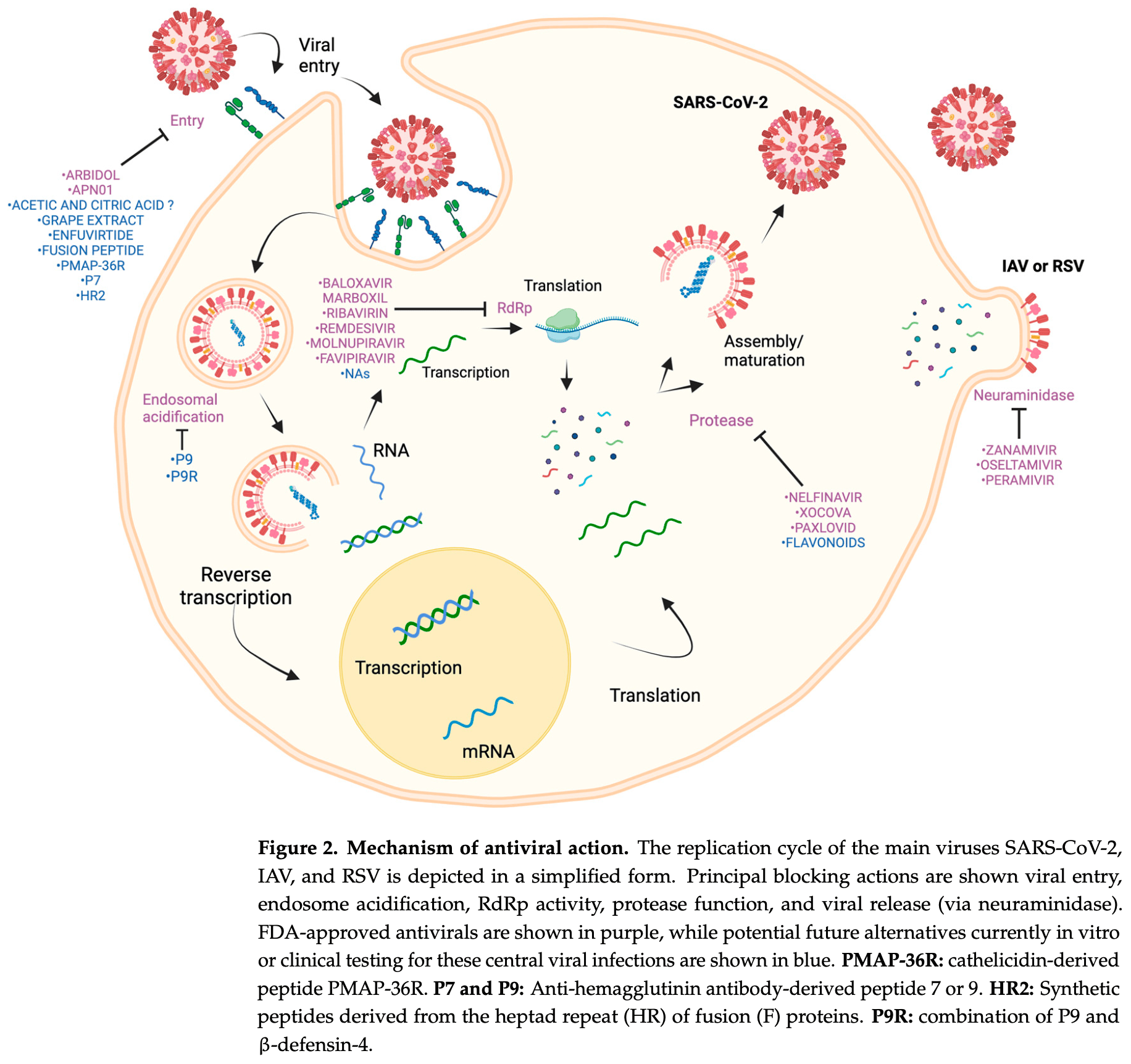
Review of the advances and challenges in developing antivirals for respiratory viruses such as SARS-CoV-2, influenza, and respiratory syncytial virus (RSV). Authors highlight therapeutic strategies targeting critical stages of the viral replication cycle, including inhibitors of viral entry, replication, and assembly.
De Jesús-González et al., 31 Dec 2024, peer-reviewed, 14 authors.
Contact: luis.dejesus@cinvestav.mx (corresponding author), flor.lihe@gmail.com, rondo_vm@yahoo.com, iridiana941901@gmail.com, 36173990@uaz.edu.mx, 37183498@uaz.edu.mx, 20202095@uaz.edu.mx, willy_salvadormtz@hotmail.com, ana.garciaher@imss.gob.mx, adrianvela18@gmail.com, jonathan.hernandez@cinvestav.mx, jose.reyesr@imss.gob.mx, osunajuanfidel.fm@uas.edu.mx, moisesleoninper@gmail.com.
Advances and Challenges in Antiviral Development for Respiratory Viruses
Pathogens, doi:10.3390/pathogens14010020
The development of antivirals for respiratory viruses has advanced markedly in response to the growing threat of pathogens such as Influenzavirus (IAV), respiratory syncytial virus (RSV), and SARS-CoV-2. This article reviews the advances and challenges in this field, highlighting therapeutic strategies that target critical stages of the viral replication cycle, including inhibitors of viral entry, replication, and assembly. In addition, innovative approaches such as inhibiting host cellular proteins to reduce viral resistance and repurposing existing drugs are explored, using advanced bioinformatics tools that optimize the identification of antiviral candidates. The analysis also covers emerging technologies such as nanomedicine and CRISPR gene editing, which promise to improve the stability and efficacy of treatments. While current antivirals offer valuable options, they face challenges such as viral evolution and the need for accessible treatments for vulnerable populations. This article underscores the importance of continued innovation in biotechnology to overcome these limitations and provide safe and effective treatments. Combining traditional and advanced approaches in developing antivirals is essential in order to address respiratory viral diseases that affect global health.
Funding: FUNDACIÓN IMSS, AC funded this research. However, the funders had no role in study design, data collection, analysis, publication decision, or article preparation.
Institutional Review Board Statement: This project was analyzed and approved by the National Committee for Scientific Research of the IMSS, with registration number R-2024-785-073 and protocol title "In silico and in vitro analysis of Repositioned Drugs to inhibit SARS-CoV-2 Replication directed against the NSP5 protease and the NSP12 RNA polymerase". Informed Consent Statement: This project does not require informed consent.
Conflicts of Interest: The authors declare no conflicts of interest.
References
Abbott, Dhamdhere, Liu, Lin, Goudy et al., Development of CRISPR as an Antiviral Strategy to Combat SARS-CoV-2 and Influenza, Cell, doi:10.1016/j.cell.2020.04.020
Abdelqader, Hasan, Alanagreh, Epidemiology of Human Bocavirus in the Middle East and North Africa: Systematic Review, Pathogens, doi:10.3390/pathogens10111456
Abe, Wannigama, Suzuki, Akaneya, Igarashi et al., Real World Effectiveness of Early Ensitrelvir Treatment in Patients with SARS-CoV-2, a Retrospective Case Series, New Microbes New Infect, doi:10.1016/j.nmni.2024.101522
Agut, Antivirales (a Excepción Del Virus de La Inmunodeficiencia Humana y La Hepatitis), EMC-Tratado Med, doi:10.1016/S1636-5410(22)46453-1
Akinosoglou, Schinas, Gogos, Oral Antiviral Treatment for COVID-19: A Comprehensive Review on Nirmatrelvir/Ritonavir, Viruses, doi:10.3390/v14112540
Al-Beltagi, Preda, Goulding, James, Pu et al., Thapsigargin Is a Broad-Spectrum Inhibitor of Major Human Respiratory Viruses: Coronavirus, Respiratory Syncytial Virus and Influenza A Virus, Viruses, doi:10.3390/v13020234
Alfano, Bigoni, Caggiano, Papi, Respiratory Syncytial Virus Infection in Older Adults: An Update, Drugs Aging, doi:10.1007/s40266-024-01118-9
Aljabr, Touzelet, Pollakis, Wu, Munday et al., Investigating the Influence of Ribavirin on Human Respiratory Syncytial Virus RNA Synthesis by Using a High-Resolution Transcriptome Sequencing Approach, J. Virol, doi:10.1128/JVI.02349-15
Arabyan, Kotsynyan, Hakobyan, Zakaryan, Antiviral Agents against African Swine Fever Virus, Virus Res, doi:10.1016/j.virusres.2019.197669
Arevalo-Romero, Chingaté-López, Camacho, Alméciga-Díaz, Ramirez-Segura, Next-Generation Treatments: Immunotherapy and Advanced Therapies for COVID-19, Heliyon, doi:10.1016/j.heliyon.2024.e26423
Avery, Hoffmann, Whalen, The Use of Aerosolized Ribavirin in Respiratory Syncytial Virus Lower Respiratory Tract Infections in Adult Immunocompromised Patients: A Systematic Review, Hosp. Pharm, doi:10.1177/0018578719836646
Baden, El Sahly, Essink, Kotloff, Frey et al., Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine, N. Engl. J. Med, doi:10.1056/NEJMoa2035389
Badia, Garcia-Vidal, Ballana, Viral-Host Dependency Factors as Therapeutic Targets to Overcome Antiviral Drug-Resistance: A Focus on Innate Immune Modulation, Front. Virol, doi:10.3389/fviro.2022.935933
Baldassi, Ambike, Feuerherd, Cheng, Peeler et al., Inhibition of SARS-CoV-2 Replication in the Lung with siRNA/VIPER Polyplexes, J. Control. Release, doi:10.1016/j.jconrel.2022.03.051
Berman, Westbrook, Feng, Gilliland, Bhat et al., The Protein Data Bank, Nucleic Acids Res, doi:10.1093/nar/28.1.235
Bizot, Bousquet, Charpié, Coquelin, Lefevre et al., Rhinovirus: A Narrative Review on Its Genetic Characteristics, Pediatric Clinical Presentations, and Pathogenesis, Front. Pediatr, doi:10.3389/fped.2021.643219
Blair, Remdesivir, A Review in COVID-19, Drugs, doi:10.1007/s40265-023-01926-0
Bobardt, Cheng, De Witte, Selvarajah, Chatterji et al., Hepatitis C Virus NS5A Anchor Peptide Disrupts Human Immunodeficiency Virus, Proc. Natl. Acad. Sci, doi:10.1073/pnas.0801388105
Bonneux, Jacoby, Ceconi, Stobbelaar, Delputte et al., Direct-Acting Antivirals for RSV Treatment, a Review, Antivir. Res, doi:10.1016/j.antiviral.2024.105948
Branche, Falsey, Parainfluenza Virus Infection, None, Semin. Respir. Crit. Care Med, doi:10.1055/s-0036-1584798
Burley, Bhikadiya, Bi, Bittrich, Chao et al., RCSB Protein Data Bank (RCSB.Org): Delivery of Experimentally-Determined PDB Structures alongside One Million Computed Structure Models of Proteins from Artificial Intelligence/Machine Learning, Nucleic Acids Res, doi:10.1093/nar/gkac1077
Carter, Iqbal, The Influenza A Virus Replication Cycle: A Comprehensive Review, Viruses, doi:10.3390/v16020316
Chachulski, Development and Application of Compound Class-Specific Benchmark Data Sets for Differentiated Assessment of Docking and Scoring Algorithm Performance
Challagulla, Schat, Doran, In Vitro Inhibition of Influenza Virus Using CRISPR/Cas13a in Chicken Cells, Methods Protoc, doi:10.3390/mps4020040
Che, Liu, Zhang, An Accurate and Universal Protein-Small Molecule Batch Docking Solution Using Autodock Vina, Results Eng, doi:10.1016/j.rineng.2023.101335
Cheng, Montero, Gastaminza, Whitten-Bauer, Wieland et al., A Virocidal Amphipathic α-Helical Peptide That Inhibits Hepatitis C Virus Infection in Vitro, Proc. Natl. Acad. Sci
Chera, Tanca, Remdesivir, The First FDA-Approved Anti-COVID-19 Treatment for Young Children, Discoveries, doi:10.15190/d.2022.10
Chilamakuri, Agarwal, COVID-19: Characteristics and Therapeutics, Cells, doi:10.3390/cells10020206
Cies, Moore, Enache, Chopra, Peramivir for Influenza A and B Viral Infections: A Pharmacokinetic Case Series, Pharmacotherapy, doi:10.1002/phar.2330
Cox, Peacock, Harvey, Hughes, Wright et al., SARS-CoV-2 Variant Evasion of Monoclonal Antibodies Based on in Vitro Studies, Nat. Rev. Microbiol, doi:10.1038/s41579-022-00809-7
Cuevas-Cianca, Romero-Castillo, Gálvez-Romero, Sánchez-Arreola, Juárez et al., Latin American Plants against Microorganisms, Plants, doi:10.3390/plants12233997
De Meyer, Bojkova, Cinatl, Van Damme, Buyck et al., Lack of Antiviral Activity of Darunavir against SARS-CoV-2, Int. J. Infect. Dis, doi:10.1016/j.ijid.2020.05.085
Falcó, Randazzo, Sánchez, Antiviral Activity of Natural Compounds for Food Safety, Food Environ. Virol, doi:10.1007/s12560-024-09605-3
Federico, Silva, Da, Hage-Melim, Gomes et al., Identification of Known Drugs As Potential SARS-CoV-2 Mpro Inhibitors Using Ligand-and Structure-Based Virtual Screening, Future Med. Chem, doi:10.4155/fmc-2021-0025
Ferreira, Dos Santos, Oliva, Andricopulo, Molecular Docking and Structure-Based Drug Design Strategies, Molecules, doi:10.3390/molecules200713384
Fintelman-Rodrigues, Sacramento, Ribeiro Lima, Souza Da Silva, Ferreira et al., Alone or in Combination with Ritonavir, Inhibits SARS-CoV-2 Replication and Proinflammatory Cytokine Production, Antimicrob. Agents Chemother, doi:10.1128/AAC.00825-20
Food, Administration, FDA Revises Letter of Authorization for the Emergency Use Authorization for Paxlovid
Forchette, Sebastian, Liu, A Comprehensive Review of COVID-19 Virology, Vaccines, Variants, and Therapeutics, Curr. Med. Sci, doi:10.1007/s11596-021-2395-1
Fowotade, Bamidele, Egbetola, Fagbamigbe, Adeagbo et al., A Randomized, Open-Label Trial of Combined Nitazoxanide and Atazanavir/Ritonavir for Mild to Moderate COVID-19, Front. Med, doi:10.3389/fmed.2022.956123
Gasmi, Mujawdiya, Lysiuk, Shanaida, Peana et al., Quercetin in the Prevention and Treatment of Coronavirus Infections: A Focus on SARS-CoV-2, Pharmaceuticals, doi:10.3390/ph15091049
Geraghty, Aliota, Bonnac, Broad-Spectrum Antiviral Strategies and Nucleoside Analogues, Viruses, doi:10.3390/v13040667
Goodsell, Zardecki, Di Costanzo, Duarte, Hudson et al., RCSB Protein Data Bank: Enabling Biomedical Research and Drug Discovery, Protein Sci, doi:10.1002/pro.3730
Gratteri, Ambrosio, Lupia, Moraca, Catalanotti et al., Molecular and Structural Aspects of Clinically Relevant Mutations of SARS-CoV-2 RNA-Dependent RNA Polymerase in Remdesivir-Treated Patients, Pharmaceuticals, doi:10.3390/ph16081143
Gregoire, Le Turnier, Gaborit, Veyrac, Lecomte et al., Lopinavir Pharmacokinetics in COVID-19 Patients, J. Antimicrob. Chemother, doi:10.1093/jac/dkaa195
Grundeis, Ansems, Dahms, Thieme, Metzendorf et al., Remdesivir for the Treatment of COVID-19, Cochrane Database Syst. Rev, doi:10.1002/14651858.CD014962.pub2
Han, Perez, Schafer, Cheng, Peet et al., Influenza Virus: Small Molecule Therapeutics and Mechanisms of Antiviral Resistance, Curr. Med. Chem, doi:10.2174/0929867324666170920165926
Handa, Okuwaki, Kawashima, Hatada, Mochizuki et al., Prediction of Binding Pose and Affinity of Nelfinavir, a SARS-CoV-2 Main Protease Repositioned Drug, by Combining Docking, Molecular Dynamics, and Fragment Molecular Orbital Calculations, J. Phys. Chem. B, doi:10.1021/acs.jpcb.3c05564
Hata, Akashi-Ueda, Takamatsu, Matsumura, Safety and Efficacy of Peramivir for Influenza Treatment, Drug Des. Dev. Ther, doi:10.2147/DDDT.S46654
Heaton, Harnessing Host-Virus Evolution in Antiviral Therapy and Immunotherapy, Clin. Transl. Immunol, doi:10.1002/cti2.1067
Huang, Shoichet, Irwin, Benchmarking Sets for Molecular Docking, J. Med. Chem, doi:10.1021/jm0608356
Hutchinson, Influenza Virus, Trends Microbiol, doi:10.1016/j.tim.2018.05.013
Ianevski, Yao, Biza, Zusinaite, Mannik et al., Identification and Tracking of Antiviral Drug Combinations, Viruses, doi:10.3390/v12101178
Ing Lorenzini, Girardin, Direct-Acting Antiviral Interactions with Opioids, Alcohol or Illicit Drugs of Abuse in HCV-Infected Patients, Liver Int, doi:10.1111/liv.14283
Ison, Portsmouth, Yoshida, Shishido, Mitchener et al., Early Treatment with Baloxavir Marboxil in High-Risk Adolescent and Adult Outpatients with Uncomplicated Influenza (CAPSTONE-2): A Randomised, Placebo-Controlled, Phase 3 Trial, Lancet Infect. Dis, doi:10.1016/S1473-3099(20)30004-9
Ivanova, Karelson, The Impact of Software Used and the Type of Target Protein on Molecular Docking Accuracy, Molecules, doi:10.3390/molecules27249041
Jackman, Antiviral Peptide Engineering for Targeting Membrane-Enveloped Viruses: Recent Progress and Future Directions, Biochim. Biophys. Acta Biomembr, doi:10.1016/j.bbamem.2021.183821
Jackman, Costa, Park, Real, Park et al., Therapeutic Treatment of Zika Virus Infection Using a Brain-Penetrating Antiviral Peptide, Nat. Mater, doi:10.1038/s41563-018-0194-2
Jadimurthy, Jagadish, Nayak, Kumar, Mohan et al., Phytochemicals as Invaluable Sources of Potent Antimicrobial Agents to Combat Antibiotic Resistance, Life, doi:10.3390/life13040948
Jakeman, Tisdale, Russell, Leone, Sweet, Efficacy of 2'-Deoxy-2'-Fluororibosides against Influenza A and B Viruses in Ferrets, Antimicrob. Agents Chemother, doi:10.1128/AAC.38.8.1864
Jesús-González, Cervantes-Salazar, Reyes-Ruiz, Osuna-Ramos, Farfán-Morales et al., The Nuclear Pore Complex: A Target for NS3 Protease of Dengue and Zika Viruses, Viruses, doi:10.3390/v12060583
Jesús-González, Del Ángel, Palacios-Rápalo, Cordero-Rivera, Rodríguez-Carlos et al., A Dual Pharmacological Strategy against COVID-19: The Therapeutic Potential of Metformin and Atorvastatin, Microorganisms, doi:10.3390/microorganisms12020383
Jones, Use of Nirsevimab for the Prevention of Respiratory Syncytial Virus Disease Among Infants and Young Children: Recommendations of the Advisory Committee on Immunization Practices-United States, 2023, MMWR Morb. Mortal. Wkly. Rep, doi:10.15585/mmwr.mm7234a4
Jordan, Stevens, Tam, Pemberton, Chaudhuri et al., Activation Pathway of a Nucleoside Analog Inhibiting Respiratory Syncytial Virus Polymerase, ACS Chem. Biol, doi:10.1021/acschembio.6b00788
Jordheim, Durantel, Zoulim, Dumontet, Advances in the Development of Nucleoside and Nucleotide Analogues for Cancer and Viral Diseases, Nat. Rev. Drug Discov, doi:10.1038/nrd4010
Kamzeeva, Aralov, Alferova, Korshun, Recent Advances in Molecular Mechanisms of Nucleoside Antivirals, Curr. Issues Mol. Biol, doi:10.3390/cimb45080433
Kang, Seong, Choi, Kim, Choe et al., In Vitro Activity of Lopinavir/Ritonavir and Hydroxychloroquine against Severe Acute Respiratory Syndrome Coronavirus 2 at Concentrations Achievable by Usual Doses, Korean J. Intern. Med, doi:10.3904/kjim.2020.157
Kim, Chung, Tamanna, Bang, Tariq et al., Comparable Efficacy of Lopinavir/Ritonavir and Remdesivir in Reducing Viral Load and Shedding Duration in Patients with COVID-19, Microorganisms, doi:10.3390/microorganisms12081696
Kim, Lew, Williams, Liu, Zhang et al., Influenza Neuraminidase Inhibitors Possessing a Novel Hydrophobic Interaction in the Enzyme Active Site: Design, Synthesis, and Structural Analysis of Carbocyclic Sialic Acid Analogues with Potent Anti-Influenza Activity, J. Am. Chem. Soc, doi:10.1021/ja963036t
Koval, Gonzalez, RSV in Transplant and Immunocompromised Patients, Clevel. Clin. J. Med, doi:10.3949/ccjm.91.s1.06
Kumari, Lu, Li, Huang, Hsu et al., A Critical Overview of Current Progress for COVID-19: Development of Vaccines, Antiviral Drugs, and Therapeutic Antibodies, J. Biomed. Sci, doi:10.1186/s12929-022-00852-9
Kumari, Sharma, Kumar, Ende, Mishina et al., Antiviral Approaches against Influenza Virus, Clin. Microbiol. Rev, doi:10.1128/cmr.00040-22
Lalezari, Henry, O'hearn, Montaner, Piliero et al., Enfuvirtide, an HIV-1 Fusion Inhibitor, for Drug-Resistant HIV Infection in North and South America, N. Engl. J. Med, doi:10.1056/NEJMoa035026
Lampejo, Influenza and Antiviral Resistance: An Overview, Eur. J. Clin. Microbiol. Infect. Dis, doi:10.1007/s10096-020-03840-9
Lee, Wu, Poh, Molecular Mechanisms of Antiviral Agents against Dengue Virus, Viruses, doi:10.3390/v15030705
Li, Chan, Lee, Clinical Implications of Antiviral Resistance in Influenza, Viruses, doi:10.3390/v7092850
Li, Huo, Yin, Tian, Huang et al., The Current State of Research on Influenza Antiviral Drug Development: Drugs in Clinical Trial and Licensed Drugs, mBio, doi:10.1128/mbio.01273-23
Liu, Zhou, Ye, Yang, Antivirals for Respiratory Viral Infections: Problems and Prospects, Semin. Respir. Crit. Care Med, doi:10.1055/s-0036-1584803
Loo, Lee, Nanotechnology-Based Therapeutics for Targeting Inflammatory Lung Diseases, Nanomedicine, doi:10.2217/nnm-2021-0447
Loo, Lee, Zhou, Recent Advances in Inhaled Nanoformulations of Vaccines and Therapeutics Targeting Respiratory Viral Infections, Pharm. Res, doi:10.1007/s11095-023-03520-1
Low, Farouk, Lal, Drug Repositioning: New Approaches and Future Prospects for Life-Debilitating Diseases and the COVID-19 Pandemic Outbreak, Viruses, doi:10.3390/v12091058
Lynch, Kajon, Adenovirus: Epidemiology, Global Spread of Novel Serotypes, and Advances in Treatment and Prevention, Semin. Respir. Crit. Care Med, doi:10.1055/s-0036-1584923
Ma, Song, Huang, Coronavirus Disease-2019 (COVID-19) and Cardiovascular Complications, J. Cardiothorac. Vasc. Anesth, doi:10.1053/j.jvca.2020.04.041
Ma, Soto, Ohigashi, Taylor, Bournas et al., Identification of the Pore-Lining Residues of the BM2 Ion Channel Protein of Influenza B Virus, J. Biol. Chem, doi:10.1074/jbc.M710302200
Mackman, Phosphoramidate Prodrugs Continue to Deliver, The Journey of Remdesivir (GS-5734) from RSV to SARS-CoV-2, ACS Med. Chem. Lett, doi:10.1021/acsmedchemlett.1c00624
Mahajan, Choudhary, Kumar, Tomar, Antiviral Strategies Targeting Host Factors and Mechanisms Obliging +ssRNA Viral Pathogens, Bioorg. Med. Chem, doi:10.1016/j.bmc.2021.116356
Marzolini, Stader, Stoeckle, Franzeck, Egli et al., Effect of Systemic Inflammatory Response to SARS-CoV-2 on Lopinavir and Hydroxychloroquine Plasma Concentrations, Antimicrob. Agents Chemother, doi:10.1128/AAC.01177-20
Mckimm-Breschkin, Jiang, Hui, Beigel, Govorkova et al., Prevention and Treatment of Respiratory Viral Infections: Presentations on Antivirals, Traditional Therapies and Host-Directed Interventions at the 5th ISIRV Antiviral Group Conference, Antivir. Res, doi:10.1016/j.antiviral.2017.11.013
Mclaughlin, Skoglund, Ison, Peramivir, An Intravenous Neuraminidase Inhibitor, Expert Opin. Pharmacother, doi:10.1517/14656566.2015.1066336
Mello, Aguayo, Rodriguez, Lee, Jordan et al., Multiple Classes of Antiviral Agents Exhibit in Vitro Activity against Human Rhinovirus Type C, Antimicrob. Agents Chemother, doi:10.1128/AAC.01746-13
Meseko, Sanicas, Asha, Sulaiman, Kumar, Antiviral Options and Therapeutics against Influenza: History, Latest Developments and Future Prospects, Front. Cell. Infect. Microbiol, doi:10.3389/fcimb.2023.1269344
Mia, Howlader, Akter, Hossain, Preclinical and Clinical Investigations of Potential Drugs and Vaccines for COVID-19 Therapy: A Comprehensive Review With Recent Update, Clin. Pathol, doi:10.1177/2632010X241263054
Monto, Robinson, Herlocher, Hinson, Elliott et al., Zanamivir in the Prevention of Influenza among Healthy Adults: A Randomized Controlled Trial, JAMA, doi:10.1001/jama.282.1.31
Morris, Huey, Lindstrom, Sanner, Belew et al., AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility, J. Comput. Chem, doi:10.1002/jcc.21256
Murillo-Zamora, Mendoza-Cano, Huerta, Ríos-Silva, Lugo-Radillo et al., Respiratory Syncytial Virus Infection: Survival Experience in a Cohort of Children Inpatients, Public Health, doi:10.1016/j.puhe.2023.06.020
Musarrat, Chouljenko, Dahal, Nabi, Chouljenko et al., The Anti-HIV Drug Nelfinavir Mesylate (Viracept) Is a Potent Inhibitor of Cell Fusion Caused by the SARSCoV-2 Spike (S) Glycoprotein Warranting Further Evaluation as an Antiviral against COVID-19 Infections, J. Med. Virol, doi:10.1002/jmv.25985
Muthukutty, Macdonald, Yoo, Combating Emerging Respiratory Viruses: Lessons and Future Antiviral Strategies, Vaccines, doi:10.3390/vaccines12111220
Nekoukar, Ala, Moradi, Hill, Davoudi Badabi et al., Comparison of the Efficacy and Safety of Atazanavir/Ritonavir Plus Hydroxychloroquine with Lopinavir/Ritonavir Plus Hydroxychloroquine in Patients with Moderate COVID-19, A Randomized, Double-Blind Clinical Trial, Iran. J. Pharm. Res. IJPR, doi:10.22037/ijpr.2021.115157.15243
Netzler, Enosi Tuipulotu, White, Norovirus Antivirals: Where Are We Now?, Med. Res. Rev, doi:10.1002/med.21545
Ng, Correia, Seagal, Degoey, Schrimpf et al., Antiviral Drug Discovery for the Treatment of COVID-19 Infections, Viruses, doi:10.3390/v14050961
Niazi, Mariam, Computer-Aided Drug Design and Drug Discovery: A Prospective Analysis, Pharmaceuticals, doi:10.3390/ph17010022
Novak, Potemkin, A New Glimpse on the Active Site of SARS-CoV-2 3CLpro, Coupled with Drug Repurposing Study, Mol. Divers, doi:10.1007/s11030-021-10355-8
Nypaver, Dehlinger, Carter, Influenza and Influenza Vaccine: A Review, J. Midwifery Womens Health, doi:10.1111/jmwh.13203
O'hanlon, Shaw, Baloxavir, Marboxil: The New Influenza Drug on the Market, Curr. Opin. Virol, doi:10.1016/j.coviro.2019.01.006
Okoli, Askin, Rabbani, Nirmatrelvir/Ritonavir Regimen for Mild/Moderately Severe COVID-19: A Rapid Review With Meta-Analysis and Trial Sequential Analysis, Ann. Fam. Med, doi:10.1370/afm.3120
Osborne, Davies, Lane, Evans, Denyer et al., Lopinavir-Ritonavir in the Treatment of COVID-19: A Dynamic Systematic Benefit-Risk Assessment, Drug Saf, doi:10.1007/s40264-020-00966-9
Outlaw, Bovier, Mears, Cajimat, Zhu et al., Inhibition of Coronavirus Entry In Vitro and Ex Vivo by a Lipid-Conjugated Peptide Derived from the SARS-CoV-2 Spike Glycoprotein HRC Domain, mBio, doi:10.1128/mBio.01935-20
Palacios-Rápalo, Cordero-Rivera, De Jesús-González, Farfan-Morales, Benitez-Vega et al., Drug Repositioning as an Antiviral Strategy Against Emerging Viruses
Palacios-Rápalo, Farfan-Morales, Cordero-Rivera, De Jesús-González, Reyes-Ruiz et al., An Ivermectin-Atorvastatin Combination Impairs Nuclear Transport Inhibiting Dengue Infection in Vitro and in Vivo, iScience, doi:10.1016/j.isci.2023.108294
Pantsar, Poso, Binding Affinity via Docking: Fact and Fiction, Molecules, doi:10.3390/molecules23081899
Peng, Wang, Wang, Wang, Progression of Antiviral Agents Targeting Viral Polymerases, Molecules, doi:10.3390/molecules27217370
Pinzi, Rastelli, Molecular Docking: Shifting Paradigms in Drug Discovery, Int. J. Mol. Sci, doi:10.3390/ijms20184331
Pircalabioru, Iliescu, Mihaescu, Cucu, Ionescu et al., Advances in the Rapid Diagnostic of Viral Respiratory Tract Infections, Front. Cell. Infect. Microbiol, doi:10.3389/fcimb.2022.807253
Polack, Thomas, Kitchin, Absalon, Gurtman et al., Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine, N. Engl. J. Med, doi:10.1056/NEJMoa2034577
Qayed, Ferreira, Silva, In Silico Study towards Repositioning of FDA-Approved Drug Candidates for Anticoronaviral Therapy: Molecular Docking, Molecular Dynamics and Binding Free Energy Calculations, Molecules, doi:10.3390/molecules27185988
Rabaan, Al-Ahmed, Haque, Sah, Tiwari et al., SARS-CoV-2, SARS-CoV, and MERS-COV: A Comparative Overview, Infez. Med
Ramesh, Govindarajulu, Parise, Neel, Shankar et al., Emerging SARS-CoV-2 Variants: A Review of Its Mutations, Its Implications and Vaccine Efficacy, Vaccines, doi:10.3390/vaccines9101195
Ramos-Martín, D'amelio, Drug Resistance: An Incessant Fight against Evolutionary Strategies of Survival, Microbiol. Res, doi:10.3390/microbiolres14020037
Receives, FDA Fast Track Designation for Ensitrelvir Fumaric Acid, an Investigational Oral Antiviral for COVID-19
Redondo, Rivero-Calle, Mascarós, Ocaña, Jimeno et al., Respiratory Syncytial Virus Vaccination Recommendations for Adults Aged 60 Years and Older: The NeumoExperts Prevention Group Position Paper, Arch. Bronconeumol, doi:10.1016/j.arbres.2024.01.004
Rehman, Rehman, Yoo, COVID-19 Challenges and Its Therapeutics, Biomed. Pharmacother, doi:10.1016/j.biopha.2021.112015
Reina, Iglesias, EDP-938, a new antiviral with inhibitory activity against the nucleoprotein of the respiratory syncytial virus, Rev. Esp. Quimioter, doi:10.37201/req/096.2022
Reyes-Ruiz, García-Hernández, Martínez-Mier, Osuna-Ramos, De Jesús-González et al., The Role of Aspartate Aminotransferase-to-Lymphocyte Ratio Index (ALRI) in Predicting Mortality in SARS-CoV-2 Infection, Microorganisms, doi:10.3390/microorganisms11122894
Rivera-Serrano, Cabanillas-Salcido, Cordero-Rivera, Jiménez-Camacho, Norzagaray-Valenzuela et al., Antiviral Effect of Microalgae Phaeodactylum Tricornutum Protein Hydrolysates against Dengue Virus Serotype 2, Mar. Drugs, doi:10.3390/md22080369
Ryu, Jeong, Kim, Kim, Park et al., Biflavonoids from Torreya Nucifera Displaying SARS-CoV 3CL(pro) Inhibition, Bioorg. Med. Chem, doi:10.1016/j.bmc.2010.09.035
Ryu, Shin, SARS-CoV-2 Infection of Airway Epithelial Cells, Immune Netw, doi:10.4110/in.2021.21.e3
Saiz, De Oya, Blázquez, Escribano-Romero, Martín-Acebes, Host-Directed Antivirals: A Realistic Alternative to Fight Zika Virus, Viruses, doi:10.3390/v10090453
Schoof, Faust, Saunders, Sangwan, Rezelj et al., An Ultrapotent Synthetic Nanobody Neutralizes SARS-CoV-2 by Stabilizing Inactive Spike, Science, doi:10.1126/science.abe3255
Schuster, Williams, Human Metapneumovirus, Microbiol. Spectr, doi:10.1128/microbiolspec.AID-0020-2014
Shamsian, Sokouti, Dastmalchi, Benchmarking Different Docking Protocols for Predicting the Binding Poses of Ligands Complexed with Cyclooxygenase Enzymes and Screening Chemical Libraries, Bioimpacts, doi:10.34172/bi.2023.29955
Sheahan, Sims, Graham, Menachery, Gralinski et al., Broad-Spectrum Antiviral GS-5734 Inhibits Both Epidemic and Zoonotic Coronaviruses, Sci. Transl. Med, doi:10.1126/scitranslmed.aal3653
Shyr, Cheng, Lo, Zheng, Drug Combination Therapy for Emerging Viral Diseases, Drug Discov. Today, doi:10.1016/j.drudis.2021.05.008
Simões, Madhi, Muller, Atanasova, Bosheva et al., Efficacy of Nirsevimab against Respiratory Syncytial Virus Lower Respiratory Tract Infections in Preterm and Term Infants, and Pharmacokinetic Extrapolation to Infants with Congenital Heart Disease and Chronic Lung Disease: A Pooled Analysis of Randomised Controlled Trials, Lancet Child Adolesc. Health, doi:10.1016/S2352-4642(22)00321-2
Smyk, Szydłowska, Szulc, Majewska, Evolution of Influenza Viruses-Drug Resistance, Treatment Options, and Prospects, Int. J. Mol. Sci, doi:10.3390/ijms232012244
Sooksawasdi Na Ayudhya, Laksono, Van Riel, The Pathogenesis and Virulence of Enterovirus-D68 Infection, Virulence, doi:10.1080/21505594.2021.1960106
Stevens, Pruijssers, Lee, Gordon, Tchesnokov et al., Mutations in the SARS-CoV-2 RNA-Dependent RNA Polymerase Confer Resistance to Remdesivir by Distinct Mechanisms, Sci. Transl. Med, doi:10.1126/scitranslmed.abo0718
Strasfeld, Chou, Antiviral Drug Resistance: Mechanisms and Clinical Implications, Infect. Dis. Clin. N. Am, doi:10.1016/j.idc.2010.01.001
Suberi, Grun, Mao, Israelow, Reschke et al., Inhalable Polymer Nanoparticles for Versatile mRNA Delivery and Mucosal Vaccination, bioRxiv, doi:10.1101/2022.03.22.485401
Suberi, Grun, Mao, Israelow, Reschke et al., Polymer nanoparticles deliver mRNA to the lung for mucosal vaccination, Sci Transl Med, doi:10.1126/scitranslmed.abq0603
Sun, Zhang, Wang, Yao, He et al., Prevention and Potential Treatment Strategies for Respiratory Syncytial Virus, Molecules, doi:10.3390/molecules29030598
Takazono, Fujita, Komeda, Miyazawa, Yoshida et al., Real-World Effectiveness of Ensitrelvir in Reducing Severe Outcomes in Outpatients at High Risk for COVID-19, Infect. Dis. Ther, doi:10.1007/s40121-024-01010-4
Terefe, Ghosh, Molecular Docking, Validation, Dynamics Simulations, and Pharmacokinetic Prediction of Phytochemicals Isolated From Croton Dichogamus Against the HIV-1 Reverse Transcriptase, Bioinform. Biol. Insights, doi:10.1177/11779322221125605
Torres, Sodero, Jofily, Silva-Jr, Key Topics in Molecular Docking for Drug Design, Int. J. Mol. Sci, doi:10.3390/ijms20184574
Trott, Olson, AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization and Multithreading, J. Comput. Chem, doi:10.1002/jcc.21334
Unoh, Uehara, Nakahara, Nobori, Yamatsu et al., Discovery of S-217622, a Noncovalent Oral SARS-CoV-2 3CL Protease Inhibitor Clinical Candidate for Treating COVID-19, J. Med. Chem, doi:10.1021/acs.jmedchem.2c00117
Van Der Oost, Westra, Jackson, Wiedenheft, Unravelling the Structural and Mechanistic Basis of CRISPR-Cas Systems, Nat. Rev. Microbiol, doi:10.1038/nrmicro3279
Vincent, Bergeron, Benjannet, Erickson, Rollin et al., Chloroquine Is a Potent Inhibitor of SARS Coronavirus Infection and Spread, Virol. J, doi:10.1186/1743-422X-2-69
Von Delft, Hall, Kwong, Purcell, Saikatendu et al., Accelerating Antiviral Drug Discovery: Lessons from COVID-19, Nat. Rev. Drug Discov, doi:10.1038/s41573-023-00692-8
Von Itzstein, Wu, Kok, Pegg, Dyason et al., Rational Design of Potent Sialidase-Based Inhibitors of Influenza Virus Replication, Nature, doi:10.1038/363418a0
Wang, Moon, Huang, Sun, Qiu, Antiviral Effects and Underlying Mechanisms of Probiotics as Promising Antivirals, Front. Cell. Infect. Microbiol, doi:10.3389/fcimb.2022.928050
Wei, Zhuo, Fu, Zeng, Wang et al., A Multisource Prompt Framework for Drug Repurposing Based on Large Language Models, BMC Biol, doi:10.1186/s12915-024-02028-3
Wrotek, Czajkowska, Jackowska, Nosocomial Infections in Patients Hospitalized with Respiratory Syncytial Virus: A Practice Review, Adv. Exp. Med. Biol, doi:10.1007/5584_2020_483
Wu, Huang, Zhang, Wu, Zhang et al., Safety, tolerability, and immunogenicity of an aerosolised adenovirus type-5 vector-based COVID-19 vaccine (Ad5-nCoV) in adults: Preliminary report of an open-label and randomised phase 1 clinical trial, Lancet Infect Dis, doi:10.1016/S1473-3099(21)00396-0
Xia, Liang, Development of Direct-Acting Antiviral and Host-Targeting Agents for Treatment of Hepatitis B Virus Infection, Gastroenterology, doi:10.1053/j.gastro.2018.07.057
Xu, Shi, Han, Ling, Jiang et al., Preventive and Therapeutic Benefits of Nelfinavir in Rhesus Macaques and Human Beings Infected with SARS-CoV-2, Sig. Transduct. Target. Ther, doi:10.1038/s41392-023-01429-0
Yoo, Antivirals for Coexistence with COVID-19: Brief Review for General Physicians, J. Korean Med. Sci, doi:10.3346/jkms.2021.36.e298
Yotsuyanagi, Ohmagari, Doi, Imamura, Sonoyama et al., A Phase 2/3 Study of S-217622 in Participants with SARS-CoV-2 Infection (Phase 3 Part), Medicine, doi:10.1097/MD.0000000000033024
Yotsuyanagi, Ohmagari, Doi, Yamato, Bac et al., Efficacy and Safety of 5-Day Oral Ensitrelvir for Patients With Mild to Moderate COVID-19, JAMA Netw. Open, doi:10.1001/jamanetworkopen.2023.54991
Yuan, Jiao, Qu, Yang, Liu, The Development of COVID-19 Treatment, Front. Immunol, doi:10.3389/fimmu.2023.1125246
Świerczy Ńska, Mirowska-Guzel, Pindelska, Antiviral Drugs in Influenza, Int. J. Environ. Res. Public Health, doi:10.3390/ijerph19053018
DOI record:
{
"DOI": "10.3390/pathogens14010020",
"ISSN": [
"2076-0817"
],
"URL": "http://dx.doi.org/10.3390/pathogens14010020",
"abstract": "<jats:p>The development of antivirals for respiratory viruses has advanced markedly in response to the growing threat of pathogens such as Influenzavirus (IAV), respiratory syncytial virus (RSV), and SARS-CoV-2. This article reviews the advances and challenges in this field, highlighting therapeutic strategies that target critical stages of the viral replication cycle, including inhibitors of viral entry, replication, and assembly. In addition, innovative approaches such as inhibiting host cellular proteins to reduce viral resistance and repurposing existing drugs are explored, using advanced bioinformatics tools that optimize the identification of antiviral candidates. The analysis also covers emerging technologies such as nanomedicine and CRISPR gene editing, which promise to improve the stability and efficacy of treatments. While current antivirals offer valuable options, they face challenges such as viral evolution and the need for accessible treatments for vulnerable populations. This article underscores the importance of continued innovation in biotechnology to overcome these limitations and provide safe and effective treatments. Combining traditional and advanced approaches in developing antivirals is essential in order to address respiratory viral diseases that affect global health.</jats:p>",
"alternative-id": [
"pathogens14010020"
],
"author": [
{
"ORCID": "https://orcid.org/0000-0003-1415-6260",
"affiliation": [
{
"name": "Unidad de Investigación Biomédica de Zacatecas, Instituto Mexicano del Seguro Social, Zacatecas 98000, Mexico"
}
],
"authenticated-orcid": false,
"family": "De Jesús-González",
"given": "Luis Adrián",
"sequence": "first"
},
{
"ORCID": "https://orcid.org/0000-0002-5726-5953",
"affiliation": [
{
"name": "Laboratorio de Virología Perinatal y Diseño Molecular de Antígenos y Biomarcadores, Departamento de Inmunobioquímica, Instituto Nacional de Perinatología, Ciudad de México 11000, Mexico"
}
],
"authenticated-orcid": false,
"family": "León-Juárez",
"given": "Moisés",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Unidad de Investigación Biomédica de Zacatecas, Instituto Mexicano del Seguro Social, Zacatecas 98000, Mexico"
}
],
"family": "Lira-Hernández",
"given": "Flor Itzel",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-1521-1519",
"affiliation": [
{
"name": "Unidad de Investigación Biomédica de Zacatecas, Instituto Mexicano del Seguro Social, Zacatecas 98000, Mexico"
}
],
"authenticated-orcid": false,
"family": "Rivas-Santiago",
"given": "Bruno",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-5108-8052",
"affiliation": [
{
"name": "Laboratorio de Virología Perinatal y Diseño Molecular de Antígenos y Biomarcadores, Departamento de Inmunobioquímica, Instituto Nacional de Perinatología, Ciudad de México 11000, Mexico"
}
],
"authenticated-orcid": false,
"family": "Velázquez-Cervantes",
"given": "Manuel Adrián",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Unidad de Investigación Biomédica de Zacatecas, Instituto Mexicano del Seguro Social, Zacatecas 98000, Mexico"
},
{
"name": "Especialidad en Medicina Familiar, Unidad Académica de Medicina Humana y Ciencias de la Salud, Universidad Autónoma de Zacatecas, Zacatecas 98160, Mexico"
},
{
"name": "Instituto Mexicano del Seguro Social, Unidad de Medicina Familiar # 4, Servicio de Medicina Familiar, Guadalupe, Zacatecas 98618, Mexico"
}
],
"family": "Méndez-Delgado",
"given": "Iridiana Monserrat",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Unidad de Investigación Biomédica de Zacatecas, Instituto Mexicano del Seguro Social, Zacatecas 98000, Mexico"
},
{
"name": "Unidad Académica de Ciencias Químicas, Universidad Autónoma de Zacatecas, Zacatecas 98160, Mexico"
}
],
"family": "Macías-Guerrero",
"given": "Daniela Itzel",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-2760-697X",
"affiliation": [
{
"name": "Department of Infectomics and Molecular Pathogenesis, Center for Research and Advanced Studies (CINVESTAV-IPN), Mexico City 07360, Mexico"
}
],
"authenticated-orcid": false,
"family": "Hernández-Castillo",
"given": "Jonathan",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0009-0002-1930-2685",
"affiliation": [
{
"name": "Unidad de Investigación Biomédica de Zacatecas, Instituto Mexicano del Seguro Social, Zacatecas 98000, Mexico"
},
{
"name": "Unidad Académica de Ciencias Químicas, Universidad Autónoma de Zacatecas, Zacatecas 98160, Mexico"
}
],
"authenticated-orcid": false,
"family": "Hernández-Rodríguez",
"given": "Ximena",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Unidad de Investigación Biomédica de Zacatecas, Instituto Mexicano del Seguro Social, Zacatecas 98000, Mexico"
},
{
"name": "Unidad Académica de Ciencias Químicas, Universidad Autónoma de Zacatecas, Zacatecas 98160, Mexico"
}
],
"family": "Calderón-Sandate",
"given": "Daniela Nahomi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Unidad de Investigación Biomédica de Zacatecas, Instituto Mexicano del Seguro Social, Zacatecas 98000, Mexico"
},
{
"name": "Especialidad en Medicina Familiar, Unidad Académica de Medicina Humana y Ciencias de la Salud, Universidad Autónoma de Zacatecas, Zacatecas 98160, Mexico"
},
{
"name": "Instituto Mexicano del Seguro Social, Unidad de Medicina Familiar # 4, Servicio de Medicina Familiar, Guadalupe, Zacatecas 98618, Mexico"
}
],
"family": "Mata-Martínez",
"given": "Willy Salvador",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-2379-8591",
"affiliation": [
{
"name": "División de Investigación en Salud, Unidad Médica de Alta Especialidad, Hospital de Especialidades No. 14, Centro Médico Nacional “Adolfo Ruiz Cortines”, Instituto Mexicano del Seguro Social (IMSS), Veracruz 91897, Mexico"
},
{
"name": "Facultad de Medicina, Región Veracruz, Universidad Veracruzana (UV), Veracruz 91700, Mexico"
}
],
"authenticated-orcid": false,
"family": "Reyes-Ruíz",
"given": "José Manuel",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-8280-9812",
"affiliation": [
{
"name": "Facultad de Medicina, Universidad Autónoma de Sinaloa, Culiacán 80019, Mexico"
}
],
"authenticated-orcid": false,
"family": "Osuna-Ramos",
"given": "Juan Fidel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Unidad de Investigación Biomédica de Zacatecas, Instituto Mexicano del Seguro Social, Zacatecas 98000, Mexico"
}
],
"family": "García-Herrera",
"given": "Ana Cristina",
"sequence": "additional"
}
],
"container-title": "Pathogens",
"container-title-short": "Pathogens",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
12,
31
]
],
"date-time": "2024-12-31T12:51:57Z",
"timestamp": 1735649517000
},
"deposited": {
"date-parts": [
[
2024,
12,
31
]
],
"date-time": "2024-12-31T13:52:28Z",
"timestamp": 1735653148000
},
"funder": [
{
"name": "FUNDACIÓN IMSS, AC"
}
],
"indexed": {
"date-parts": [
[
2024,
12,
31
]
],
"date-time": "2024-12-31T14:10:28Z",
"timestamp": 1735654228940,
"version": "3.32.0"
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2024,
12,
31
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2025,
1
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
12,
31
]
],
"date-time": "2024-12-31T00:00:00Z",
"timestamp": 1735603200000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2076-0817/14/1/20/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "20",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2024,
12,
31
]
]
},
"published-online": {
"date-parts": [
[
2024,
12,
31
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1055/s-0036-1584803",
"article-title": "Antivirals for Respiratory Viral Infections: Problems and Prospects",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "640",
"journal-title": "Semin. Respir. Crit. Care Med.",
"key": "ref_1",
"volume": "37",
"year": "2016"
},
{
"DOI": "10.3389/fcimb.2022.807253",
"doi-asserted-by": "crossref",
"key": "ref_2",
"unstructured": "Pircalabioru, G.G., Iliescu, F.S., Mihaescu, G., Cucu, A.I., Ionescu, O.N., Popescu, M., Simion, M., Burlibasa, L., Tica, M., and Chifiriuc, M.C. (2022). Advances in the Rapid Diagnostic of Viral Respiratory Tract Infections. Front. Cell. Infect. Microbiol., 12."
},
{
"DOI": "10.3390/vaccines12111220",
"doi-asserted-by": "crossref",
"key": "ref_3",
"unstructured": "Muthukutty, P., MacDonald, J., and Yoo, S.Y. (2024). Combating Emerging Respiratory Viruses: Lessons and Future Antiviral Strategies. Vaccines, 12."
},
{
"DOI": "10.3390/microorganisms11122894",
"doi-asserted-by": "crossref",
"key": "ref_4",
"unstructured": "Reyes-Ruiz, J.M., García-Hernández, O., Martínez-Mier, G., Osuna-Ramos, J.F., De Jesús-González, L.A., Farfan-Morales, C.N., Palacios-Rápalo, S.N., Cordero-Rivera, C.D., Ordoñez-Rodríguez, T., and del Ángel, R.M. (2023). The Role of Aspartate Aminotransferase-to-Lymphocyte Ratio Index (ALRI) in Predicting Mortality in SARS-CoV-2 Infection. Microorganisms, 11."
},
{
"DOI": "10.1007/s40266-024-01118-9",
"article-title": "Respiratory Syncytial Virus Infection in Older Adults: An Update",
"author": "Alfano",
"doi-asserted-by": "crossref",
"first-page": "487",
"journal-title": "Drugs Aging",
"key": "ref_5",
"volume": "41",
"year": "2024"
},
{
"DOI": "10.1016/j.tim.2018.05.013",
"article-title": "Influenza Virus",
"author": "Hutchinson",
"doi-asserted-by": "crossref",
"first-page": "809",
"journal-title": "Trends Microbiol.",
"key": "ref_6",
"volume": "26",
"year": "2018"
},
{
"DOI": "10.1016/j.arbres.2024.01.004",
"article-title": "Respiratory Syncytial Virus Vaccination Recommendations for Adults Aged 60 Years and Older: The NeumoExperts Prevention Group Position Paper",
"author": "Redondo",
"doi-asserted-by": "crossref",
"first-page": "161",
"journal-title": "Arch. Bronconeumol.",
"key": "ref_7",
"volume": "60",
"year": "2024"
},
{
"DOI": "10.3390/microorganisms12020383",
"doi-asserted-by": "crossref",
"key": "ref_8",
"unstructured": "De Jesús-González, L.A., del Ángel, R.M., Palacios-Rápalo, S.N., Cordero-Rivera, C.D., Rodríguez-Carlos, A., Trujillo-Paez, J.V., Farfan-Morales, C.N., Osuna-Ramos, J.F., Reyes-Ruiz, J.M., and Rivas-Santiago, B. (2024). A Dual Pharmacological Strategy against COVID-19: The Therapeutic Potential of Metformin and Atorvastatin. Microorganisms, 12."
},
{
"DOI": "10.3390/md22080369",
"doi-asserted-by": "crossref",
"key": "ref_9",
"unstructured": "Rivera-Serrano, B.V., Cabanillas-Salcido, S.L., Cordero-Rivera, C.D., Jiménez-Camacho, R., Norzagaray-Valenzuela, C.D., Calderón-Zamora, L., De Jesús-González, L.A., Reyes-Ruiz, J.M., Farfan-Morales, C.N., and Romero-Utrilla, A. (2024). Antiviral Effect of Microalgae Phaeodactylum Tricornutum Protein Hydrolysates against Dengue Virus Serotype 2. Mar. Drugs, 22."
},
{
"DOI": "10.3390/molecules29030598",
"doi-asserted-by": "crossref",
"key": "ref_10",
"unstructured": "Sun, B.-W., Zhang, P.-P., Wang, Z.-H., Yao, X., He, M.-L., Bai, R.-T., Che, H., Lin, J., Xie, T., and Hui, Z. (2024). Prevention and Potential Treatment Strategies for Respiratory Syncytial Virus. Molecules, 29."
},
{
"DOI": "10.3389/fviro.2022.935933",
"doi-asserted-by": "crossref",
"key": "ref_11",
"unstructured": "Badia, R., Garcia-Vidal, E., and Ballana, E. (2022). Viral-Host Dependency Factors as Therapeutic Targets to Overcome Antiviral Drug-Resistance: A Focus on Innate Immune Modulation. Front. Virol., 2."
},
{
"DOI": "10.1007/s11030-021-10355-8",
"article-title": "A New Glimpse on the Active Site of SARS-CoV-2 3CLpro, Coupled with Drug Repurposing Study",
"author": "Novak",
"doi-asserted-by": "crossref",
"first-page": "2631",
"journal-title": "Mol. Divers.",
"key": "ref_12",
"volume": "26",
"year": "2022"
},
{
"DOI": "10.1016/j.virusres.2019.197669",
"article-title": "Antiviral Agents against African Swine Fever Virus",
"author": "Arabyan",
"doi-asserted-by": "crossref",
"first-page": "197669",
"journal-title": "Virus Res.",
"key": "ref_13",
"volume": "270",
"year": "2019"
},
{
"DOI": "10.3389/fcimb.2022.928050",
"doi-asserted-by": "crossref",
"key": "ref_14",
"unstructured": "Wang, Y., Moon, A., Huang, J., Sun, Y., and Qiu, H.-J. (2022). Antiviral Effects and Underlying Mechanisms of Probiotics as Promising Antivirals. Front. Cell. Infect. Microbiol., 12."
},
{
"DOI": "10.1016/S1636-5410(22)46453-1",
"article-title": "Antivirales (a Excepción Del Virus de La Inmunodeficiencia Humana y La Hepatitis)",
"author": "Agut",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "EMC-Tratado Med.",
"key": "ref_15",
"volume": "26",
"year": "2022"
},
{
"DOI": "10.3390/v15030705",
"doi-asserted-by": "crossref",
"key": "ref_16",
"unstructured": "Lee, M.F., Wu, Y.S., and Poh, C.L. (2023). Molecular Mechanisms of Antiviral Agents against Dengue Virus. Viruses, 15."
},
{
"DOI": "10.20944/preprints201807.0359.v1",
"doi-asserted-by": "crossref",
"key": "ref_17",
"unstructured": "Saiz, J.-C., de Oya, N.J., Blázquez, A.-B., Escribano-Romero, E., and Martín-Acebes, M.A. (2018). Host-Directed Antivirals: A Realistic Alternative to Fight Zika Virus. Viruses, 10."
},
{
"DOI": "10.3390/v13040667",
"doi-asserted-by": "crossref",
"key": "ref_18",
"unstructured": "Geraghty, R.J., Aliota, M.T., and Bonnac, L.F. (2021). Broad-Spectrum Antiviral Strategies and Nucleoside Analogues. Viruses, 13."
},
{
"DOI": "10.1053/j.gastro.2018.07.057",
"article-title": "Development of Direct-Acting Antiviral and Host-Targeting Agents for Treatment of Hepatitis B Virus Infection",
"author": "Xia",
"doi-asserted-by": "crossref",
"first-page": "311",
"journal-title": "Gastroenterology",
"key": "ref_19",
"volume": "156",
"year": "2019"
},
{
"DOI": "10.1111/liv.14283",
"article-title": "Direct-Acting Antiviral Interactions with Opioids, Alcohol or Illicit Drugs of Abuse in HCV-Infected Patients",
"author": "Girardin",
"doi-asserted-by": "crossref",
"first-page": "32",
"journal-title": "Liver Int.",
"key": "ref_20",
"volume": "40",
"year": "2020"
},
{
"DOI": "10.1002/med.21545",
"article-title": "Norovirus Antivirals: Where Are We Now?",
"author": "Netzler",
"doi-asserted-by": "crossref",
"first-page": "860",
"journal-title": "Med. Res. Rev.",
"key": "ref_21",
"volume": "39",
"year": "2019"
},
{
"DOI": "10.3390/v12060583",
"doi-asserted-by": "crossref",
"key": "ref_22",
"unstructured": "De Jesús-González, L.A., Cervantes-Salazar, M., Reyes-Ruiz, J.M., Osuna-Ramos, J.F., Farfán-Morales, C.N., Palacios-Rápalo, S.N., Pérez-Olais, J.H., Cordero-Rivera, C.D., Hurtado-Monzón, A.M., and Ruíz-Jiménez, F. (2020). The Nuclear Pore Complex: A Target for NS3 Protease of Dengue and Zika Viruses. Viruses, 12."
},
{
"DOI": "10.1016/j.isci.2023.108294",
"article-title": "An Ivermectin–Atorvastatin Combination Impairs Nuclear Transport Inhibiting Dengue Infection in Vitro and in Vivo",
"doi-asserted-by": "crossref",
"first-page": "108294",
"journal-title": "iScience",
"key": "ref_23",
"volume": "26",
"year": "2023"
},
{
"DOI": "10.1016/j.bmc.2021.116356",
"doi-asserted-by": "crossref",
"key": "ref_24",
"unstructured": "Mahajan, S., Choudhary, S., Kumar, P., and Tomar, S. (2021). Antiviral Strategies Targeting Host Factors and Mechanisms Obliging +ssRNA Viral Pathogens. Bioorg. Med. Chem., 46."
},
{
"DOI": "10.3390/molecules27217370",
"doi-asserted-by": "crossref",
"key": "ref_25",
"unstructured": "Peng, S., Wang, H., Wang, Z., and Wang, Q. (2022). Progression of Antiviral Agents Targeting Viral Polymerases. Molecules, 27."
},
{
"DOI": "10.1055/s-0036-1584923",
"article-title": "Adenovirus: Epidemiology, Global Spread of Novel Serotypes, and Advances in Treatment and Prevention",
"author": "Lynch",
"doi-asserted-by": "crossref",
"first-page": "586",
"journal-title": "Semin. Respir. Crit. Care Med.",
"key": "ref_26",
"volume": "37",
"year": "2016"
},
{
"DOI": "10.3390/pathogens10111456",
"doi-asserted-by": "crossref",
"key": "ref_27",
"unstructured": "Abdelqader, R., Hasan, H., and Alanagreh, L. (2021). Epidemiology of Human Bocavirus in the Middle East and North Africa: Systematic Review. Pathogens, 10."
},
{
"DOI": "10.3389/fped.2021.643219",
"doi-asserted-by": "crossref",
"key": "ref_28",
"unstructured": "Bizot, E., Bousquet, A., Charpié, M., Coquelin, F., Lefevre, S., Le Lorier, J., Patin, M., Sée, P., Sarfati, E., and Walle, S. (2021). Rhinovirus: A Narrative Review on Its Genetic Characteristics, Pediatric Clinical Presentations, and Pathogenesis. Front. Pediatr., 9."
},
{
"DOI": "10.1080/21505594.2021.1960106",
"article-title": "The Pathogenesis and Virulence of Enterovirus-D68 Infection",
"author": "Laksono",
"doi-asserted-by": "crossref",
"first-page": "2060",
"journal-title": "Virulence",
"key": "ref_29",
"volume": "12",
"year": "2021"
},
{
"article-title": "SARS-CoV-2, SARS-CoV, and MERS-COV: A Comparative Overview",
"author": "Rabaan",
"first-page": "174",
"journal-title": "Infez. Med.",
"key": "ref_30",
"volume": "28",
"year": "2020"
},
{
"DOI": "10.1053/j.jvca.2020.04.041",
"article-title": "Coronavirus Disease-2019 (COVID-19) and Cardiovascular Complications",
"author": "Ma",
"doi-asserted-by": "crossref",
"first-page": "1860",
"journal-title": "J. Cardiothorac. Vasc. Anesth.",
"key": "ref_31",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1016/j.puhe.2023.06.020",
"article-title": "Respiratory Syncytial Virus Infection: Survival Experience in a Cohort of Children Inpatients",
"author": "Huerta",
"doi-asserted-by": "crossref",
"first-page": "181",
"journal-title": "Public Health",
"key": "ref_32",
"volume": "221",
"year": "2023"
},
{
"DOI": "10.1007/5584_2020_483",
"article-title": "Nosocomial Infections in Patients Hospitalized with Respiratory Syncytial Virus: A Practice Review",
"author": "Wrotek",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Adv. Exp. Med. Biol.",
"key": "ref_33",
"volume": "1271",
"year": "2020"
},
{
"DOI": "10.1128/microbiolspec.AID-0020-2014",
"doi-asserted-by": "crossref",
"key": "ref_34",
"unstructured": "Schuster, J.E., and Williams, J.V. (2014). Human Metapneumovirus. Microbiol. Spectr., 2."
},
{
"DOI": "10.1055/s-0036-1584798",
"article-title": "Parainfluenza Virus Infection",
"author": "Branche",
"doi-asserted-by": "crossref",
"first-page": "538",
"journal-title": "Semin. Respir. Crit. Care Med.",
"key": "ref_35",
"volume": "37",
"year": "2016"
},
{
"DOI": "10.1111/jmwh.13203",
"article-title": "Influenza and Influenza Vaccine: A Review",
"author": "Nypaver",
"doi-asserted-by": "crossref",
"first-page": "45",
"journal-title": "J. Midwifery Womens Health",
"key": "ref_36",
"volume": "66",
"year": "2021"
},
{
"DOI": "10.3390/v16020316",
"doi-asserted-by": "crossref",
"key": "ref_37",
"unstructured": "Carter, T., and Iqbal, M. (2024). The Influenza A Virus Replication Cycle: A Comprehensive Review. Viruses, 16."
},
{
"DOI": "10.1128/mbio.01273-23",
"article-title": "The Current State of Research on Influenza Antiviral Drug Development: Drugs in Clinical Trial and Licensed Drugs",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "e01273-23",
"journal-title": "mBio",
"key": "ref_38",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.2174/0929867324666170920165926",
"article-title": "Influenza Virus: Small Molecule Therapeutics and Mechanisms of Antiviral Resistance",
"author": "Han",
"doi-asserted-by": "crossref",
"first-page": "5115",
"journal-title": "Curr. Med. Chem.",
"key": "ref_39",
"volume": "25",
"year": "2018"
},
{
"DOI": "10.3390/v7092850",
"article-title": "Clinical Implications of Antiviral Resistance in Influenza",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "4929",
"journal-title": "Viruses",
"key": "ref_40",
"volume": "7",
"year": "2015"
},
{
"DOI": "10.3389/fcimb.2023.1269344",
"doi-asserted-by": "crossref",
"key": "ref_41",
"unstructured": "Meseko, C., Sanicas, M., Asha, K., Sulaiman, L., and Kumar, B. (2023). Antiviral Options and Therapeutics against Influenza: History, Latest Developments and Future Prospects. Front. Cell. Infect. Microbiol., 13."
},
{
"DOI": "10.1038/363418a0",
"article-title": "Rational Design of Potent Sialidase-Based Inhibitors of Influenza Virus Replication",
"author": "Wu",
"doi-asserted-by": "crossref",
"first-page": "418",
"journal-title": "Nature",
"key": "ref_42",
"volume": "363",
"year": "1993"
},
{
"DOI": "10.1074/jbc.M710302200",
"article-title": "Identification of the Pore-Lining Residues of the BM2 Ion Channel Protein of Influenza B Virus",
"author": "Ma",
"doi-asserted-by": "crossref",
"first-page": "15921",
"journal-title": "J. Biol. Chem.",
"key": "ref_43",
"volume": "283",
"year": "2008"
},
{
"DOI": "10.3390/ijerph19053018",
"doi-asserted-by": "crossref",
"key": "ref_44",
"unstructured": "Świerczyńska, M., Mirowska-Guzel, D.M., and Pindelska, E. (2022). Antiviral Drugs in Influenza. Int. J. Environ. Res. Public Health, 19."
},
{
"DOI": "10.1001/jama.282.1.31",
"article-title": "Zanamivir in the Prevention of Influenza among Healthy Adults: A Randomized Controlled Trial",
"author": "Monto",
"doi-asserted-by": "crossref",
"first-page": "31",
"journal-title": "JAMA",
"key": "ref_45",
"volume": "282",
"year": "1999"
},
{
"key": "ref_46",
"unstructured": "(2024, November 08). CDC Treating Flu with Antiviral Drugs, Available online: https://www.cdc.gov/flu/treatment/antiviral-drugs.html."
},
{
"DOI": "10.1021/ja963036t",
"article-title": "Influenza Neuraminidase Inhibitors Possessing a Novel Hydrophobic Interaction in the Enzyme Active Site: Design, Synthesis, and Structural Analysis of Carbocyclic Sialic Acid Analogues with Potent Anti-Influenza Activity",
"author": "Kim",
"doi-asserted-by": "crossref",
"first-page": "681",
"journal-title": "J. Am. Chem. Soc.",
"key": "ref_47",
"volume": "119",
"year": "1997"
},
{
"DOI": "10.1517/14656566.2015.1066336",
"article-title": "Peramivir: An Intravenous Neuraminidase Inhibitor",
"author": "McLaughlin",
"doi-asserted-by": "crossref",
"first-page": "1889",
"journal-title": "Expert Opin. Pharmacother.",
"key": "ref_48",
"volume": "16",
"year": "2015"
},
{
"key": "ref_49",
"unstructured": "U.S. Food & Drug Administration (2018). Rapivab (Peramivir) Information, FDA."
},
{
"DOI": "10.2147/DDDT.S46654",
"article-title": "Safety and Efficacy of Peramivir for Influenza Treatment",
"author": "Hata",
"doi-asserted-by": "crossref",
"first-page": "2017",
"journal-title": "Drug Des. Dev. Ther.",
"key": "ref_50",
"volume": "8",
"year": "2014"
},
{
"DOI": "10.1002/phar.2330",
"article-title": "Peramivir for Influenza A and B Viral Infections: A Pharmacokinetic Case Series",
"author": "Cies",
"doi-asserted-by": "crossref",
"first-page": "1060",
"journal-title": "Pharmacotherapy",
"key": "ref_51",
"volume": "39",
"year": "2019"
},
{
"DOI": "10.1016/j.coviro.2019.01.006",
"article-title": "Baloxavir Marboxil: The New Influenza Drug on the Market",
"author": "Shaw",
"doi-asserted-by": "crossref",
"first-page": "14",
"journal-title": "Curr. Opin. Virol.",
"key": "ref_52",
"volume": "35",
"year": "2019"
},
{
"DOI": "10.1016/S1473-3099(20)30004-9",
"article-title": "Early Treatment with Baloxavir Marboxil in High-Risk Adolescent and Adult Outpatients with Uncomplicated Influenza (CAPSTONE-2): A Randomised, Placebo-Controlled, Phase 3 Trial",
"author": "Ison",
"doi-asserted-by": "crossref",
"first-page": "1204",
"journal-title": "Lancet Infect. Dis.",
"key": "ref_53",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1016/j.antiviral.2017.11.013",
"article-title": "Prevention and Treatment of Respiratory Viral Infections: Presentations on Antivirals, Traditional Therapies and Host-Directed Interventions at the 5th ISIRV Antiviral Group Conference",
"author": "Jiang",
"doi-asserted-by": "crossref",
"first-page": "118",
"journal-title": "Antivir. Res.",
"key": "ref_54",
"volume": "149",
"year": "2018"
},
{
"DOI": "10.37201/req/096.2022",
"article-title": "[EDP-938, a new antiviral with inhibitory activity against the nucleoprotein of the respiratory syncytial virus]",
"author": "Reina",
"doi-asserted-by": "crossref",
"first-page": "26",
"journal-title": "Rev. Esp. Quimioter.",
"key": "ref_55",
"volume": "36",
"year": "2023"
},
{
"DOI": "10.1016/j.antiviral.2024.105948",
"article-title": "Direct-Acting Antivirals for RSV Treatment, a Review",
"author": "Bonneux",
"doi-asserted-by": "crossref",
"first-page": "105948",
"journal-title": "Antivir. Res.",
"key": "ref_56",
"volume": "229",
"year": "2024"
},
{
"DOI": "10.1128/JVI.02349-15",
"article-title": "Investigating the Influence of Ribavirin on Human Respiratory Syncytial Virus RNA Synthesis by Using a High-Resolution Transcriptome Sequencing Approach",
"author": "Aljabr",
"doi-asserted-by": "crossref",
"first-page": "4876",
"journal-title": "J. Virol.",
"key": "ref_57",
"volume": "90",
"year": "2016"
},
{
"DOI": "10.1177/0018578719836646",
"article-title": "The Use of Aerosolized Ribavirin in Respiratory Syncytial Virus Lower Respiratory Tract Infections in Adult Immunocompromised Patients: A Systematic Review",
"author": "Avery",
"doi-asserted-by": "crossref",
"first-page": "224",
"journal-title": "Hosp. Pharm.",
"key": "ref_58",
"volume": "55",
"year": "2020"
},
{
"DOI": "10.3949/ccjm.91.s1.06",
"article-title": "RSV in Transplant and Immunocompromised Patients",
"author": "Koval",
"doi-asserted-by": "crossref",
"first-page": "S34",
"journal-title": "Clevel. Clin. J. Med.",
"key": "ref_59",
"volume": "91",
"year": "2024"
},
{
"DOI": "10.1016/S2352-4642(22)00321-2",
"article-title": "Efficacy of Nirsevimab against Respiratory Syncytial Virus Lower Respiratory Tract Infections in Preterm and Term Infants, and Pharmacokinetic Extrapolation to Infants with Congenital Heart Disease and Chronic Lung Disease: A Pooled Analysis of Randomised Controlled Trials",
"author": "Madhi",
"doi-asserted-by": "crossref",
"first-page": "180",
"journal-title": "Lancet Child Adolesc. Health",
"key": "ref_60",
"volume": "7",
"year": "2023"
},
{
"key": "ref_61",
"unstructured": "(2024, November 08). CDC Respiratory Syncytial Virus (RSV) Vaccine Safety, Available online: https://www.cdc.gov/vaccine-safety/vaccines/rsv.html."
},
{
"DOI": "10.3346/jkms.2021.36.e298",
"article-title": "Antivirals for Coexistence with COVID-19: Brief Review for General Physicians",
"author": "Yoo",
"doi-asserted-by": "crossref",
"first-page": "e298",
"journal-title": "J. Korean Med. Sci.",
"key": "ref_62",
"volume": "36",
"year": "2021"
},
{
"DOI": "10.1016/j.biopha.2021.112015",
"doi-asserted-by": "crossref",
"key": "ref_63",
"unstructured": "Rehman, S.U., Rehman, S.U., and Yoo, H.H. (2021). COVID-19 Challenges and Its Therapeutics. Biomed. Pharmacother., 142."
},
{
"DOI": "10.1007/s11596-021-2395-1",
"article-title": "A Comprehensive Review of COVID-19 Virology, Vaccines, Variants, and Therapeutics",
"author": "Forchette",
"doi-asserted-by": "crossref",
"first-page": "1037",
"journal-title": "Curr. Med. Sci.",
"key": "ref_64",
"volume": "41",
"year": "2021"
},
{
"article-title": "Remdesivir for the Treatment of COVID-19",
"author": "Grundeis",
"first-page": "CD014962",
"journal-title": "Cochrane Database Syst. Rev.",
"key": "ref_65",
"volume": "1",
"year": "2023"
},
{
"DOI": "10.15190/d.2022.10",
"article-title": "Remdesivir: The First FDA-Approved Anti-COVID-19 Treatment for Young Children",
"author": "Chera",
"doi-asserted-by": "crossref",
"first-page": "e151",
"journal-title": "Discoveries",
"key": "ref_66",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1007/s40265-023-01926-0",
"article-title": "Remdesivir: A Review in COVID-19",
"author": "Blair",
"doi-asserted-by": "crossref",
"first-page": "1215",
"journal-title": "Drugs",
"key": "ref_67",
"volume": "83",
"year": "2023"
},
{
"DOI": "10.3390/v14050961",
"doi-asserted-by": "crossref",
"key": "ref_68",
"unstructured": "Ng, T.I., Correia, I., Seagal, J., DeGoey, D.A., Schrimpf, M.R., Hardee, D.J., Noey, E.L., and Kati, W.M. (2022). Antiviral Drug Discovery for the Treatment of COVID-19 Infections. Viruses, 14."
},
{
"DOI": "10.3390/cells10020206",
"doi-asserted-by": "crossref",
"key": "ref_69",
"unstructured": "Chilamakuri, R., and Agarwal, S. (2021). COVID-19: Characteristics and Therapeutics. Cells, 10."
},
{
"DOI": "10.3389/fimmu.2023.1125246",
"doi-asserted-by": "crossref",
"key": "ref_70",
"unstructured": "Yuan, Y., Jiao, B., Qu, L., Yang, D., and Liu, R. (2023). The Development of COVID-19 Treatment. Front. Immunol., 14."
},
{
"DOI": "10.1177/2632010X241263054",
"article-title": "Preclinical and Clinical Investigations of Potential Drugs and Vaccines for COVID-19 Therapy: A Comprehensive Review With Recent Update",
"author": "Mia",
"doi-asserted-by": "crossref",
"first-page": "2632010X241263054",
"journal-title": "Clin. Pathol.",
"key": "ref_71",
"volume": "17",
"year": "2024"
},
{
"DOI": "10.3390/v14112540",
"doi-asserted-by": "crossref",
"key": "ref_72",
"unstructured": "Akinosoglou, K., Schinas, G., and Gogos, C. (2022). Oral Antiviral Treatment for COVID-19: A Comprehensive Review on Nirmatrelvir/Ritonavir. Viruses, 14."
},
{
"DOI": "10.1186/s12929-022-00852-9",
"doi-asserted-by": "crossref",
"key": "ref_73",
"unstructured": "Kumari, M., Lu, R.-M., Li, M.-C., Huang, J.-L., Hsu, F.-F., Ko, S.-H., Ke, F.-Y., Su, S.-C., Liang, K.-H., and Yuan, J.P.-Y. (2022). A Critical Overview of Current Progress for COVID-19: Development of Vaccines, Antiviral Drugs, and Therapeutic Antibodies. J. Biomed. Sci., 29."
},
{
"DOI": "10.1370/afm.3120",
"article-title": "Nirmatrelvir/Ritonavir Regimen for Mild/Moderately Severe COVID-19: A Rapid Review With Meta-Analysis and Trial Sequential Analysis",
"author": "Okoli",
"doi-asserted-by": "crossref",
"first-page": "336",
"journal-title": "Ann. Fam. Med.",
"key": "ref_74",
"volume": "22",
"year": "2024"
},
{
"key": "ref_75",
"unstructured": "(2024, December 11). Xocova® (Ensitrelvir Fumaric Acid) Tablets 125 mg Approved in Japan for the Treatment of SARS-CoV-2 Infection, Under the Emergency Regulatory Approval System. Available online: https://www.shionogi.com/global/en/news/2022/11/e20221122.html."
},
{
"key": "ref_76",
"unstructured": "(2024, December 11). Notice Regarding the Signing of a Basic Agreement with the Ministry of Health, Labor and Welfare for Domestic Supply of S-217622, a Therapeutic Drug for COVID-19. Available online: https://www.shionogi.com/global/en/news/2022/03/e-20220325.html."
},
{
"key": "ref_77",
"unstructured": "(2024, December 11). Shionogi Receives U.S. FDA Fast Track Designation for Ensitrelvir Fumaric Acid, an Investigational Oral Antiviral for COVID-19. Available online: https://www.shionogi.com/global/en/news/2023/04/20230404.html."
},
{
"DOI": "10.1001/jamanetworkopen.2023.54991",
"article-title": "Efficacy and Safety of 5-Day Oral Ensitrelvir for Patients With Mild to Moderate COVID-19",
"author": "Yotsuyanagi",
"doi-asserted-by": "crossref",
"first-page": "e2354991",
"journal-title": "JAMA Netw. Open",
"key": "ref_78",
"volume": "7",
"year": "2024"
},
{
"DOI": "10.1097/MD.0000000000033024",
"article-title": "A Phase 2/3 Study of S-217622 in Participants with SARS-CoV-2 Infection (Phase 3 Part)",
"author": "Yotsuyanagi",
"doi-asserted-by": "crossref",
"first-page": "e33024",
"journal-title": "Medicine",
"key": "ref_79",
"volume": "102",
"year": "2023"
},
{
"DOI": "10.1021/acs.jmedchem.2c00117",
"article-title": "Discovery of S-217622, a Noncovalent Oral SARS-CoV-2 3CL Protease Inhibitor Clinical Candidate for Treating COVID-19",
"author": "Unoh",
"doi-asserted-by": "crossref",
"first-page": "6499",
"journal-title": "J. Med. Chem.",
"key": "ref_80",
"volume": "65",
"year": "2022"
},
{
"key": "ref_81",
"unstructured": "(2024, December 11). NIH Trial to Evaluate Shionogi Antiviral in Adults Hospitalized with COVID-19, Available online: https://www.nih.gov/news-events/news-releases/nih-trial-evaluate-shionogi-antiviral-adults-hospitalized-covid-19."
},
{
"DOI": "10.1007/s40121-024-01010-4",
"article-title": "Real-World Effectiveness of Ensitrelvir in Reducing Severe Outcomes in Outpatients at High Risk for COVID-19",
"author": "Takazono",
"doi-asserted-by": "crossref",
"first-page": "1821",
"journal-title": "Infect. Dis. Ther.",
"key": "ref_82",
"volume": "13",
"year": "2024"
},
{
"DOI": "10.1016/j.nmni.2024.101522",
"article-title": "Real World Effectiveness of Early Ensitrelvir Treatment in Patients with SARS-CoV-2, a Retrospective Case Series",
"author": "Abe",
"doi-asserted-by": "crossref",
"first-page": "101522",
"journal-title": "New Microbes New Infect.",
"key": "ref_83",
"volume": "62",
"year": "2024"
},
{
"DOI": "10.1002/jmv.25985",
"article-title": "The Anti-HIV Drug Nelfinavir Mesylate (Viracept) Is a Potent Inhibitor of Cell Fusion Caused by the SARSCoV-2 Spike (S) Glycoprotein Warranting Further Evaluation as an Antiviral against COVID-19 Infections",
"author": "Musarrat",
"doi-asserted-by": "crossref",
"first-page": "2087",
"journal-title": "J. Med. Virol.",
"key": "ref_84",
"volume": "92",
"year": "2020"
},
{
"DOI": "10.1038/s41392-023-01429-0",
"article-title": "Preventive and Therapeutic Benefits of Nelfinavir in Rhesus Macaques and Human Beings Infected with SARS-CoV-2",
"author": "Xu",
"doi-asserted-by": "crossref",
"first-page": "169",
"journal-title": "Sig. Transduct. Target. Ther.",
"key": "ref_85",
"volume": "8",
"year": "2023"
},
{
"DOI": "10.1021/acs.jpcb.3c05564",
"article-title": "Prediction of Binding Pose and Affinity of Nelfinavir, a SARS-CoV-2 Main Protease Repositioned Drug, by Combining Docking, Molecular Dynamics, and Fragment Molecular Orbital Calculations",
"author": "Handa",
"doi-asserted-by": "crossref",
"first-page": "2249",
"journal-title": "J. Phys. Chem. B",
"key": "ref_86",
"volume": "128",
"year": "2024"
},
{
"DOI": "10.3904/kjim.2020.157",
"article-title": "In Vitro Activity of Lopinavir/Ritonavir and Hydroxychloroquine against Severe Acute Respiratory Syndrome Coronavirus 2 at Concentrations Achievable by Usual Doses",
"author": "Kang",
"doi-asserted-by": "crossref",
"first-page": "728",
"journal-title": "Korean J. Intern. Med.",
"key": "ref_87",
"volume": "35",
"year": "2020"
},
{
"DOI": "10.3390/microorganisms12081696",
"doi-asserted-by": "crossref",
"key": "ref_88",
"unstructured": "Kim, C.-M., Chung, J.K., Tamanna, S., Bang, M.-S., Tariq, M., Lee, Y.M., Seo, J.-W., Kim, D.Y., Yun, N.R., and Seo, J. (2024). Comparable Efficacy of Lopinavir/Ritonavir and Remdesivir in Reducing Viral Load and Shedding Duration in Patients with COVID-19. Microorganisms, 12."
},
{
"DOI": "10.1093/jac/dkaa195",
"article-title": "Lopinavir Pharmacokinetics in COVID-19 Patients",
"author": "Gregoire",
"doi-asserted-by": "crossref",
"first-page": "2702",
"journal-title": "J. Antimicrob. Chemother.",
"key": "ref_89",
"volume": "75",
"year": "2020"
},
{
"DOI": "10.1128/AAC.01177-20",
"article-title": "Effect of Systemic Inflammatory Response to SARS-CoV-2 on Lopinavir and Hydroxychloroquine Plasma Concentrations",
"author": "Marzolini",
"doi-asserted-by": "crossref",
"first-page": "10-1128",
"journal-title": "Antimicrob. Agents Chemother.",
"key": "ref_90",
"volume": "64",
"year": "2020"
},
{
"DOI": "10.1007/s40264-020-00966-9",
"article-title": "Lopinavir-Ritonavir in the Treatment of COVID-19: A Dynamic Systematic Benefit-Risk Assessment",
"author": "Osborne",
"doi-asserted-by": "crossref",
"first-page": "809",
"journal-title": "Drug Saf.",
"key": "ref_91",
"volume": "43",
"year": "2020"
},
{
"key": "ref_92",
"unstructured": "U.S. Food & Drug Administration (2024). FDA Revises Letter of Authorization for the Emergency Use Authorization for Paxlovid, FDA."
},
{
"key": "ref_93",
"unstructured": "(2024, July 17). Drug Approval Package, Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/99/20-945_Ritonovir.cfm."
},
{
"article-title": "Comparison of the Efficacy and Safety of Atazanavir/Ritonavir Plus Hydroxychloroquine with Lopinavir/Ritonavir Plus Hydroxychloroquine in Patients with Moderate COVID-19, A Randomized, Double-Blind Clinical Trial",
"author": "Nekoukar",
"first-page": "278",
"journal-title": "Iran. J. Pharm. Res. IJPR",
"key": "ref_94",
"volume": "20",
"year": "2021"
},
{
"DOI": "10.3389/fmed.2022.956123",
"doi-asserted-by": "crossref",
"key": "ref_95",
"unstructured": "Fowotade, A., Bamidele, F., Egbetola, B., Fagbamigbe, A.F., Adeagbo, B.A., Adefuye, B.O., Olagunoye, A., Ojo, T.O., Adebiyi, A.O., and Olagunju, O.I. (2022). A Randomized, Open-Label Trial of Combined Nitazoxanide and Atazanavir/Ritonavir for Mild to Moderate COVID-19. Front. Med., 9."
},
{
"DOI": "10.1016/j.ijid.2020.05.085",
"article-title": "Lack of Antiviral Activity of Darunavir against SARS-CoV-2",
"author": "Bojkova",
"doi-asserted-by": "crossref",
"first-page": "7",
"journal-title": "Int. J. Infect. Dis.",
"key": "ref_96",
"volume": "97",
"year": "2020"
},
{
"DOI": "10.1128/AAC.00825-20",
"article-title": "Atazanavir, Alone or in Combination with Ritonavir, Inhibits SARS-CoV-2 Replication and Proinflammatory Cytokine Production",
"author": "Sacramento",
"doi-asserted-by": "crossref",
"first-page": "e00825-20",
"journal-title": "Antimicrob. Agents Chemother.",
"key": "ref_97",
"volume": "64",
"year": "2020"
},
{
"DOI": "10.1186/1743-422X-2-69",
"article-title": "Chloroquine Is a Potent Inhibitor of SARS Coronavirus Infection and Spread",
"author": "Vincent",
"doi-asserted-by": "crossref",
"first-page": "69",
"journal-title": "Virol. J.",
"key": "ref_98",
"volume": "2",
"year": "2005"
},
{
"key": "ref_99",
"unstructured": "Organización Panamericana de Salud (PAHO) (2020). COVID-19: Chloroquine and Hydroxychloroquine Research, PAHO. Rapid Review-28 March 2020."
},
{
"DOI": "10.1016/j.idc.2010.01.001",
"article-title": "Antiviral Drug Resistance: Mechanisms and Clinical Implications",
"author": "Strasfeld",
"doi-asserted-by": "crossref",
"first-page": "413",
"journal-title": "Infect. Dis. Clin. N. Am.",
"key": "ref_100",
"volume": "24",
"year": "2010"
},
{
"DOI": "10.3390/ijms232012244",
"doi-asserted-by": "crossref",
"key": "ref_101",
"unstructured": "Smyk, J.M., Szydłowska, N., Szulc, W., and Majewska, A. (2022). Evolution of Influenza Viruses—Drug Resistance, Treatment Options, and Prospects. Int. J. Mol. Sci., 23."
},
{
"DOI": "10.1128/cmr.00040-22",
"article-title": "Antiviral Approaches against Influenza Virus",
"author": "Kumari",
"doi-asserted-by": "crossref",
"first-page": "e00040-22",
"journal-title": "Clin. Microbiol. Rev.",
"key": "ref_102",
"volume": "36",
"year": "2023"
},
{
"DOI": "10.1126/scitranslmed.abo0718",
"article-title": "Mutations in the SARS-CoV-2 RNA-Dependent RNA Polymerase Confer Resistance to Remdesivir by Distinct Mechanisms",
"author": "Stevens",
"doi-asserted-by": "crossref",
"first-page": "eabo0718",
"journal-title": "Sci. Transl. Med.",
"key": "ref_103",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.3390/ph16081143",
"doi-asserted-by": "crossref",
"key": "ref_104",
"unstructured": "Gratteri, C., Ambrosio, F.A., Lupia, A., Moraca, F., Catalanotti, B., Costa, G., Bellocchi, M., Carioti, L., Salpini, R., and Ceccherini-Silberstein, F. (2023). Molecular and Structural Aspects of Clinically Relevant Mutations of SARS-CoV-2 RNA-Dependent RNA Polymerase in Remdesivir-Treated Patients. Pharmaceuticals, 16."
},
{
"DOI": "10.1007/s10096-020-03840-9",
"article-title": "Influenza and Antiviral Resistance: An Overview",
"author": "Lampejo",
"doi-asserted-by": "crossref",
"first-page": "1201",
"journal-title": "Eur. J. Clin. Microbiol. Infect. Dis.",
"key": "ref_105",
"volume": "39",
"year": "2020"
},
{
"DOI": "10.1038/s41579-022-00809-7",
"article-title": "SARS-CoV-2 Variant Evasion of Monoclonal Antibodies Based on in Vitro Studies",
"author": "Cox",
"doi-asserted-by": "crossref",
"first-page": "112",
"journal-title": "Nat. Rev. Microbiol.",
"key": "ref_106",
"volume": "21",
"year": "2023"
},
{
"DOI": "10.3390/vaccines9101195",
"doi-asserted-by": "crossref",
"key": "ref_107",
"unstructured": "Ramesh, S., Govindarajulu, M., Parise, R.S., Neel, L., Shankar, T., Patel, S., Lowery, P., Smith, F., Dhanasekaran, M., and Moore, T. (2021). Emerging SARS-CoV-2 Variants: A Review of Its Mutations, Its Implications and Vaccine Efficacy. Vaccines, 9."
},
{
"DOI": "10.1016/j.drudis.2021.05.008",
"article-title": "Drug Combination Therapy for Emerging Viral Diseases",
"author": "Shyr",
"doi-asserted-by": "crossref",
"first-page": "2367",
"journal-title": "Drug Discov. Today",
"key": "ref_108",
"volume": "26",
"year": "2021"
},
{
"DOI": "10.1038/s41573-023-00692-8",
"article-title": "Accelerating Antiviral Drug Discovery: Lessons from COVID-19",
"author": "Hall",
"doi-asserted-by": "crossref",
"first-page": "585",
"journal-title": "Nat. Rev. Drug Discov.",
"key": "ref_109",
"volume": "22",
"year": "2023"
},
{
"DOI": "10.3390/microbiolres14020037",
"article-title": "Drug Resistance: An Incessant Fight against Evolutionary Strategies of Survival",
"doi-asserted-by": "crossref",
"first-page": "507",
"journal-title": "Microbiol. Res.",
"key": "ref_110",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.1002/cti2.1067",
"article-title": "Harnessing Host–Virus Evolution in Antiviral Therapy and Immunotherapy",
"author": "Heaton",
"doi-asserted-by": "crossref",
"first-page": "e1067",
"journal-title": "Clin. Transl. Immunol.",
"key": "ref_111",
"volume": "8",
"year": "2019"
},
{
"DOI": "10.1016/j.heliyon.2024.e26423",
"article-title": "Next-Generation Treatments: Immunotherapy and Advanced Therapies for COVID-19",
"author": "Camacho",
"doi-asserted-by": "crossref",
"first-page": "e26423",
"journal-title": "Heliyon",
"key": "ref_112",
"volume": "10",
"year": "2024"
},
{
"DOI": "10.3390/ijms20184331",
"doi-asserted-by": "crossref",
"key": "ref_113",
"unstructured": "Pinzi, L., and Rastelli, G. (2019). Molecular Docking: Shifting Paradigms in Drug Discovery. Int. J. Mol. Sci., 20."
},
{
"DOI": "10.3390/v12091058",
"doi-asserted-by": "crossref",
"key": "ref_114",
"unstructured": "Low, Z.Y., Farouk, I.A., and Lal, S.K. (2020). Drug Repositioning: New Approaches and Future Prospects for Life-Debilitating Diseases and the COVID-19 Pandemic Outbreak. Viruses, 12."
},
{
"DOI": "10.3390/molecules200713384",
"article-title": "Molecular Docking and Structure-Based Drug Design Strategies",
"author": "Ferreira",
"doi-asserted-by": "crossref",
"first-page": "13384",
"journal-title": "Molecules",
"key": "ref_115",
"volume": "20",
"year": "2015"
},
{
"DOI": "10.3390/ijms20184574",
"doi-asserted-by": "crossref",
"key": "ref_116",
"unstructured": "Torres, P.H.M., Sodero, A.C.R., Jofily, P., and Silva-Jr, F.P. (2019). Key Topics in Molecular Docking for Drug Design. Int. J. Mol. Sci., 20."
},
{
"DOI": "10.3390/v12101178",
"doi-asserted-by": "crossref",
"key": "ref_117",
"unstructured": "Ianevski, A., Yao, R., Biza, S., Zusinaite, E., Mannik, A., Kivi, G., Planken, A., Kurg, K., Tombak, E.-M., and Ustav, M. (2020). Identification and Tracking of Antiviral Drug Combinations. Viruses, 12."
},
{
"DOI": "10.3390/molecules27249041",
"doi-asserted-by": "crossref",
"key": "ref_118",
"unstructured": "Ivanova, L., and Karelson, M. (2022). The Impact of Software Used and the Type of Target Protein on Molecular Docking Accuracy. Molecules, 27."
},
{
"DOI": "10.1016/j.rineng.2023.101335",
"article-title": "An Accurate and Universal Protein-Small Molecule Batch Docking Solution Using Autodock Vina",
"author": "Che",
"doi-asserted-by": "crossref",
"first-page": "101335",
"journal-title": "Results Eng.",
"key": "ref_119",
"volume": "19",
"year": "2023"
},
{
"DOI": "10.1002/jcc.21334",
"article-title": "AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization and Multithreading",
"author": "Trott",
"doi-asserted-by": "crossref",
"first-page": "455",
"journal-title": "J. Comput. Chem.",
"key": "ref_120",
"volume": "31",
"year": "2010"
},
{
"DOI": "10.1186/s12915-024-02028-3",
"doi-asserted-by": "crossref",
"key": "ref_121",
"unstructured": "Wei, J., Zhuo, L., Fu, X., Zeng, X., Wang, L., Zou, Q., and Cao, D. (2024). DrugReAlign: A Multisource Prompt Framework for Drug Repurposing Based on Large Language Models. BMC Biol., 22."
},
{
"DOI": "10.3390/ph17010022",
"doi-asserted-by": "crossref",
"key": "ref_122",
"unstructured": "Niazi, S.K., and Mariam, Z. (2024). Computer-Aided Drug Design and Drug Discovery: A Prospective Analysis. Pharmaceuticals, 17."
},
{
"key": "ref_123",
"unstructured": "Chachulski, L. (2024). Development and Application of Compound Class-Specific Benchmark Data Sets for Differentiated Assessment of Docking and Scoring Algorithm Performance. [Ph.D. Dissertation, Jacobs University Bremen]."
},
{
"DOI": "10.1021/jm0608356",
"article-title": "Benchmarking Sets for Molecular Docking",
"author": "Huang",
"doi-asserted-by": "crossref",
"first-page": "6789",
"journal-title": "J. Med. Chem.",
"key": "ref_124",
"volume": "49",
"year": "2006"
},
{
"DOI": "10.34172/bi.2023.29955",
"doi-asserted-by": "crossref",
"key": "ref_125",
"unstructured": "Shamsian, S., Sokouti, B., and Dastmalchi, S. (2024). Benchmarking Different Docking Protocols for Predicting the Binding Poses of Ligands Complexed with Cyclooxygenase Enzymes and Screening Chemical Libraries. Bioimpacts, 14."
},
{
"DOI": "10.1002/jcc.21256",
"article-title": "AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility",
"author": "Morris",
"doi-asserted-by": "crossref",
"first-page": "2785",
"journal-title": "J. Comput. Chem.",
"key": "ref_126",
"volume": "30",
"year": "2009"
},
{
"DOI": "10.1093/nar/28.1.235",
"article-title": "The Protein Data Bank",
"author": "Berman",
"doi-asserted-by": "crossref",
"first-page": "235",
"journal-title": "Nucleic Acids Res.",
"key": "ref_127",
"volume": "28",
"year": "2000"
},
{
"DOI": "10.1002/pro.3730",
"article-title": "RCSB Protein Data Bank: Enabling Biomedical Research and Drug Discovery",
"author": "Goodsell",
"doi-asserted-by": "crossref",
"first-page": "52",
"journal-title": "Protein Sci.",
"key": "ref_128",
"volume": "29",
"year": "2020"
},
{
"DOI": "10.1093/nar/gkac1077",
"article-title": "RCSB Protein Data Bank (RCSB.Org): Delivery of Experimentally-Determined PDB Structures alongside One Million Computed Structure Models of Proteins from Artificial Intelligence/Machine Learning",
"author": "Burley",
"doi-asserted-by": "crossref",
"first-page": "D488",
"journal-title": "Nucleic Acids Res.",
"key": "ref_129",
"volume": "51",
"year": "2023"
},
{
"DOI": "10.1007/978-3-031-68419-7",
"doi-asserted-by": "crossref",
"key": "ref_130",
"unstructured": "Pujol, F.H., and Paniz-Mondolfi, A.E. (2024). Drug Repositioning as an Antiviral Strategy Against Emerging Viruses. Emerging Viruses in Latin America: Contemporary Virology, Springer Nature Switzerland."
},
{
"DOI": "10.1177/11779322221125605",
"doi-asserted-by": "crossref",
"key": "ref_131",
"unstructured": "Terefe, E.M., and Ghosh, A. (2022). Molecular Docking, Validation, Dynamics Simulations, and Pharmacokinetic Prediction of Phytochemicals Isolated From Croton Dichogamus Against the HIV-1 Reverse Transcriptase. Bioinform. Biol. Insights, 16."
},
{
"DOI": "10.3390/molecules27185988",
"doi-asserted-by": "crossref",
"key": "ref_132",
"unstructured": "Qayed, W.S., Ferreira, R.S., and Silva, J.R.A. (2022). In Silico Study towards Repositioning of FDA-Approved Drug Candidates for Anticoronaviral Therapy: Molecular Docking, Molecular Dynamics and Binding Free Energy Calculations. Molecules, 27."
},
{
"DOI": "10.3390/molecules23081899",
"doi-asserted-by": "crossref",
"key": "ref_133",
"unstructured": "Pantsar, T., and Poso, A. (2018). Binding Affinity via Docking: Fact and Fiction. Molecules, 23."
},
{
"DOI": "10.4155/fmc-2021-0025",
"article-title": "Identification of Known Drugs As Potential SARS-CoV-2 Mpro Inhibitors Using Ligand- and Structure-Based Virtual Screening",
"author": "Federico",
"doi-asserted-by": "crossref",
"first-page": "1353",
"journal-title": "Future Med. Chem.",
"key": "ref_134",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1007/s11095-023-03520-1",
"article-title": "Recent Advances in Inhaled Nanoformulations of Vaccines and Therapeutics Targeting Respiratory Viral Infections",
"author": "Loo",
"doi-asserted-by": "crossref",
"first-page": "1015",
"journal-title": "Pharm. Res.",
"key": "ref_135",
"volume": "40",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2035389",
"article-title": "Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine",
"author": "Baden",
"doi-asserted-by": "crossref",
"first-page": "403",
"journal-title": "N. Engl. J. Med.",
"key": "ref_136",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1016/j.jconrel.2022.03.051",
"article-title": "Inhibition of SARS-CoV-2 Replication in the Lung with siRNA/VIPER Polyplexes",
"author": "Baldassi",
"doi-asserted-by": "crossref",
"first-page": "661",
"journal-title": "J. Control. Release",
"key": "ref_137",
"volume": "345",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2034577",
"article-title": "Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine",
"author": "Polack",
"doi-asserted-by": "crossref",
"first-page": "2603",
"journal-title": "N. Engl. J. Med.",
"key": "ref_138",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.2217/nnm-2021-0447",
"article-title": "Nanotechnology-Based Therapeutics for Targeting Inflammatory Lung Diseases",
"author": "Loo",
"doi-asserted-by": "crossref",
"first-page": "865",
"journal-title": "Nanomedicine",
"key": "ref_139",
"volume": "17",
"year": "2022"
},
{
"DOI": "10.1016/S1473-3099(21)00396-0",
"article-title": "Safety, tolerability, and immunogenicity of an aerosolised adenovirus type-5 vector-based COVID-19 vaccine (Ad5-nCoV) in adults: Preliminary report of an open-label and randomised phase 1 clinical trial",
"author": "Wu",
"doi-asserted-by": "crossref",
"first-page": "1654",
"journal-title": "Lancet Infect Dis.",
"key": "ref_140",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1126/science.abe3255",
"article-title": "An Ultrapotent Synthetic Nanobody Neutralizes SARS-CoV-2 by Stabilizing Inactive Spike",
"author": "Schoof",
"doi-asserted-by": "crossref",
"first-page": "1473",
"journal-title": "Science",
"key": "ref_141",
"volume": "370",
"year": "2020"
},
{
"DOI": "10.1126/scitranslmed.abq0603",
"article-title": "Polymer nanoparticles deliver mRNA to the lung for mucosal vaccination",
"author": "Suberi",
"doi-asserted-by": "crossref",
"first-page": "eabq0603",
"journal-title": "Sci Transl Med.",
"key": "ref_142",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.1038/nrmicro3279",
"article-title": "Unravelling the Structural and Mechanistic Basis of CRISPR–Cas Systems",
"author": "Westra",
"doi-asserted-by": "crossref",
"first-page": "479",
"journal-title": "Nat. Rev. Microbiol.",
"key": "ref_143",
"volume": "12",
"year": "2014"
},
{
"article-title": "SARS-CoV-2 Infection of Airway Epithelial Cells",
"author": "Ryu",
"first-page": "e3",
"journal-title": "Immune Netw.",
"key": "ref_144",
"volume": "2",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.04.020",
"article-title": "Development of CRISPR as an Antiviral Strategy to Combat SARS-CoV-2 and Influenza",
"author": "Abbott",
"doi-asserted-by": "crossref",
"first-page": "865",
"journal-title": "Cell",
"key": "ref_145",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.3390/mps4020040",
"doi-asserted-by": "crossref",
"key": "ref_146",
"unstructured": "Challagulla, A., Schat, K.A., and Doran, T.J. (2021). In Vitro Inhibition of Influenza Virus Using CRISPR/Cas13a in Chicken Cells. Methods Protoc., 4."
},
{
"DOI": "10.15585/mmwr.mm7234a4",
"article-title": "Use of Nirsevimab for the Prevention of Respiratory Syncytial Virus Disease Among Infants and Young Children: Recommendations of the Advisory Committee on Immunization Practices—United States, 2023",
"author": "Jones",
"doi-asserted-by": "crossref",
"first-page": "920",
"journal-title": "MMWR Morb. Mortal. Wkly. Rep.",
"key": "ref_147",
"volume": "72",
"year": "2023"
},
{
"DOI": "10.1101/2022.03.22.485401",
"doi-asserted-by": "crossref",
"key": "ref_148",
"unstructured": "Suberi, A., Grun, M.K., Mao, T., Israelow, B., Reschke, M., Grundler, J., Akhtar, L., Lee, T., Shin, K., and Piotrowski-Daspit, A.S. (2022). Inhalable Polymer Nanoparticles for Versatile mRNA Delivery and Mucosal Vaccination. bioRxiv."
},
{
"DOI": "10.1021/acschembio.6b00788",
"article-title": "Activation Pathway of a Nucleoside Analog Inhibiting Respiratory Syncytial Virus Polymerase",
"author": "Jordan",
"doi-asserted-by": "crossref",
"first-page": "83",
"journal-title": "ACS Chem. Biol.",
"key": "ref_149",
"volume": "12",
"year": "2017"
},
{
"DOI": "10.1128/AAC.01746-13",
"article-title": "Multiple Classes of Antiviral Agents Exhibit in Vitro Activity against Human Rhinovirus Type C",
"author": "Mello",
"doi-asserted-by": "crossref",
"first-page": "1546",
"journal-title": "Antimicrob. Agents Chemother.",
"key": "ref_150",
"volume": "58",
"year": "2014"
},
{
"DOI": "10.1128/AAC.38.8.1864",
"article-title": "Efficacy of 2’-Deoxy-2’-Fluororibosides against Influenza A and B Viruses in Ferrets",
"author": "Jakeman",
"doi-asserted-by": "crossref",
"first-page": "1864",
"journal-title": "Antimicrob. Agents Chemother.",
"key": "ref_151",
"volume": "38",
"year": "1994"
},
{
"DOI": "10.1126/scitranslmed.aal3653",
"article-title": "Broad-Spectrum Antiviral GS-5734 Inhibits Both Epidemic and Zoonotic Coronaviruses",
"author": "Sheahan",
"doi-asserted-by": "crossref",
"first-page": "eaal3653",
"journal-title": "Sci. Transl. Med.",
"key": "ref_152",
"volume": "9",
"year": "2017"
},
{
"DOI": "10.1021/acsmedchemlett.1c00624",
"article-title": "Phosphoramidate Prodrugs Continue to Deliver, The Journey of Remdesivir (GS-5734) from RSV to SARS-CoV-2",
"author": "Mackman",
"doi-asserted-by": "crossref",
"first-page": "338",
"journal-title": "ACS Med. Chem. Lett.",
"key": "ref_153",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.3390/cimb45080433",
"article-title": "Recent Advances in Molecular Mechanisms of Nucleoside Antivirals",
"author": "Kamzeeva",
"doi-asserted-by": "crossref",
"first-page": "6851",
"journal-title": "Curr. Issues Mol. Biol.",
"key": "ref_154",
"volume": "45",
"year": "2023"
},
{
"DOI": "10.1038/nrd4010",
"article-title": "Advances in the Development of Nucleoside and Nucleotide Analogues for Cancer and Viral Diseases",
"author": "Jordheim",
"doi-asserted-by": "crossref",
"first-page": "447",
"journal-title": "Nat. Rev. Drug Discov.",
"key": "ref_155",
"volume": "12",
"year": "2013"
},
{
"DOI": "10.1007/s12560-024-09605-3",
"article-title": "Antiviral Activity of Natural Compounds for Food Safety",
"author": "Randazzo",
"doi-asserted-by": "crossref",
"first-page": "280",
"journal-title": "Food Environ. Virol.",
"key": "ref_156",
"volume": "16",
"year": "2024"
},
{
"DOI": "10.1016/j.bmc.2010.09.035",
"article-title": "Biflavonoids from Torreya Nucifera Displaying SARS-CoV 3CL(pro) Inhibition",
"author": "Ryu",
"doi-asserted-by": "crossref",
"first-page": "7940",
"journal-title": "Bioorg. Med. Chem.",
"key": "ref_157",
"volume": "18",
"year": "2010"
},
{
"DOI": "10.3390/ph15091049",
"doi-asserted-by": "crossref",
"key": "ref_158",
"unstructured": "Gasmi, A., Mujawdiya, P.K., Lysiuk, R., Shanaida, M., Peana, M., Gasmi Benahmed, A., Beley, N., Kovalska, N., and Bjørklund, G. (2022). Quercetin in the Prevention and Treatment of Coronavirus Infections: A Focus on SARS-CoV-2. Pharmaceuticals, 15."
},
{
"DOI": "10.3390/life13040948",
"doi-asserted-by": "crossref",
"key": "ref_159",
"unstructured": "Jadimurthy, R., Jagadish, S., Nayak, S.C., Kumar, S., Mohan, C.D., and Rangappa, K.S. (2023). Phytochemicals as Invaluable Sources of Potent Antimicrobial Agents to Combat Antibiotic Resistance. Life, 13."
},
{
"DOI": "10.3390/plants12233997",
"doi-asserted-by": "crossref",
"key": "ref_160",
"unstructured": "Cuevas-Cianca, S.I., Romero-Castillo, C., Gálvez-Romero, J.L., Sánchez-Arreola, E., Juárez, Z.N., and Hernández, L.R. (2023). Latin American Plants against Microorganisms. Plants, 12."
},
{
"DOI": "10.3390/v13020234",
"doi-asserted-by": "crossref",
"key": "ref_161",
"unstructured": "Al-Beltagi, S., Preda, C.A., Goulding, L.V., James, J., Pu, J., Skinner, P., Jiang, Z., Wang, B.L., Yang, J., and Banyard, A.C. (2021). Thapsigargin Is a Broad-Spectrum Inhibitor of Major Human Respiratory Viruses: Coronavirus, Respiratory Syncytial Virus and Influenza A Virus. Viruses, 13."
},
{
"DOI": "10.1016/j.bbamem.2021.183821",
"doi-asserted-by": "crossref",
"key": "ref_162",
"unstructured": "Jackman, J.A. (2022). Antiviral Peptide Engineering for Targeting Membrane-Enveloped Viruses: Recent Progress and Future Directions. Biochim. Biophys. Acta Biomembr., 1864."
},
{
"DOI": "10.1056/NEJMoa035026",
"article-title": "Enfuvirtide, an HIV-1 Fusion Inhibitor, for Drug-Resistant HIV Infection in North and South America",
"author": "Lalezari",
"doi-asserted-by": "crossref",
"first-page": "2175",
"journal-title": "N. Engl. J. Med.",
"key": "ref_163",
"volume": "348",
"year": "2003"
},
{
"DOI": "10.1128/mBio.01935-20",
"article-title": "Inhibition of Coronavirus Entry In Vitro and Ex Vivo by a Lipid-Conjugated Peptide Derived from the SARS-CoV-2 Spike Glycoprotein HRC Domain",
"author": "Outlaw",
"doi-asserted-by": "crossref",
"first-page": "e01935-20",
"journal-title": "mBio",
"key": "ref_164",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1073/pnas.0801388105",
"article-title": "Hepatitis C Virus NS5A Anchor Peptide Disrupts Human Immunodeficiency Virus",
"author": "Bobardt",
"doi-asserted-by": "crossref",
"first-page": "5525",
"journal-title": "Proc. Natl. Acad. Sci. USA",
"key": "ref_165",
"volume": "105",
"year": "2008"
},
{
"DOI": "10.1038/s41563-018-0194-2",
"article-title": "Therapeutic Treatment of Zika Virus Infection Using a Brain-Penetrating Antiviral Peptide",
"author": "Jackman",
"doi-asserted-by": "crossref",
"first-page": "971",
"journal-title": "Nat. Mater.",
"key": "ref_166",
"volume": "17",
"year": "2018"
},
{
"DOI": "10.1073/pnas.0712380105",
"article-title": "A Virocidal Amphipathic α-Helical Peptide That Inhibits Hepatitis C Virus Infection in Vitro",
"author": "Cheng",
"doi-asserted-by": "crossref",
"first-page": "3088",
"journal-title": "Proc. Natl. Acad. Sci. USA",
"key": "ref_167",
"volume": "105",
"year": "2008"
}
],
"reference-count": 167,
"references-count": 167,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2076-0817/14/1/20"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Advances and Challenges in Antiviral Development for Respiratory Viruses",
"type": "journal-article",
"volume": "14"
}