Chlorhexidine mouthwash reduces the salivary viral load of SARS‐CoV‐2: A randomized clinical trial
et al., Oral Diseases, doi:10.1111/odi.14086, Dec 2021
53rd treatment shown to reduce risk in
November 2023, now with p < 0.00000000001 from 5 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
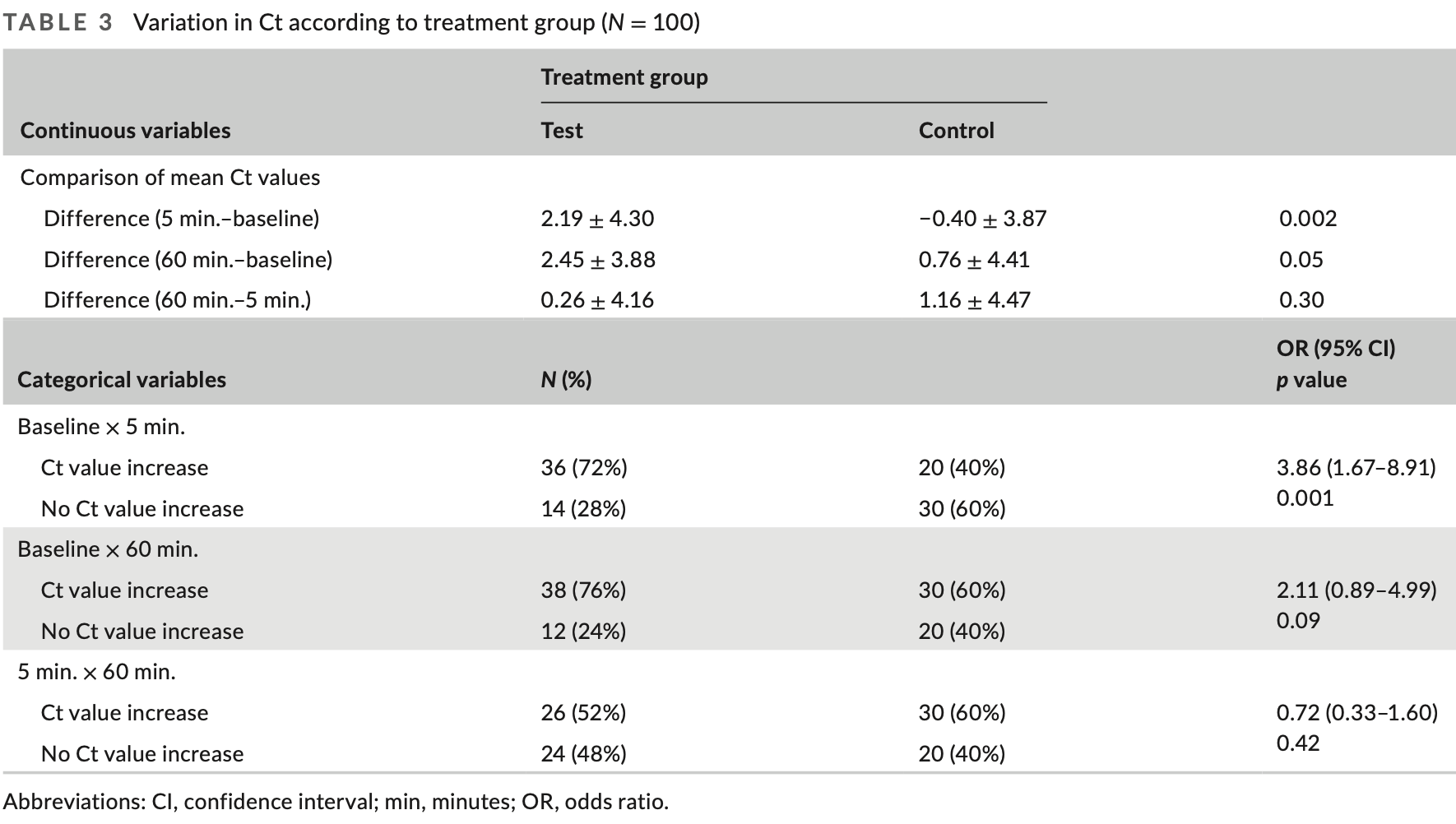
RCT 100 outpatients in Brazil showing a reduction in salivary viral load with 0.12% chlorhexidine gluconate mouthwash. The test group gargled and rinsed with 15ml of the mouthwash for 1 minute, while the control group used a placebo. Saliva samples were collected at baseline, 5 minutes, and 60 minutes after using the mouthwash. The reduction in viral load was significantly greater in the test group at 5 and 60 minutes compared to the control group.
Analysis of short-term changes in viral load using PCR may not detect
effective treatments because PCR is unable to differentiate between intact
infectious virus and non-infectious or destroyed virus particles. For example
Tarragó-Gil, Alemany perform RCTs with cetylpyridinium chloride
(CPC) mouthwash that show no difference in PCR viral load, however there was
significantly increased detection of SARS-CoV-2 nucleocapsid protein,
indicating viral lysis. CPC inactivates SARS-CoV-2 by degrading its membrane,
exposing the nucleocapsid of the virus. To better estimate changes in viral
load and infectivity, methods like viral culture that can
differentiate intact vs. degraded virus are preferred.
This study is excluded in meta-analysis:
study only provides short-term viral load results.
|
viral load, 69.0% lower, relative load 0.31, p = 0.04, treatment mean 2.45 (±3.88) n=50, control mean 0.76 (±4.41) n=50, 60 minutes.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Costa et al., 11 Dec 2021, Double Blind Randomized Controlled Trial, placebo-controlled, Brazil, peer-reviewed, 6 authors.
Chlorhexidine mouthwash reduces the salivary viral load of SARS‐CoV‐2: A randomized clinical trial
Oral Diseases, doi:10.1111/odi.14086
Objectives: This study aimed to evaluate the effect of 0.12% chlorhexidine gluconate on the salivary load of SARS-CoV-2.
Materials and Methods: A randomized, double-blind, placebo-controlled trial was performed on 100 participants positive for SARS-CoV-2. In the test group (n = 50), volunteers gargled with a mouthwash containing 15 ml of 0.12% chlorhexidine gluconate for 1 min, while the control group (n = 50) used a placebo. Saliva samples were obtained before (baseline) and 5 and 60 min after using the solutions. Real-time reverse transcription polymerase chain reaction assays (qRT-PCR) were carried out and the cycle threshold (Ct) was computed. The chi-square test and t-test were used for group comparison (p ≤ 0.05).
Results: The differences in Ct values between the 5-min evaluation and baseline (test group: 2.19 ± 4.30; control: -0.40 ± 3.87, p = 0.002) and between 60 min and baseline (test group: 2.45 ± 3.88; control: 0.76 ± 4.41, p = 0.05) were significantly greater in the test group, revealing a reduction of viral load. Furthermore, there was a reduction in the load of SARS-CoV-2 in 72% of the volunteers using chlorhexidine versus 30% in the control group (p = 0.001). Conclusions: Chlorhexidine gluconate (0.12%) was effective in reducing salivary SARS-CoV-2 load for at least 60 min.
TA B L E 4 Association between SARS-CoV-2 salivary viral load reduction from baseline and demographic data, cigarette smoking, health status and COVID-19 symptoms (N = 100)
PATI E NT CO N S E NT S TATE M E NT Participants signed the consent form.
PE R M I SS I O N TO R E PRO D U CE M ATE R I A L FRO M OTH E R S O U RCE S There is no material from other sources.
CLI N I C A L TR I A L R EG I S TR ATI O N It was registered on clinicaltrial.gov.br on 03/17/2021, protocol UTN U1111-1264-8920.
PE E R R E V I E W The peer review history for this article is available at https://publo ns.com/publo n/10.1111/odi.14086.
References
Bidra, Pelletier, Westover, Frank, Brown et al., Comparison of in vitro inactivation of SARS CoV-2 with hydrogen peroxide and povidone-iodine oral antiseptic rinses, Journal of Prosthodontics: Official Journal of the American College of Prosthodontists, doi:10.1111/jopr.13220
Blumenthal, Causino, Chang, Culpepper, Marder et al., The duration of ambulatory visits to physicians, The Journal of Family Practice
Burton, Clarkson, Goulao, Glenny, Mcbain et al., Antimicrobial mouthwashes (gargling) and nasal sprays administered to patients with suspected or confirmed COVID-19 infection to improve patient outcomes and to protect healthcare workers treating them, doi:10.1002/14651858.CD013627.pub2
Davies, Buczkowski, Welch, Green, Mawer et al., Effective in vitro inactivation of SARS-CoV-2 by commercially available mouthwashes, The Journal of General Virology, doi:10.1099/jgv.0.001578
Fe R E N C E S Addy, Jenkins, Newcombe, The effect of some chlorhexidine-containing mouthrinses on salivary bacterial counts, Journal of Clinical Periodontology, doi:10.1111/j.1600-051x.1991.tb01694.x
Ganzeboom, Schröder, The international standard classification of education 2011
Geneva, Cuzzo, Fazili, Javaid, Normal body temperature: A systematic review, Open Forum Infectious Diseases, doi:10.1093/ofid/ofz032
Gorbalenya, Baker, Baric, Groot, Drosten et al., Coronaviridae study group of the international committee on taxonomy of viruses, the species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2, Nat Microbiol, doi:10.1038/s41564-020-0695-z
Jain, Grover, Singh, Sharma, Das et al., Chlorhexidine: An effective anticovid mouth rinse, Journal of Indian Society of Periodontology, doi:10.4103/jisp.jisp_824_20
Joynt, Wu, Understanding COVID-19: what does viral RNA load really mean? The Lancet, Infectious Diseases, doi:10.1016/S1473-3099(20)30237-1
Koletsi, Belibasakis, Eliades, Interventions to reduce aerosolized microbes in dental practice: A systematic review with network meta-analysis of randomized controlled trials, Journal of Dental Research, doi:10.1177/0022034520943574
Komine, Yamaguchi, Okamoto, Yamamoto, Virucidal activity of oral care products against SARS-CoV-2 in vitro, Journal of Oral and Maxillofacial Surgery, Medicine, and Pathology, doi:10.1016/j.ajoms.2021.02.002
Letko, Marzi, Munster, Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses, Nature Microbiology, doi:10.1038/s41564-020-0688-y
Magleby, Westblade, Trzebucki, Simon, Rajan et al., Impact of SARS-CoV-2 viral load on risk of intubation and mortality among hospitalized patients with coronavirus disease 2019, Clinical Infectious Diseases : an Official Publication of the Infectious Diseases Society of America, doi:10.1093/cid/ciaa851
Nunes, None
Nunes, None
O'donnell, Thomas, Stanton, Maillard, Murphy et al., Potential role of oral rinses targeting the viral lipid envelope in SARS-CoV-2 infection, Function, doi:10.1093/function/zqaa002
Peng, Xu, Li, Cheng, Zhou et al., PubChem Compound Summary for CID 9552081, Chlorhexidine gluconate, International Journal of Oral Science, doi:10.1038/s41368-020-0075-9
Ramos, None
Seneviratne, Balan, Ko, Udawatte, Lai et al., Efficacy of commercial mouth-rinses on SARS-CoV-2 viral load in saliva: Randomized control trial in Singapore, Infection, doi:10.1007/s15010-020-01563-9
Shah, Singhal, Davar, Thakkar, No correlation between Ct values and severity of disease or mortality in patients with COVID 19 disease, Indian Journal of Medical Microbiology, doi:10.1016/j.ijmmb.2020.10.021
Souza De Santana, None
Stanley, Number of patients and patient visits; usual length of patient appointment. Bureau of economic research and statistics, Journal of the American Dental Association, doi:10.1161/CIRCULATIONAHA.105.594945
To, Tsang, Yip, Chan, Wu et al., Consistent detection of 2019 novel coronavirus in saliva, Clinical Infectious Diseases : an Official Publication of the Infectious Diseases Society of America, doi:10.1093/cid/ciaa149
Vaz, Santana, Netto, Pedroso, Wang et al., Saliva is a reliable, non-invasive specimen for SARS-CoV-2 detection, The Brazilian Journal of Infectious Diseases: an Official Publication of the Brazilian Society of Infectious Diseases, doi:10.1016/j.bjid.2020.08.001
Xu, Wang, Hoskin, Cugini, Markowitz et al., Differential effects of antiseptic mouth rinses on SARS-CoV-2 Infectivity in vitro, Pathogens, doi:10.3390/pathogens10030272
Yoon, Yoon, Song, Yoon, Lim et al., Clinical significance of a high SARS-CoV-2 viral load in the saliva, Journal of Korean Medical Science, doi:10.3346/jkms.2020.35.e195
DOI record:
{
"DOI": "10.1111/odi.14086",
"ISSN": [
"1354-523X",
"1601-0825"
],
"URL": "http://dx.doi.org/10.1111/odi.14086",
"abstract": "<jats:title>Abstract</jats:title><jats:sec><jats:title>Objectives</jats:title><jats:p>This study aimed to evaluate the effect of 0.12% chlorhexidine gluconate on the salivary load of SARS‐CoV‐2.</jats:p></jats:sec><jats:sec><jats:title>Materials and Methods</jats:title><jats:p>A randomized, double‐blind, placebo‐controlled trial was performed on 100 participants positive for SARS‐CoV‐2. In the test group <jats:italic>(n</jats:italic> = 50), volunteers gargled with a mouthwash containing 15 ml of 0.12% chlorhexidine gluconate for 1 min, while the control group (<jats:italic>n</jats:italic> = 50) used a placebo. Saliva samples were obtained before (baseline) and 5 and 60 min after using the solutions. Real‐time reverse transcription polymerase chain reaction assays (qRT‐PCR) were carried out and the cycle threshold (Ct) was computed. The chi‐square test and <jats:italic>t</jats:italic>‐test were used for group comparison (<jats:italic>p </jats:italic>≤ 0.05).</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>The differences in Ct values between the 5‐min evaluation and baseline (test group: 2.19 ± 4.30; control: −0.40 ± 3.87, <jats:italic>p </jats:italic>= 0.002) and between 60 min and baseline (test group: 2.45 ± 3.88; control: 0.76 ± 4.41, <jats:italic>p </jats:italic>= 0.05) were significantly greater in the test group, revealing a reduction of viral load. Furthermore, there was a reduction in the load of SARS‐CoV‐2 in 72% of the volunteers using chlorhexidine versus 30% in the control group (<jats:italic>p </jats:italic>= 0.001).</jats:p></jats:sec><jats:sec><jats:title>Conclusions</jats:title><jats:p>Chlorhexidine gluconate (0.12%) was effective in reducing salivary SARS‐CoV‐2 load for at least 60 min.</jats:p></jats:sec>",
"alternative-id": [
"10.1111/odi.14086"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2021-08-20"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2021-11-09"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2021-12-11"
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-1702-5573",
"affiliation": [
{
"name": "Postgraduate Program in Dentistry and Health Federal University of Bahia Salvador Brazil"
}
],
"authenticated-orcid": false,
"family": "Costa",
"given": "Denis Damião",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-4673-6991",
"affiliation": [
{
"name": "Infectious Diseases Research Laboratory Professor Edgard Santos Hospital Federal University of Bahia Salvador Brazil"
},
{
"name": "Postgraduate Program in Medicine and Health Federal University of Bahia Salvador Brazil"
}
],
"authenticated-orcid": false,
"family": "Brites",
"given": "Carlos",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7556-9670",
"affiliation": [
{
"name": "Postgraduate Program in Medicine and Health Federal University of Bahia Salvador Brazil"
}
],
"authenticated-orcid": false,
"family": "Vaz",
"given": "Sara Nunes",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0829-6817",
"affiliation": [
{
"name": "Postgraduate Program in Medicine and Health Federal University of Bahia Salvador Brazil"
}
],
"authenticated-orcid": false,
"family": "de Santana",
"given": "Daniele Souza",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7225-5879",
"affiliation": [
{
"name": "Postgraduate Program in Dentistry and Health Federal University of Bahia Salvador Brazil"
},
{
"name": "Department of Oral Pathology School of Dentistry Federal University of Bahia Salvador Brazil"
}
],
"authenticated-orcid": false,
"family": "dos Santos",
"given": "Jean Nunes",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8907-0483",
"affiliation": [
{
"name": "Postgraduate Program in Dentistry and Health Federal University of Bahia Salvador Brazil"
},
{
"name": "Department of Periodontology School of Dentistry Federal University of Bahia Salvador Brazil"
}
],
"authenticated-orcid": false,
"family": "Cury",
"given": "Patricia Ramos",
"sequence": "additional"
}
],
"container-title": "Oral Diseases",
"container-title-short": "Oral Diseases",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2021,
11,
27
]
],
"date-time": "2021-11-27T04:20:30Z",
"timestamp": 1637986830000
},
"deposited": {
"date-parts": [
[
2023,
8,
22
]
],
"date-time": "2023-08-22T02:05:12Z",
"timestamp": 1692669912000
},
"funder": [
{
"DOI": "10.13039/501100006181",
"award": [
"0009/2014"
],
"doi-asserted-by": "publisher",
"name": "Fundação de Amparo à Pesquisa do Estado da Bahia"
},
{
"DOI": "10.13039/501100003593",
"award": [
"303861/2018‐5",
"407711/2018‐0"
],
"doi-asserted-by": "publisher",
"name": "Conselho Nacional de Desenvolvimento Científico e Tecnológico"
}
],
"indexed": {
"date-parts": [
[
2024,
3,
12
]
],
"date-time": "2024-03-12T01:59:14Z",
"timestamp": 1710208754851
},
"is-referenced-by-count": 23,
"issue": "S2",
"issued": {
"date-parts": [
[
2021,
12,
11
]
]
},
"journal-issue": {
"issue": "S2",
"published-print": {
"date-parts": [
[
2022,
11
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://onlinelibrary.wiley.com/termsAndConditions#vor",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
12,
11
]
],
"date-time": "2021-12-11T00:00:00Z",
"timestamp": 1639180800000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1111/odi.14086",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1111/odi.14086",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1111/odi.14086",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"page": "2500-2508",
"prefix": "10.1111",
"published": {
"date-parts": [
[
2021,
12,
11
]
]
},
"published-online": {
"date-parts": [
[
2021,
12,
11
]
]
},
"published-print": {
"date-parts": [
[
2022,
11
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1111/j.1600‐051x.1991.tb01694.x",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_2_1"
},
{
"DOI": "10.1111/jopr.13220",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_3_1"
},
{
"article-title": "The duration of ambulatory visits to physicians",
"author": "Blumenthal D.",
"first-page": "264",
"issue": "4",
"journal-title": "The Journal of Family Practice",
"key": "e_1_2_12_4_1",
"volume": "48",
"year": "1999"
},
{
"DOI": "10.1002/14651858.CD013627.pub2",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_5_1"
},
{
"key": "#cr-split#-e_1_2_12_6_1.1",
"unstructured": "Centers for Disease Control and Prevention - CDC. (2020).Guidance for dental settings: interim infection prevention and control guidance for dental settings during the coronavirus disease 2019 (COVID‐19) pandemic"
},
{
"key": "#cr-split#-e_1_2_12_6_1.2",
"unstructured": "[Accessed 2021 Jan 02]. Available from:https://www.cdc.gov/coronavirus/2019‐ncov/hcp/dental‐settings.html"
},
{
"key": "#cr-split#-e_1_2_12_7_1.1",
"unstructured": "Centers of Disease Control and Prevention - CDC. (2020).CDC 2019‐Novel Coronavirus (2019‐nCoV) Real‐Time RT‐PCR Diagnostic Panel"
},
{
"key": "#cr-split#-e_1_2_12_7_1.2",
"unstructured": "[Accessed 2021 Jan 05]. Available from:https://www.fda.gov/media/134922/download"
},
{
"DOI": "10.1099/jgv.0.001578",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_8_1"
},
{
"key": "e_1_2_12_9_1",
"unstructured": "Ganzeboom H. B. G. &Schröder H.(2015).The international standard classification of education 2011 [ISCED–2011]. In: Nederland in context: verschillen en overeenkomsten. Proceedings vijfde Nederlandse Workshop European Social Survey 11–34. [Accessed 2021 Jan 10]."
},
{
"DOI": "10.1093/ofid/ofz032",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_10_1"
},
{
"DOI": "10.1038/s41564‐020‐0695‐z",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_11_1"
},
{
"DOI": "10.4103/jisp.jisp_824_20",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_12_1"
},
{
"DOI": "10.1016/S1473‐3099(20)30237‐1",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_13_1"
},
{
"DOI": "10.1177/0022034520943574",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_14_1"
},
{
"DOI": "10.1016/j.ajoms.2021.02.002",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_15_1"
},
{
"DOI": "10.1038/s41564‐020‐0688‐y",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_16_1"
},
{
"DOI": "10.1093/cid/ciaa851",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_17_1"
},
{
"DOI": "10.1093/function/zqaa002",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_18_1"
},
{
"DOI": "10.1038/s41368‐020‐0075‐9",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_19_1"
},
{
"key": "#cr-split#-e_1_2_12_20_1.1",
"unstructured": "PubChem.(2004).Bethesda (MD): National Library of Medicine (US) National Center for Biotechnology Information"
},
{
"key": "#cr-split#-e_1_2_12_20_1.2",
"unstructured": "[Accessed 2021 June 13]. Available from:https://pubchem.ncbi.nlm.nih.gov/compound/Chlorhexidine‐gluconate"
},
{
"key": "#cr-split#-e_1_2_12_20_1.3",
"unstructured": "PubChem.(2004).Bethesda (MD): National Library of Medicine"
},
{
"key": "#cr-split#-e_1_2_12_20_1.4",
"unstructured": "(US) National Center for Biotechnology Information; PubChem Compound Summary for CID 9552081 Chlorhexidine gluconate; [Accessed 2021 June 13]. Available from:https://pubchem.ncbi.nlm.nih.gov/compound/Chlorhexidine‐gluconate"
},
{
"DOI": "10.1007/s15010‐020‐01563‐9",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_21_1"
},
{
"DOI": "10.1016/j.ijmmb.2020.10.021",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_22_1"
},
{
"DOI": "10.1161/CIRCULATIONAHA.105.594945",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_23_1"
},
{
"article-title": "Number of patients and patient visits; usual length of patient appointment. Bureau of economic research and statistics",
"author": "Survey of dental practice",
"first-page": "154",
"issue": "1",
"journal-title": "Journal of the American Dental Association (1939)",
"key": "e_1_2_12_24_1",
"volume": "85",
"year": "1972"
},
{
"DOI": "10.1093/cid/ciaa149",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_25_1"
},
{
"DOI": "10.1016/j.bjid.2020.08.001",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_26_1"
},
{
"DOI": "10.3390/pathogens10030272",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_27_1"
},
{
"DOI": "10.3346/jkms.2020.35.e195",
"doi-asserted-by": "publisher",
"key": "e_1_2_12_28_1"
}
],
"reference-count": 32,
"references-count": 32,
"relation": {
"has-review": [
{
"asserted-by": "object",
"id": "10.1111/ODI.14086/v1/decision1",
"id-type": "doi"
},
{
"asserted-by": "object",
"id": "10.1111/ODI.14086/v2/review2",
"id-type": "doi"
},
{
"asserted-by": "object",
"id": "10.1111/ODI.14086/v2/decision1",
"id-type": "doi"
},
{
"asserted-by": "object",
"id": "10.1111/ODI.14086/v2/response1",
"id-type": "doi"
},
{
"asserted-by": "object",
"id": "10.1111/ODI.14086/v1/review1",
"id-type": "doi"
},
{
"asserted-by": "object",
"id": "10.1111/ODI.14086/v1/review2",
"id-type": "doi"
},
{
"asserted-by": "object",
"id": "10.1111/ODI.14086/v2/review1",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1111/odi.14086"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Dentistry",
"Otorhinolaryngology"
],
"subtitle": [],
"title": "Chlorhexidine mouthwash reduces the salivary viral load of SARS‐CoV‐2: A randomized clinical trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy",
"volume": "28"
}
