Effectiveness of Regdanvimab at Preventing the Need for Oxygen Therapy in Patients with Mild-to-Moderate COVID-19: A Retrospective Cohort Study
et al., Infection & Chemotherapy, doi:10.3947/ic.2021.0140, Mar 2022
39th treatment shown to reduce risk in
March 2022, now with p = 0.00049 from 12 studies, recognized in 27 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
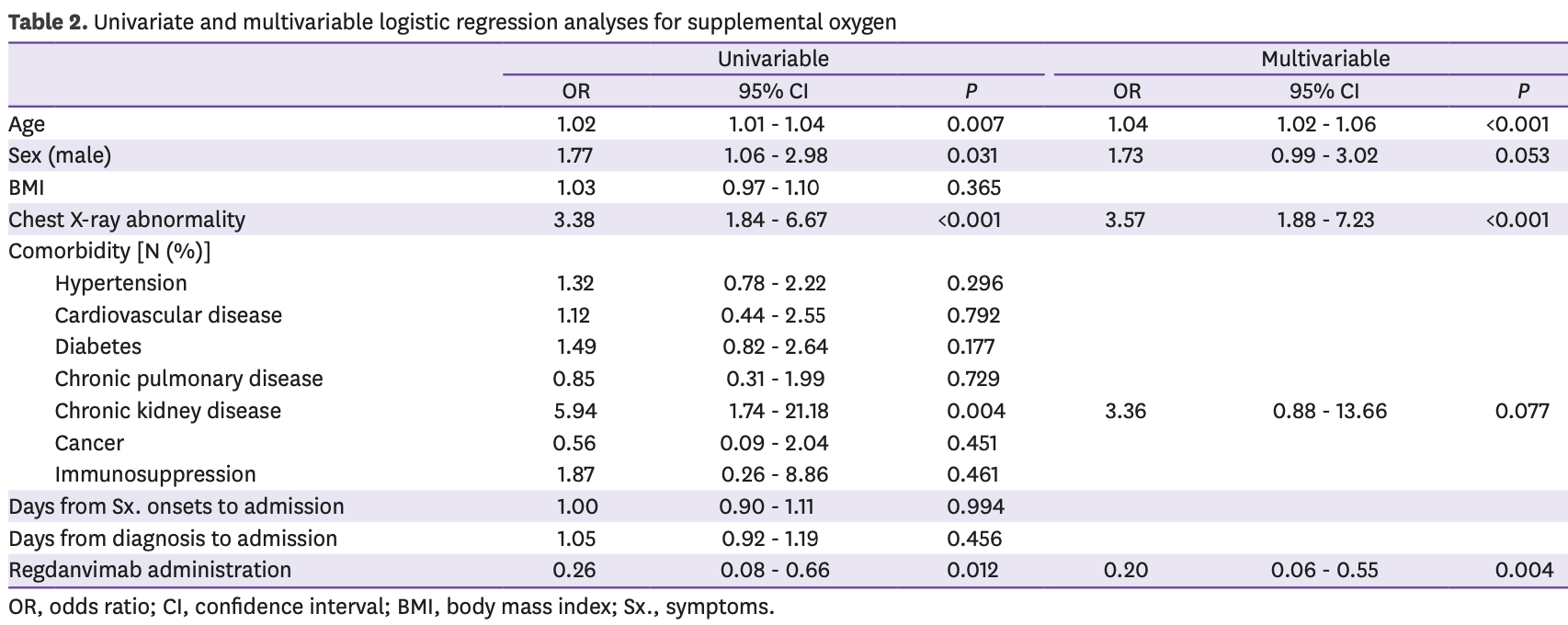
Retrospective 398 hospitalized mild-to-moderate COVID-19 patients in South Korea eligible for regdanvimab treatment. 65 patients received regdanvimab, with significantly lower supplemental oxygen requirements (6.2% vs 20.1% in controls). After adjusting for potential confounders, regdanvimab remained associated with lower risk of requiring supplemental oxygen (OR 0.20). There was no significant difference in mortality or hospitalization time in unadjusted results.
Efficacy is variant dependent. In Vitro research suggests a lack of efficacy for omicron BA.2, BA.4, BA.51, ХВВ.1.9.1, XBB.1.9.3, XBB.1.5.24, XBB.1.16, XBB.2.9, BQ.1.1.45, CL.1, and CH.1.12.
|
risk of death, 85.7% lower, RR 0.14, p = 1.00, treatment 0 of 65 (0.0%), control 5 of 333 (1.5%), NNT 67, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of oxygen therapy, 76.2% lower, RR 0.24, p = 0.004, treatment 4 of 65 (6.2%), control 67 of 333 (20.1%), NNT 7.2, odds ratio converted to relative risk.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Choi et al., 8 Mar 2022, retrospective, South Korea, peer-reviewed, 3 authors.
Contact: eunylee0903@gmail.com.
Effectiveness of Regdanvimab at Preventing the Need for Oxygen Therapy in Patients with Mild-to-Moderate COVID-19: A Retrospective Cohort Study
Infection & Chemotherapy, doi:10.3947/ic.2021.0140
Background: Monoclonal antibodies are a treatment option for patients with mild-tomoderate coronavirus disease . We investigated the effectiveness of regdanvimab, an anti-severe acute respiratory syndrome coronavirus-2 monoclonal antibody approved in Korea, in the treatment of patients with mild-to-moderate COVID-19. Materials and Methods: Medical records of patients who were admitted to a COVID-19 designated hospital during the study period of February 1 to June 31 and met the indications for administration of regdanvimab were reviewed to assess baseline characteristics and clinical outcomes such as supplemental oxygen requirements, mortality, and length of hospitalization. Multivariable logistic regression analysis was conducted to identify factors associated with requiring supplemental oxygen. Subgroup analysis was performed according to the presence of pneumonia confirmed on a chest X-ray. Results: Three hundred ninety-eight COVID-19 patients were included in the study, and 65 (16.3%) of them were administered regdanvimab. The proportion of patients requiring supplemental oxygen was significantly lower in the regdanvimab group than in the control group (6.2% vs. 20.1%, P = 0.007). There was no significant difference in mortality (0% vs. 1.5%, P >0.999) and the length of hospitalization (median: 10 days vs. 10 days, P = 0.267) between two groups. The multivariable analysis demonstrated that administration of regdanvimab was independently associated with lower oxygen supplement [odds ratio (OR): 0.20, 95% confidence interval (CI): 0.06 -0.55, P = 0.004] after adjustment of potential risk factors related to supplemental oxygen including age, sex, chest X-ray abnormality, and underlying chronic kidney disease. Among the patients with pneumonia radiologically, administration of regdanvimab was also associated with lower risk of oxygen supplement (OR: 0.13, 95% CI: 0.02 -0.46, P = 0.007). Conclusion: Regdanvimab use was related to lower need for supplemental oxygen in patients with mild-to-moderate COVID-19 for the indications for administration of regdanvimab.
Conflict of Interest No conflict of interest.
SUPPLEMENTARY MATERIALS Supplementary Table 1 Characteristics of 5 patients with fatal outcomes in the control group Click here to view Supplementary Table 2 Baseline characteristics and clinical outcomes of COVID-19 patients who match the indications of regdanvimab administration by Chest X-ray abnormalities Click here to view Supplementary Table 3 Univariate and multivariable logistic regression analyses for supplemental oxygen by chest X-ray abnormalities Click here to view
References
Barnes, Jette, Abernathy, Dam, Esswein et al., SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies, Nature, doi:10.1038/s41586-020-2852-1
Cai, Chen, Wang, Luo, Liu et al., Obesity and COVID-19 severity in a designated hospital in Shenzhen, China, Diabetes Care, doi:10.2337/dc20-0576
Cdc, Team, Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 -United States, February 12-March 28, 2020, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm6913e2
Cele, Jackson, Khoury, Khan, Moyo-Gwete et al., Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization, Nature, doi:10.1038/s41586-021-04387-1
Cherian, Potdar, Jadhav, Yadav, Gupta et al., SARS-CoV-2 spike mutations, L452R, T478K, E484Q and P681R, in the second wave of COVID-19 in Maharashtra, India, Microorganisms, doi:10.3390/microorganisms9071542
Dougan, Nirula, Azizad, Mocherla, Gottlieb et al., BLAZE-1 Investigators. Bamlanivimab plus Etesevimab in mild or moderate Covid-19, N Engl J Med, doi:10.1056/NEJMoa2102685
Eom, Ison, Streinu-Cercel, Săndulescu, Preotescu et al., Efficacy and safety of ct-p59 plus standard of care: A phase 2/3 randomized, double-blind, placebo-controlled trial in outpatients with mild-to-moderate SARS-CoV-2 infection, Research Square
Gansevoort, Hilbrands, CKD is a key risk factor for COVID-19 mortality, Nat Rev Nephrol, doi:10.1038/s41581-020-00349-4
Guan, Ni, Hu, Liang, Ou et al., China medical treatment expert group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China, N Engl J Med, doi:10.1056/NEJMoa2002032
Gupta, Gonzalez-Rojas, Juarez, Casal, Moya et al., Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody Sotrovimab, N Engl J Med, doi:10.1056/NEJMoa2107934
Huang, Wang, Li, Ren, Zhao et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet, doi:10.1016/S0140-6736(20)30183-5
Kim, Kim, Kim, Kim, Lee et al., Clinical characteristics and outcomes of COVID-19 cohort patients in Daegu metropolitan city outbreak in 2020, J Korean Med Sci, doi:10.3346/jkms.2021.36.e12
Kim, Park, Lee, Kim, Kim et al., July 2021 status and characteristics of the covid-19 variant virus outbreak in the Republic of Korea, Public Healthy Weekly Report
Kim, Ryu, Lee, Kim, Seo et al., A therapeutic neutralizing antibody targeting receptor binding domain of SARS-CoV-2 spike protein, Nat Commun, doi:10.1038/s41467-020-20602-5
Lee, Lee, Ko, Hyun, Kim et al., Effectiveness of regdanvimab treatment in high-risk COVID-19 patients to prevent progression to severe disease, Front Immunol, doi:10.3389/fimmu.2021.772320
Lee, Song, Lim, Kim, Chai et al., Operation and management of Seoul metropolitan city community treatment center for mild condition COVID-19 patients, J Korean Med Sci, doi:10.3346/jkms.2020.35.e367
Liu, Iketani, Guo, Chan, Wang et al., Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2, Nature, doi:10.1038/s41586-021-04388-0
Planas, Saunders, Maes, Guivel-Benhassine, Planchais et al., Considerable escape of SARS-CoV-2 Omicron to antibody neutralization, Nature, doi:10.1038/s41586-021-04389-z
Planas, Veyer, Baidaliuk, Staropoli, Guivel-Benhassine et al., Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization, Nature, doi:10.1038/s41586-021-03777-9
Ryu, Woo, Kang, Noh, Kim et al., The in vitro and in vivo potency of ct-p59 against delta and its associated variants of SARS-CoV-2, bioRxiv
Vanblargan, Errico, Halfmann, Zost, Crowe et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies, Nat Med, doi:10.1038/s41591-021-01678-y
Weinreich, Sivapalasingam, Norton, Ali, Gao et al., REGEN-COV antibody combination and outcomes in outpatients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2108163
Weinreich, Sivapalasingam, Norton, Ali, Gao et al., Trial Investigators. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2035002
Wu, Chen, Cai, Xia, Zhou et al., Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China, JAMA Intern Med, doi:10.1001/jamainternmed.2020.0994
Wu, Zhao, Yu, Chen, Song et al., A new coronavirus associated with human respiratory disease in China, Nature, doi:10.1038/s41586-020-2008-3
Zhou, Yu, Du, Fan, Liu et al., Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, Lancet, doi:10.1016/S0140-6736(20)30566-3
Zhu, Zhang, Li, Yang, Song et al., China novel coronavirus investigating and research team. A novel coronavirus from patients with pneumonia in China, 2019, N Engl J Med, doi:10.1056/NEJMoa2001017
DOI record:
{
"DOI": "10.3947/ic.2021.0140",
"ISSN": [
"2093-2340",
"2092-6448"
],
"URL": "http://dx.doi.org/10.3947/ic.2021.0140",
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"value": "2021-12-14"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"value": "2022-02-27"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published online",
"name": "published_online",
"value": "2022-03-08"
},
{
"group": {
"label": "Copyright and Licensing",
"name": "Copyright_and_licensing"
},
"label": "Copyright",
"name": "copyright",
"value": "Copyright © 2022 by The Korean Society of Infectious Diseases, Korean Society for Antimicrobial Therapy, and The Korean Society for AIDS"
},
{
"explanation": {
"URL": "https://creativecommons.org/licenses/by-nc/4.0/"
},
"group": {
"label": "Copyright and Licensing",
"name": "Copyright_and_licensing"
},
"label": "License",
"name": "license",
"value": "This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-3842-0524",
"affiliation": [
{
"name": "Division of Infectious Diseases, Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea."
}
],
"authenticated-orcid": false,
"family": "Choi",
"given": "Seong Jin",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-0550-1897",
"affiliation": [
{
"name": "Division of Infectious Diseases, Department of Internal Medicine, Boramae Medical Center, Seoul, Korea."
},
{
"name": "Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea."
}
],
"authenticated-orcid": false,
"family": "Park",
"given": "Sang-Won",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8280-3605",
"affiliation": [
{
"name": "Division of Infectious Diseases, Department of Internal Medicine, Boramae Medical Center, Seoul, Korea."
},
{
"name": "Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea."
}
],
"authenticated-orcid": false,
"family": "Lee",
"given": "Eunyoung",
"sequence": "additional"
}
],
"container-title": "Infection & Chemotherapy",
"container-title-short": "Infect Chemother",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"icjournal.org"
]
},
"created": {
"date-parts": [
[
2022,
3,
16
]
],
"date-time": "2022-03-16T02:07:12Z",
"timestamp": 1647396432000
},
"deposited": {
"date-parts": [
[
2022,
4,
1
]
],
"date-time": "2022-04-01T06:16:06Z",
"timestamp": 1648793766000
},
"indexed": {
"date-parts": [
[
2023,
10,
25
]
],
"date-time": "2023-10-25T05:50:10Z",
"timestamp": 1698213010604
},
"is-referenced-by-count": 9,
"issue": "1",
"issued": {
"date-parts": [
[
2022
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2022
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
1,
1
]
],
"date-time": "2022-01-01T00:00:00Z",
"timestamp": 1640995200000
}
}
],
"link": [
{
"URL": "https://icjournal.org/pdf/10.3947/ic.2021.0140",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://icjournal.org/DOIx.php?id=10.3947/ic.2021.0140",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://icjournal.org/DOIx.php?id=10.3947/ic.2021.0140",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "2343",
"original-title": [],
"page": "91",
"prefix": "10.3947",
"published": {
"date-parts": [
[
2022
]
]
},
"published-print": {
"date-parts": [
[
2022
]
]
},
"publisher": "Korean Society of Infectious Diseases and Korean Society for Chemotherapy",
"reference": [
{
"DOI": "10.1038/s41586-020-2008-3",
"author": "Wu",
"doi-asserted-by": "crossref",
"first-page": "265",
"journal-title": "Nature",
"key": "10.3947/ic.2021.0140_ref1",
"volume": "579",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2001017",
"author": "Zhu",
"doi-asserted-by": "crossref",
"first-page": "727",
"journal-title": "N Engl J Med",
"key": "10.3947/ic.2021.0140_ref2",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2002032",
"author": "Guan",
"doi-asserted-by": "crossref",
"first-page": "1708",
"journal-title": "N Engl J Med",
"key": "10.3947/ic.2021.0140_ref3",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"author": "Huang",
"doi-asserted-by": "crossref",
"first-page": "497",
"journal-title": "Lancet",
"key": "10.3947/ic.2021.0140_ref4",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1001/jamainternmed.2020.0994",
"author": "Wu",
"doi-asserted-by": "crossref",
"first-page": "934",
"journal-title": "JAMA Intern Med",
"key": "10.3947/ic.2021.0140_ref5",
"volume": "180",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "1054",
"journal-title": "Lancet",
"key": "10.3947/ic.2021.0140_ref6",
"volume": "395",
"year": "2020"
},
{
"key": "10.3947/ic.2021.0140_ref7",
"unstructured": "World Health Organization (WHO). Therapeutics and covid-19: living guideline. Accessed 30 December 2021. Available at: https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2021.3"
},
{
"DOI": "10.1056/NEJMoa2102685",
"author": "Dougan",
"doi-asserted-by": "crossref",
"first-page": "1382",
"journal-title": "N Engl J Med",
"key": "10.3947/ic.2021.0140_ref8",
"volume": "385",
"year": "2021"
},
{
"key": "10.3947/ic.2021.0140_ref9",
"unstructured": "National Institutes of Health (NIH). COVID-19 treatment guidelines. Accessed 10 February 2022. Avaiable at: https://www.covid19treatmentguidelines.nih.gov/therapies/anti-sars-cov-2-antibody-products/anti-sars-cov-2-monoclonal-antibodies/"
},
{
"DOI": "10.1056/NEJMoa2107934",
"author": "Gupta",
"doi-asserted-by": "crossref",
"first-page": "1941",
"journal-title": "N Engl J Med",
"key": "10.3947/ic.2021.0140_ref10",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2035002",
"author": "Weinreich",
"doi-asserted-by": "crossref",
"first-page": "238",
"journal-title": "N Engl J Med",
"key": "10.3947/ic.2021.0140_ref11",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2108163",
"author": "Weinreich",
"doi-asserted-by": "crossref",
"first-page": "e81",
"journal-title": "N Engl J Med",
"key": "10.3947/ic.2021.0140_ref12",
"volume": "385",
"year": "2021"
},
{
"author": "Eom",
"journal-title": "Research Square",
"key": "10.3947/ic.2021.0140_ref13",
"year": "2021"
},
{
"DOI": "10.3346/jkms.2021.36.e12",
"author": "Kim",
"doi-asserted-by": "crossref",
"first-page": "e12",
"journal-title": "J Korean Med Sci",
"key": "10.3947/ic.2021.0140_ref14",
"volume": "36",
"year": "2021"
},
{
"DOI": "10.15585/mmwr.mm6913e2",
"author": "CDC COVID-19 Response Team",
"doi-asserted-by": "crossref",
"first-page": "382",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "10.3947/ic.2021.0140_ref15",
"volume": "69",
"year": "2020"
},
{
"DOI": "10.1038/s41581-020-00349-4",
"author": "Gansevoort",
"doi-asserted-by": "crossref",
"first-page": "705",
"journal-title": "Nat Rev Nephrol",
"key": "10.3947/ic.2021.0140_ref16",
"volume": "16",
"year": "2020"
},
{
"DOI": "10.2337/dc20-0576",
"author": "Cai",
"doi-asserted-by": "crossref",
"first-page": "1392",
"journal-title": "Diabetes Care",
"key": "10.3947/ic.2021.0140_ref17",
"volume": "43",
"year": "2020"
},
{
"DOI": "10.3346/jkms.2020.35.e367",
"author": "Lee",
"doi-asserted-by": "crossref",
"first-page": "e367",
"journal-title": "J Korean Med Sci",
"key": "10.3947/ic.2021.0140_ref18",
"volume": "35",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2021.772320",
"author": "Lee",
"doi-asserted-by": "crossref",
"first-page": "772320",
"journal-title": "Front Immunol",
"key": "10.3947/ic.2021.0140_ref19",
"volume": "12",
"year": "2021"
},
{
"author": "Kim",
"first-page": "2555",
"journal-title": "Public Healthy Weekly Report",
"key": "10.3947/ic.2021.0140_ref20",
"volume": "14",
"year": "2021"
},
{
"DOI": "10.3390/microorganisms9071542",
"author": "Cherian",
"doi-asserted-by": "crossref",
"first-page": "1542",
"journal-title": "Microorganisms",
"key": "10.3947/ic.2021.0140_ref21",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1038/s41586-021-04388-0",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "676",
"journal-title": "Nature",
"key": "10.3947/ic.2021.0140_ref22",
"volume": "602",
"year": "2022"
},
{
"DOI": "10.1038/s41586-021-03777-9",
"author": "Planas",
"doi-asserted-by": "crossref",
"first-page": "276",
"journal-title": "Nature",
"key": "10.3947/ic.2021.0140_ref23",
"volume": "596",
"year": "2021"
},
{
"DOI": "10.1038/s41586-021-04387-1",
"author": "Cele",
"doi-asserted-by": "crossref",
"first-page": "654",
"journal-title": "Nature",
"key": "10.3947/ic.2021.0140_ref24",
"volume": "602",
"year": "2022"
},
{
"DOI": "10.1038/s41586-021-04389-z",
"author": "Planas",
"doi-asserted-by": "crossref",
"first-page": "671",
"journal-title": "Nature",
"key": "10.3947/ic.2021.0140_ref25",
"volume": "602",
"year": "2022"
},
{
"DOI": "10.1038/s41467-020-20602-5",
"author": "Kim",
"doi-asserted-by": "crossref",
"first-page": "288",
"journal-title": "Nat Commun",
"key": "10.3947/ic.2021.0140_ref26",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1038/s41586-020-2852-1",
"author": "Barnes",
"doi-asserted-by": "crossref",
"first-page": "682",
"journal-title": "Nature",
"key": "10.3947/ic.2021.0140_ref27",
"volume": "588",
"year": "2020"
},
{
"author": "Ryu",
"journal-title": "bioRxiv",
"key": "10.3947/ic.2021.0140_ref28",
"year": "2021"
},
{
"author": "VanBlargan",
"first-page": "1",
"journal-title": "Nat Med",
"key": "10.3947/ic.2021.0140_ref29",
"year": "2022"
}
],
"reference-count": 29,
"references-count": 29,
"relation": {},
"resource": {
"primary": {
"URL": "https://icjournal.org/DOIx.php?id=10.3947/ic.2021.0140"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology (medical)",
"Infectious Diseases"
],
"subtitle": [],
"title": "Effectiveness of Regdanvimab at Preventing the Need for Oxygen Therapy in Patients with Mild-to-Moderate COVID-19: A Retrospective Cohort Study",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.3947/crossmark_policy",
"volume": "54"
}