Evaluation of the Efficacy of Methylene Blue Administration in SARS-CoV-2-Affected Patients: A Proof-of-Concept Phase 2, Randomized, Placebo-Controlled, Single-Blind Clinical Trial
et al., Scientia Pharmaceutica, doi:10.3390/scipharm92040056, NCT04635605, Oct 2024
RCT with 29 mild COVID-19 outpatients in Switzerland, showing no significant difference with methylene blue treatment. There was a trend towards faster viral clearance. There were no serious adverse events. The trial was discontinued early at the interim analysis due to declining case numbers.
3 preclinical studies support the efficacy of mebendazole for COVID-19:
1 in vitro study3
1.
Agamah et al., Network-based multi-omics-disease-drug associations reveal drug repurposing candidates for COVID-19 disease phases, ScienceOpen, doi:10.58647/DRUGARXIV.PR000010.v1.
Barda et al., 14 Oct 2024, Single Blind Randomized Controlled Trial, placebo-controlled, Switzerland, peer-reviewed, 10 authors, study period 3 December, 2020 - 20 May, 2021, trial NCT04635605 (history).
Contact: beatrice.barda@chuv.ch (corresponding author), brunodimari@gmail.com, claudia.dibartolomeo@hin.ch, maurizia.bissig@hin.ch, adriana.baserga@bluewin.ch, antonella.robatto@hin.ch, lorenzo.magenta@hin.ch, rossella.forlenza@hin.ch, andreas.cerny@hin.ch, emiliano.soldini@supsi.ch.
Evaluation of the Efficacy of Methylene Blue Administration in SARS-CoV-2-Affected Patients: A Proof-of-Concept Phase 2, Randomized, Placebo-Controlled, Single-Blind Clinical Trial
Scientia Pharmaceutica, doi:10.3390/scipharm92040056
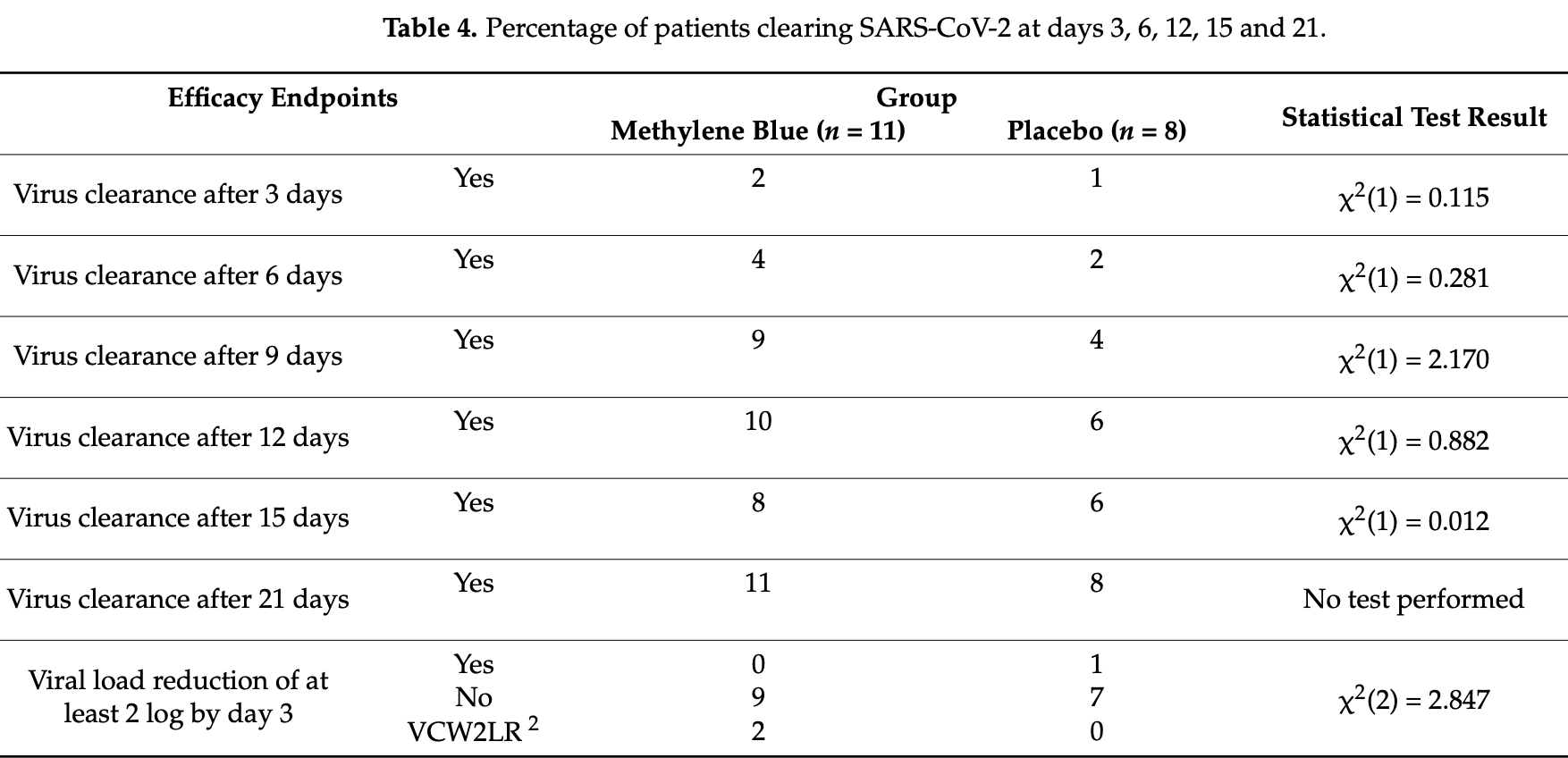
The SARS-CoV-2 pandemic has revolutionized the scientific and medical world in recent years. Methylene blue (MB) is a well-known molecule. The aim of our study was to assess the efficacy of MB against early-phase SARS-CoV-2 infections. All patients with a positive swab for SARS-CoV-2 were eligible for the trial. The intervention was a starting dose of 200 mg MB or placebo in the morning and 100 mg in the evening on the first day and afterwards the standard daily dose of 200 mg. Patients were followed up for safety and efficacy until day 84. We analyzed 21 patients for the safety profile and 19 for the efficacy objective: of these, there were 11 in the MB group and 8 in the placebo one. In both groups, patients had undetectable RNA from day 3 and 10 out of 11 subjects in the MB group were virus free by day 12 vs. 6 out of 8 in the placebo one. None of the patients experienced serious adverse events. MB has proved to be a safe and well-tolerated drug. We did not find superiority of efficacy or viral clearance of MB compared to the placebo. Given the good in vitro efficacy, larger studies are needed to assess MB efficacy against COVID-19 in vivo.
Conflicts of Interest: The authors declare no conflicts of interest.
References
Cao, Gao, Bao, Feng, Mei et al., VV116 versus Nirmatrelvir-Ritonavir for Oral Treatment of COVID-19, N. Engl. J. Med, doi:10.1056/NEJMoa2208822
Di Stefano, Radicioni, Vaccani, Fransioli, Longo et al., Methylene blue MMX ® tablets for chromoendoscopy. Bioavailability, colon staining and safety in healthy volunteers undergoing a full colonoscopy, Contemp. Clin. Trials, doi:10.1016/j.cct.2018.06.001
Gautret, Lagier, Parola, Hoang, Meddeb et al., Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label non-randomized clinical trial, Int. J. Antimicrob. Agents, doi:10.1016/j.ijantimicag.2020.105949
Henry, Summa, Patrick, Schwartz, A cohort of cancer patients with no reported cases of SARS-CoV-2 infection: The possible preventive role of Methylene Blue, Substantia, doi:10.13128/Substantia-888
Hotez, Nuzhath, Callaghan, Colwell, COVID-19 vaccine decisions: Considering the choices and opportunities, Microbes Infect, doi:10.1016/j.micinf.2021.104811
Knoll, Wonodi, Oxford-AstraZeneca COVID-19 vaccine efficacy, Lancet, doi:10.1016/S0140-6736(20)32623-4
Lobo, Rodrigues-Santos, Pereira, Núñez, Trêpa et al., Photodynamic disinfection of SARS-CoV-2 clinical samples using a methylene blue formulation, Photochem. Photobiol. Sci, doi:10.1007/s43630-022-00202-6
Lu, Nagbanshi, Goldau, Jorge, Meissner et al., Efficacy and safety of methylene blue in the treatment of malaria: A systematic review, BMC Med, doi:10.1186/s12916-018-1045-3
Mccarthy, Paxlovid as a potential treatment for long COVID, Expert Opin. Pharmacother, doi:10.1080/14656566.2023.2262387
Meo, Bukhari, Akram, Meo, Klonoff, COVID-19 vaccines: Comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines, Eur. Rev. Med. Pharmacol. Sci, doi:10.26355/eurrev_202102_24877
Meo, Klonoff, Akram, Efficacy of chloroquine and hydroxychloroquine in the treatment of COVID-19, Eur. Rev. Med. Pharmacol. Sci, doi:10.26355/eurrev_202004_21038
Schultze, Aschenbrenner, COVID-19 and the human innate immune system, Cell, doi:10.1016/j.cell.2021.02.029
Singh, Chauhan, Kakkar, Hydroxychloroquine for the treatment and prophylaxis of COVID-19: The journey so far and the road ahead, Eur. J. Pharmacol, doi:10.1016/j.ejphar.2020.173717
Thomas, Moreira, Jr, Kitchin, Absalon et al., Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine through 6 Months, N. Engl. J. Med, doi:10.1056/NEJMoa2110345
DOI record:
{
"DOI": "10.3390/scipharm92040056",
"ISSN": [
"2218-0532"
],
"URL": "http://dx.doi.org/10.3390/scipharm92040056",
"abstract": "<jats:p>The SARS-CoV-2 pandemic has revolutionized the scientific and medical world in recent years. Methylene blue (MB) is a well-known molecule. The aim of our study was to assess the efficacy of MB against early-phase SARS-CoV-2 infections. All patients with a positive swab for SARS-CoV-2 were eligible for the trial. The intervention was a starting dose of 200 mg MB or placebo in the morning and 100 mg in the evening on the first day and afterwards the standard daily dose of 200 mg. Patients were followed up for safety and efficacy until day 84. We analyzed 21 patients for the safety profile and 19 for the efficacy objective: of these, there were 11 in the MB group and 8 in the placebo one. In both groups, patients had undetectable RNA from day 3 and 10 out of 11 subjects in the MB group were virus free by day 12 vs. 6 out of 8 in the placebo one. None of the patients experienced serious adverse events. MB has proved to be a safe and well-tolerated drug. We did not find superiority of efficacy or viral clearance of MB compared to the placebo. Given the good in vitro efficacy, larger studies are needed to assess MB efficacy against COVID-19 in vivo.</jats:p>",
"alternative-id": [
"scipharm92040056"
],
"author": [
{
"affiliation": [
{
"name": "Fondazione Epatocentro Ticino, 6900 Lugano, Switzerland"
}
],
"family": "Barda",
"given": "Beatrice",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Fondazione Epatocentro Ticino, 6900 Lugano, Switzerland"
}
],
"family": "Di Mari",
"given": "Bruno",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9577-5567",
"affiliation": [
{
"name": "Competence Centre for Healthcare Practices and Policies, Department of Business Economics, Health and Social Care, University of Applied Sciences and Arts of Southern Switzerland, 6928 Manno, Switzerland"
}
],
"authenticated-orcid": false,
"family": "Soldini",
"given": "Emiliano",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Fondazione Epatocentro Ticino, 6900 Lugano, Switzerland"
}
],
"family": "Di Bartolomeo",
"given": "Claudia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Fondazione Epatocentro Ticino, 6900 Lugano, Switzerland"
}
],
"family": "Bissig",
"given": "Maurizia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Fondazione Epatocentro Ticino, 6900 Lugano, Switzerland"
}
],
"family": "Baserga",
"given": "Adriana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Fondazione Epatocentro Ticino, 6900 Lugano, Switzerland"
}
],
"family": "Robatto",
"given": "Antonella",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Fondazione Epatocentro Ticino, 6900 Lugano, Switzerland"
}
],
"family": "Magenta",
"given": "Lorenzo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Fondazione Epatocentro Ticino, 6900 Lugano, Switzerland"
}
],
"family": "Forlenza",
"given": "Rossella",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Fondazione Epatocentro Ticino, 6900 Lugano, Switzerland"
}
],
"family": "Cerny",
"given": "Andreas",
"sequence": "additional"
}
],
"container-title": "Scientia Pharmaceutica",
"container-title-short": "Sci. Pharm.",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
10,
14
]
],
"date-time": "2024-10-14T16:44:31Z",
"timestamp": 1728924271000
},
"deposited": {
"date-parts": [
[
2024,
10,
16
]
],
"date-time": "2024-10-16T04:23:36Z",
"timestamp": 1729052616000
},
"indexed": {
"date-parts": [
[
2024,
10,
17
]
],
"date-time": "2024-10-17T04:07:56Z",
"timestamp": 1729138076461,
"version": "3.27.0"
},
"is-referenced-by-count": 0,
"issue": "4",
"issued": {
"date-parts": [
[
2024,
10,
14
]
]
},
"journal-issue": {
"issue": "4",
"published-online": {
"date-parts": [
[
2024,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
10,
14
]
],
"date-time": "2024-10-14T00:00:00Z",
"timestamp": 1728864000000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2218-0532/92/4/56/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "56",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2024,
10,
14
]
]
},
"published-online": {
"date-parts": [
[
2024,
10,
14
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1016/S0140-6736(20)32623-4",
"article-title": "Oxford-AstraZeneca COVID-19 vaccine efficacy",
"author": "Knoll",
"doi-asserted-by": "crossref",
"first-page": "72",
"journal-title": "Lancet",
"key": "ref_1",
"volume": "397",
"year": "2021"
},
{
"article-title": "COVID-19 vaccines: Comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines",
"author": "Meo",
"first-page": "1663",
"journal-title": "Eur. Rev. Med. Pharmacol. Sci.",
"key": "ref_2",
"volume": "25",
"year": "2021"
},
{
"DOI": "10.1016/j.micinf.2021.104811",
"article-title": "COVID-19 vaccine decisions: Considering the choices and opportunities",
"author": "Hotez",
"doi-asserted-by": "crossref",
"first-page": "104811",
"journal-title": "Microbes Infect.",
"key": "ref_3",
"volume": "23",
"year": "2021"
},
{
"DOI": "10.1016/j.ijantimicag.2020.105949",
"article-title": "Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label non-randomized clinical trial",
"author": "Gautret",
"doi-asserted-by": "crossref",
"first-page": "105949",
"journal-title": "Int. J. Antimicrob. Agents",
"key": "ref_4",
"volume": "56",
"year": "2020"
},
{
"article-title": "Efficacy of chloroquine and hydroxychloroquine in the treatment of COVID-19",
"author": "Meo",
"first-page": "4539",
"journal-title": "Eur. Rev. Med. Pharmacol. Sci.",
"key": "ref_5",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1016/j.ejphar.2020.173717",
"article-title": "Hydroxychloroquine for the treatment and prophylaxis of COVID-19: The journey so far and the road ahead",
"author": "Singh",
"doi-asserted-by": "crossref",
"first-page": "173717",
"journal-title": "Eur. J. Pharmacol.",
"key": "ref_6",
"volume": "890",
"year": "2020"
},
{
"DOI": "10.1080/14656566.2023.2262387",
"article-title": "Paxlovid as a potential treatment for long COVID",
"author": "McCarthy",
"doi-asserted-by": "crossref",
"first-page": "1839",
"journal-title": "Expert Opin. Pharmacother.",
"key": "ref_7",
"volume": "24",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2208822",
"article-title": "VV116 versus Nirmatrelvir–Ritonavir for Oral Treatment of COVID-19",
"author": "Cao",
"doi-asserted-by": "crossref",
"first-page": "406",
"journal-title": "N. Engl. J. Med.",
"key": "ref_8",
"volume": "388",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2110345",
"article-title": "Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine through 6 Months",
"author": "Thomas",
"doi-asserted-by": "crossref",
"first-page": "1761",
"journal-title": "N. Engl. J. Med.",
"key": "ref_9",
"volume": "385",
"year": "2021"
},
{
"article-title": "A cohort of cancer patients with no reported cases of SARS-CoV-2 infection: The possible preventive role of Methylene Blue",
"author": "Henry",
"first-page": "888",
"journal-title": "Substantia",
"key": "ref_10",
"volume": "4",
"year": "2020"
},
{
"DOI": "10.1007/s43630-022-00202-6",
"article-title": "Photodynamic disinfection of SARS-CoV-2 clinical samples using a methylene blue formulation",
"author": "Lobo",
"doi-asserted-by": "crossref",
"first-page": "1101",
"journal-title": "Photochem. Photobiol. Sci.",
"key": "ref_11",
"volume": "21",
"year": "2022"
},
{
"DOI": "10.1016/j.cct.2018.06.001",
"article-title": "Methylene blue MMX® tablets for chromoendoscopy. Bioavailability, colon staining and safety in healthy volunteers undergoing a full colonoscopy",
"author": "Radicioni",
"doi-asserted-by": "crossref",
"first-page": "96",
"journal-title": "Contemp. Clin. Trials",
"key": "ref_12",
"volume": "71",
"year": "2018"
},
{
"DOI": "10.1186/s12916-018-1045-3",
"doi-asserted-by": "crossref",
"key": "ref_13",
"unstructured": "Lu, G., Nagbanshi, M., Goldau, N., Jorge, M.M., Meissner, P., Jahn, A., Mockenhaupt, F.P., and Müller, O. (2018). Efficacy and safety of methylene blue in the treatment of malaria: A systematic review. BMC Med., 16."
},
{
"DOI": "10.1016/j.cell.2021.02.029",
"article-title": "COVID-19 and the human innate immune system",
"author": "Schultze",
"doi-asserted-by": "crossref",
"first-page": "1671",
"journal-title": "Cell",
"key": "ref_14",
"volume": "184",
"year": "2021"
}
],
"reference-count": 14,
"references-count": 14,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2218-0532/92/4/56"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Evaluation of the Efficacy of Methylene Blue Administration in SARS-CoV-2-Affected Patients: A Proof-of-Concept Phase 2, Randomized, Placebo-Controlled, Single-Blind Clinical Trial",
"type": "journal-article",
"volume": "92"
}