VV116 versus Nirmatrelvir–Ritonavir for Oral Treatment of Covid-19
et al., New England Journal of Medicine, doi:10.1056/NEJMoa2208822, NCT05341609, Dec 2022
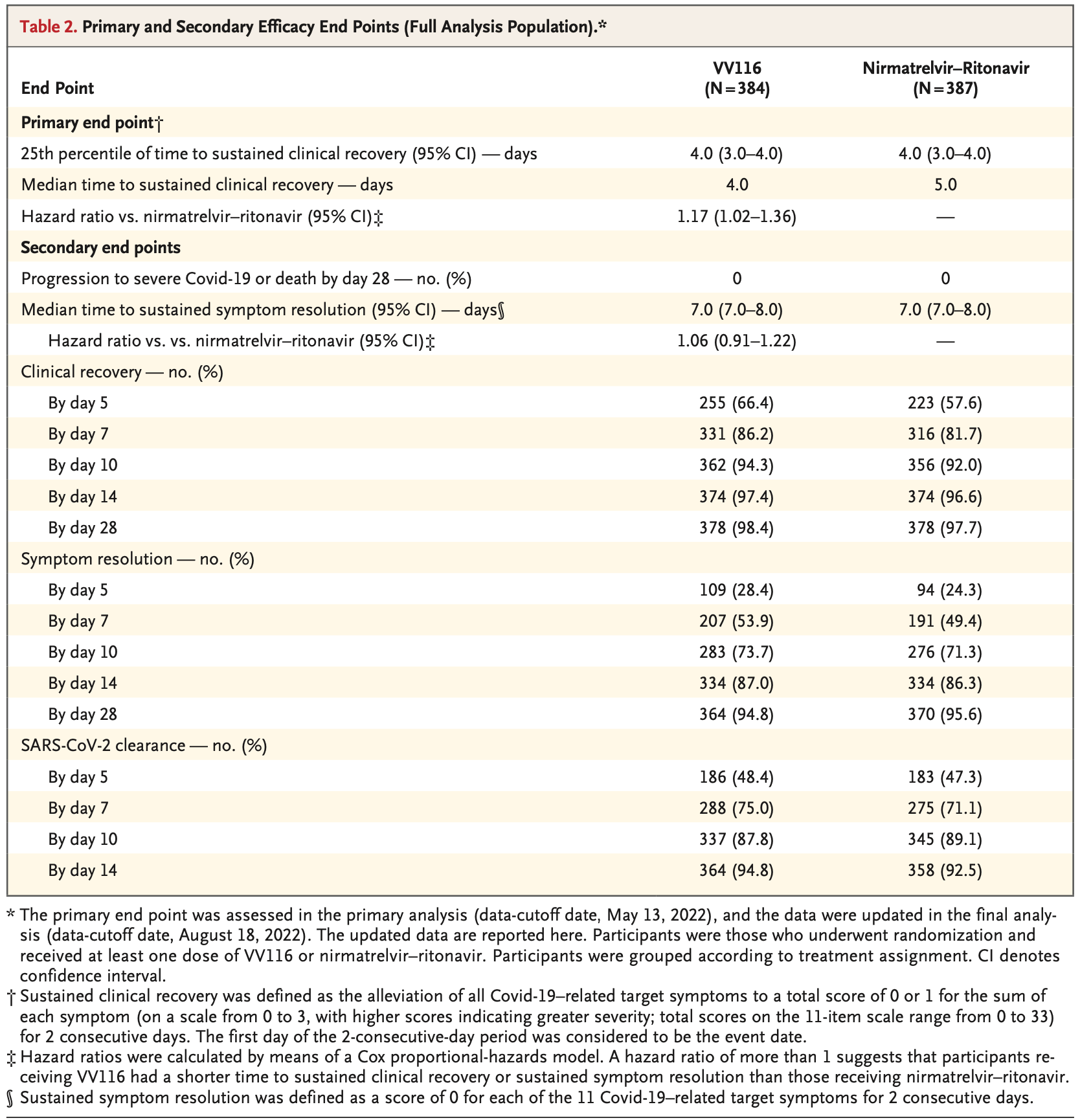
RCT 771 hospitalized patients with mild-to-moderate COVID-19 and high risk of progression showing non-inferior time to sustained clinical recovery with 5 days of VV116 compared to 5 days of paxlovid. No deaths or progression to severe disease occurred in either group. VV116 had a lower incidence of adverse events than paxlovid.
Cao et al., 28 Dec 2022, Single Blind Randomized Controlled Trial, China, peer-reviewed, 22 authors, study period 4 April, 2022 - 2 May, 2022, trial NCT05341609 (history).
Contact: zhaorensurgeon@aliyun.com.
VV116 versus Nirmatrelvir–Ritonavir for Oral Treatment of Covid-19
New England Journal of Medicine, doi:10.1056/nejmoa2208822
BACKGROUND Nirmatrelvir-ritonavir has been authorized for emergency use by many countries for the treatment of coronavirus disease 2019 (Covid-19). However, the supply falls short of the global demand, which creates a need for more options. VV116 is an oral antiviral agent with potent activity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
METHODS We conducted a phase 3, noninferiority, observer-blinded, randomized trial during the outbreak caused by the B.1.1.529 (omicron) variant of SARS-CoV-2. Symptomatic adults with mild-to-moderate Covid-19 with a high risk of progression were assigned to receive a 5-day course of either VV116 or nirmatrelvir-ritonavir. The primary end point was the time to sustained clinical recovery through day 28. Sustained clinical recovery was defined as the alleviation of all Covid-19-related target symptoms to a total score of 0 or 1 for the sum of each symptom (on a scale from 0 to 3, with higher scores indicating greater severity; total scores on the 11item scale range from 0 to 33) for 2 consecutive days. A lower boundary of the two-sided 95% confidence interval for the hazard ratio of more than 0.8 was considered to indicate noninferiority (with a hazard ratio of >1 indicating a shorter time to sustained clinical recovery with VV116 than with nirmatrelvir-ritonavir).
RESULTS A total of 822 participants underwent randomization, and 771 received VV116 (384 participants) or nirmatrelvir-ritonavir (387 participants). The noninferiority of VV116 to nirmatrelvir-ritonavir with respect to the time to sustained clinical recovery was established in the primary analysis (hazard ratio, 1.17; 95% confidence interval [CI], 1.01 to 1.35) and was maintained in the final analysis (median, 4 days with VV116 and 5 days with nirmatrelvir-ritonavir; hazard ratio, 1.17; 95% CI, 1.02 to 1.36). In the final analysis, the time to sustained symptom resolution (score of 0 for each of the 11 Covid-19-related target symptoms for 2 consecutive days) and to a first negative SARS-CoV-2 test did not differ substantially between the two groups. No participants in either group had died or had had progression to severe Covid-19 by day 28. The incidence of adverse events was lower in the VV116 group than in the nirmatrelvir-ritonavir group (67.4% vs. 77.3%).
CONCLUSIONS Among adults with mild-to-moderate Covid-19 who were at risk for progression, VV116 was noninferior to nirmatrelvir-ritonavir with respect to the time to sustained clinical recovery, with fewer safety concerns.
Appendix The authors' full names and academic degrees are as follows: Zhujun Cao
References
Agarwal, Rochwerg, Lamontagne, A living WHO guideline on drugs for covid-19, BMJ
Cai, Deng, Yang, Modeling transmission of SARS-CoV-2 Omicron in China, Nat Med
Cao, Li, Yang, The adenosine analog prodrug ATV006 is orally bioavailable and has preclinical efficacy against parental SARS-CoV-2 and variants, Sci Transl Med
Cao, Wang, Jian, Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies, Nature
Couzin-Frankel, Antiviral pills could change pandemic's course, Science
De Waal, Cohen, Maartens, Systematic review of antiretroviral-associated lipodystrophy: lipoatrophy, but not central fat gain, is an antiretroviral adverse drug reaction, PLoS One
Dryden-Peterson, Kim, Kim, Nirmatrelvir plus ritonavir for early COVID-19 and hospitalization in a large US health system, doi:10.1101/2022.06.14.22276393v2
Flemming, Omicron, the great escape artist, Nat Rev Immunol
Ganatra, Dani, Ahmad, Oral nirmatrelvir and ritonavir in nonhospitalized vaccinated patients with Covid-19, Clin Infect Dis
Gottlieb, Nirula, Chen, Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial, JAMA
Gottlieb, Vaca, Paredes, Early remdesivir to prevent progression to severe Covid-19 in outpatients, N Engl J Med
Hammond, Leister-Tebbe, Gardner, Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19
Menni, Valdes, Polidori, Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: a prospective observational study from the ZOE COVID Study, Lancet
Najjar-Debbiny, Gronich, Weber, Effectiveness of paxlovid in reducing severe COVID-19 and mortality in high risk patients, Clin Infect Dis
Owen, Allerton, Anderson, An oral SARS-CoV-2 M pro inhibitor clinical candidate for the treatment of COVID-19, Science
Qian, Wang, Zhang, Safety, tolerability, and pharmacokinetics of VV116, an oral nucleoside analog against SARS-CoV-2, in Chinese healthy subjects, Acta Pharmacol Sin
Schäfer, Martinez, Won, Therapeutic treatment with an oral pro-drug of the remdesivir parental nucleoside is protective against SARS-CoV-2 pathogenesis in mice, Sci Transl Med
Shen, Lin, An open, prospective cohort study of VV116 in Chinese participants infected with SARS-CoV-2 omicron variants, Emerg Microbes Infect
Viana, Moyo, Amoako, Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa, Nature
Wei, Hu, Zhang, Potency and pharmacokinetics of GS-441524 derivatives against SARS-CoV-2, Bioorg Med Chem
Weinreich, Sivapalasingam, Norton, REGEN-COV antibody combination and outcomes in outpatients with Covid-19, N Engl J Med
Xie, Yin, Zhang, Design and development of an oral remdesivir derivative VV116 against SARS-CoV-2, Cell Res
Ye, Li, Shao, Fighting omicron epidemic in China: real-world big data from Fangcang shelter hospital during the outbreak in Shanghai 2022, J Infect
Zhang, Zhang, Chen, Shanghai's life-saving efforts against the cur-rent omicron wave of the COVID-19 pandemic, Lancet
DOI record:
{
"DOI": "10.1056/nejmoa2208822",
"ISSN": [
"0028-4793",
"1533-4406"
],
"URL": "http://dx.doi.org/10.1056/NEJMoa2208822",
"alternative-id": [
"10.1056/NEJMoa2208822"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-8684-2107",
"affiliation": [
{
"name": "From the Department of Infectious Diseases, Shanghai Institute of Virology (Z. Cao, H.G., W.W., Q.X.), the Department of Emergency Medicine, Shanghai Innovation Center for Digital Medicine (W.G.), the Clinical Research Center, Shanghai National Center for Translational Medicine, State Key Laboratory of Medical Genomics (P.C., Y.X.), the Departments of General Surgery (Y.J., Z.S., Y.S., R.Z.) and Gastroenterology (J.S.), the Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine..."
}
],
"authenticated-orcid": false,
"family": "Cao",
"given": "Zhujun",
"sequence": "first"
},
{
"affiliation": [
{
"name": "From the Department of Infectious Diseases, Shanghai Institute of Virology (Z. Cao, H.G., W.W., Q.X.), the Department of Emergency Medicine, Shanghai Innovation Center for Digital Medicine (W.G.), the Clinical Research Center, Shanghai National Center for Translational Medicine, State Key Laboratory of Medical Genomics (P.C., Y.X.), the Departments of General Surgery (Y.J., Z.S., Y.S., R.Z.) and Gastroenterology (J.S.), the Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine..."
}
],
"family": "Gao",
"given": "Weiyi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Department of Infectious Diseases, Shanghai Institute of Virology (Z. Cao, H.G., W.W., Q.X.), the Department of Emergency Medicine, Shanghai Innovation Center for Digital Medicine (W.G.), the Clinical Research Center, Shanghai National Center for Translational Medicine, State Key Laboratory of Medical Genomics (P.C., Y.X.), the Departments of General Surgery (Y.J., Z.S., Y.S., R.Z.) and Gastroenterology (J.S.), the Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine..."
}
],
"family": "Bao",
"given": "Hong",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Department of Infectious Diseases, Shanghai Institute of Virology (Z. Cao, H.G., W.W., Q.X.), the Department of Emergency Medicine, Shanghai Innovation Center for Digital Medicine (W.G.), the Clinical Research Center, Shanghai National Center for Translational Medicine, State Key Laboratory of Medical Genomics (P.C., Y.X.), the Departments of General Surgery (Y.J., Z.S., Y.S., R.Z.) and Gastroenterology (J.S.), the Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine..."
}
],
"family": "Feng",
"given": "Haiyan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Department of Infectious Diseases, Shanghai Institute of Virology (Z. Cao, H.G., W.W., Q.X.), the Department of Emergency Medicine, Shanghai Innovation Center for Digital Medicine (W.G.), the Clinical Research Center, Shanghai National Center for Translational Medicine, State Key Laboratory of Medical Genomics (P.C., Y.X.), the Departments of General Surgery (Y.J., Z.S., Y.S., R.Z.) and Gastroenterology (J.S.), the Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine..."
}
],
"family": "Mei",
"given": "Shuya",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Department of Infectious Diseases, Shanghai Institute of Virology (Z. Cao, H.G., W.W., Q.X.), the Department of Emergency Medicine, Shanghai Innovation Center for Digital Medicine (W.G.), the Clinical Research Center, Shanghai National Center for Translational Medicine, State Key Laboratory of Medical Genomics (P.C., Y.X.), the Departments of General Surgery (Y.J., Z.S., Y.S., R.Z.) and Gastroenterology (J.S.), the Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine..."
}
],
"family": "Chen",
"given": "Peizhan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Department of Infectious Diseases, Shanghai Institute of Virology (Z. Cao, H.G., W.W., Q.X.), the Department of Emergency Medicine, Shanghai Innovation Center for Digital Medicine (W.G.), the Clinical Research Center, Shanghai National Center for Translational Medicine, State Key Laboratory of Medical Genomics (P.C., Y.X.), the Departments of General Surgery (Y.J., Z.S., Y.S., R.Z.) and Gastroenterology (J.S.), the Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine..."
}
],
"family": "Gao",
"given": "Yueqiu",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Department of Infectious Diseases, Shanghai Institute of Virology (Z. Cao, H.G., W.W., Q.X.), the Department of Emergency Medicine, Shanghai Innovation Center for Digital Medicine (W.G.), the Clinical Research Center, Shanghai National Center for Translational Medicine, State Key Laboratory of Medical Genomics (P.C., Y.X.), the Departments of General Surgery (Y.J., Z.S., Y.S., R.Z.) and Gastroenterology (J.S.), the Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine..."
}
],
"family": "Cui",
"given": "Zhilei",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Department of Infectious Diseases, Shanghai Institute of Virology (Z. Cao, H.G., W.W., Q.X.), the Department of Emergency Medicine, Shanghai Innovation Center for Digital Medicine (W.G.), the Clinical Research Center, Shanghai National Center for Translational Medicine, State Key Laboratory of Medical Genomics (P.C., Y.X.), the Departments of General Surgery (Y.J., Z.S., Y.S., R.Z.) and Gastroenterology (J.S.), the Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine..."
}
],
"family": "Zhang",
"given": "Qin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Department of Infectious Diseases, Shanghai Institute of Virology (Z. Cao, H.G., W.W., Q.X.), the Department of Emergency Medicine, Shanghai Innovation Center for Digital Medicine (W.G.), the Clinical Research Center, Shanghai National Center for Translational Medicine, State Key Laboratory of Medical Genomics (P.C., Y.X.), the Departments of General Surgery (Y.J., Z.S., Y.S., R.Z.) and Gastroenterology (J.S.), the Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine..."
}
],
"family": "Meng",
"given": "Xianmin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Department of Infectious Diseases, Shanghai Institute of Virology (Z. Cao, H.G., W.W., Q.X.), the Department of Emergency Medicine, Shanghai Innovation Center for Digital Medicine (W.G.), the Clinical Research Center, Shanghai National Center for Translational Medicine, State Key Laboratory of Medical Genomics (P.C., Y.X.), the Departments of General Surgery (Y.J., Z.S., Y.S., R.Z.) and Gastroenterology (J.S.), the Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine..."
}
],
"family": "Gui",
"given": "Honglian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Department of Infectious Diseases, Shanghai Institute of Virology (Z. Cao, H.G., W.W., Q.X.), the Department of Emergency Medicine, Shanghai Innovation Center for Digital Medicine (W.G.), the Clinical Research Center, Shanghai National Center for Translational Medicine, State Key Laboratory of Medical Genomics (P.C., Y.X.), the Departments of General Surgery (Y.J., Z.S., Y.S., R.Z.) and Gastroenterology (J.S.), the Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine..."
}
],
"family": "Wang",
"given": "Weijing",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Department of Infectious Diseases, Shanghai Institute of Virology (Z. Cao, H.G., W.W., Q.X.), the Department of Emergency Medicine, Shanghai Innovation Center for Digital Medicine (W.G.), the Clinical Research Center, Shanghai National Center for Translational Medicine, State Key Laboratory of Medical Genomics (P.C., Y.X.), the Departments of General Surgery (Y.J., Z.S., Y.S., R.Z.) and Gastroenterology (J.S.), the Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine..."
}
],
"family": "Jiang",
"given": "Yimei",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Department of Infectious Diseases, Shanghai Institute of Virology (Z. Cao, H.G., W.W., Q.X.), the Department of Emergency Medicine, Shanghai Innovation Center for Digital Medicine (W.G.), the Clinical Research Center, Shanghai National Center for Translational Medicine, State Key Laboratory of Medical Genomics (P.C., Y.X.), the Departments of General Surgery (Y.J., Z.S., Y.S., R.Z.) and Gastroenterology (J.S.), the Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine..."
}
],
"family": "Song",
"given": "Zijia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Department of Infectious Diseases, Shanghai Institute of Virology (Z. Cao, H.G., W.W., Q.X.), the Department of Emergency Medicine, Shanghai Innovation Center for Digital Medicine (W.G.), the Clinical Research Center, Shanghai National Center for Translational Medicine, State Key Laboratory of Medical Genomics (P.C., Y.X.), the Departments of General Surgery (Y.J., Z.S., Y.S., R.Z.) and Gastroenterology (J.S.), the Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine..."
}
],
"family": "Shi",
"given": "Yiqing",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Department of Infectious Diseases, Shanghai Institute of Virology (Z. Cao, H.G., W.W., Q.X.), the Department of Emergency Medicine, Shanghai Innovation Center for Digital Medicine (W.G.), the Clinical Research Center, Shanghai National Center for Translational Medicine, State Key Laboratory of Medical Genomics (P.C., Y.X.), the Departments of General Surgery (Y.J., Z.S., Y.S., R.Z.) and Gastroenterology (J.S.), the Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine..."
}
],
"family": "Sun",
"given": "Jing",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Department of Infectious Diseases, Shanghai Institute of Virology (Z. Cao, H.G., W.W., Q.X.), the Department of Emergency Medicine, Shanghai Innovation Center for Digital Medicine (W.G.), the Clinical Research Center, Shanghai National Center for Translational Medicine, State Key Laboratory of Medical Genomics (P.C., Y.X.), the Departments of General Surgery (Y.J., Z.S., Y.S., R.Z.) and Gastroenterology (J.S.), the Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine..."
}
],
"family": "Zhang",
"given": "Yifei",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Department of Infectious Diseases, Shanghai Institute of Virology (Z. Cao, H.G., W.W., Q.X.), the Department of Emergency Medicine, Shanghai Innovation Center for Digital Medicine (W.G.), the Clinical Research Center, Shanghai National Center for Translational Medicine, State Key Laboratory of Medical Genomics (P.C., Y.X.), the Departments of General Surgery (Y.J., Z.S., Y.S., R.Z.) and Gastroenterology (J.S.), the Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine..."
}
],
"family": "Xie",
"given": "Qing",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Department of Infectious Diseases, Shanghai Institute of Virology (Z. Cao, H.G., W.W., Q.X.), the Department of Emergency Medicine, Shanghai Innovation Center for Digital Medicine (W.G.), the Clinical Research Center, Shanghai National Center for Translational Medicine, State Key Laboratory of Medical Genomics (P.C., Y.X.), the Departments of General Surgery (Y.J., Z.S., Y.S., R.Z.) and Gastroenterology (J.S.), the Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine..."
}
],
"family": "Xu",
"given": "Yiping",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Department of Infectious Diseases, Shanghai Institute of Virology (Z. Cao, H.G., W.W., Q.X.), the Department of Emergency Medicine, Shanghai Innovation Center for Digital Medicine (W.G.), the Clinical Research Center, Shanghai National Center for Translational Medicine, State Key Laboratory of Medical Genomics (P.C., Y.X.), the Departments of General Surgery (Y.J., Z.S., Y.S., R.Z.) and Gastroenterology (J.S.), the Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine..."
}
],
"family": "Ning",
"given": "Guang",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Department of Infectious Diseases, Shanghai Institute of Virology (Z. Cao, H.G., W.W., Q.X.), the Department of Emergency Medicine, Shanghai Innovation Center for Digital Medicine (W.G.), the Clinical Research Center, Shanghai National Center for Translational Medicine, State Key Laboratory of Medical Genomics (P.C., Y.X.), the Departments of General Surgery (Y.J., Z.S., Y.S., R.Z.) and Gastroenterology (J.S.), the Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine..."
}
],
"family": "Gao",
"given": "Yuan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From the Department of Infectious Diseases, Shanghai Institute of Virology (Z. Cao, H.G., W.W., Q.X.), the Department of Emergency Medicine, Shanghai Innovation Center for Digital Medicine (W.G.), the Clinical Research Center, Shanghai National Center for Translational Medicine, State Key Laboratory of Medical Genomics (P.C., Y.X.), the Departments of General Surgery (Y.J., Z.S., Y.S., R.Z.) and Gastroenterology (J.S.), the Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine..."
}
],
"family": "Zhao",
"given": "Ren",
"sequence": "additional"
}
],
"container-title": "New England Journal of Medicine",
"container-title-short": "N Engl J Med",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
12,
28
]
],
"date-time": "2022-12-28T22:00:43Z",
"timestamp": 1672264843000
},
"deposited": {
"date-parts": [
[
2022,
12,
28
]
],
"date-time": "2022-12-28T22:00:54Z",
"timestamp": 1672264854000
},
"funder": [
{
"award": [
"shslczdzk01103"
],
"name": "Shanghai Municipal Key Clinical Specialty"
},
{
"DOI": "10.13039/501100003399",
"award": [
"22YJ1900600"
],
"doi-asserted-by": "publisher",
"name": "Science and Technology Commission of Shanghai Municipality"
},
{
"award": [
"LG-YJ-202204-01"
],
"name": "Lingang Laboratory emergency project"
},
{
"name": "Vigonvita Life Science Co., Ltd"
},
{
"DOI": "10.13039/501100012166",
"award": [
"2021YFC0865000"
],
"doi-asserted-by": "publisher",
"name": "National Key Research and Development Program of China"
},
{
"DOI": "10.13039/501100001809",
"award": [
"82000588",
"82088102"
],
"doi-asserted-by": "publisher",
"name": "National Natural Science Foundation of China"
},
{
"award": [
"SHDC2022CRS031"
],
"name": "Shanghai Shenkang Three-Year Action grant"
}
],
"indexed": {
"date-parts": [
[
2022,
12,
29
]
],
"date-time": "2022-12-29T05:53:16Z",
"timestamp": 1672293196575
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
12,
28
]
]
},
"language": "en",
"license": [
{
"URL": "http://www.nejmgroup.org/legal/terms-of-use.htm",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
12,
28
]
],
"date-time": "2022-12-28T00:00:00Z",
"timestamp": 1672185600000
}
}
],
"link": [
{
"URL": "http://www.nejm.org/doi/pdf/10.1056/NEJMoa2208822",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "150",
"original-title": [],
"prefix": "10.1056",
"published": {
"date-parts": [
[
2022,
12,
28
]
]
},
"published-online": {
"date-parts": [
[
2022,
12,
28
]
]
},
"publisher": "Massachusetts Medical Society",
"reference": [
{
"DOI": "10.1016/S0140-6736(21)02796-3",
"doi-asserted-by": "publisher",
"key": "r1"
},
{
"DOI": "10.1016/S0140-6736(22)00484-6",
"doi-asserted-by": "publisher",
"key": "r2"
},
{
"DOI": "10.1038/s41586-021-04385-3",
"doi-asserted-by": "publisher",
"key": "r3"
},
{
"DOI": "10.1038/s41577-022-00676-6",
"doi-asserted-by": "publisher",
"key": "r4"
},
{
"DOI": "10.1038/s41586-022-04411-y",
"doi-asserted-by": "publisher",
"key": "r5"
},
{
"DOI": "10.1038/s41591-022-01855-7",
"doi-asserted-by": "publisher",
"key": "r6"
},
{
"DOI": "10.1126/science.acx9605",
"doi-asserted-by": "publisher",
"key": "r7"
},
{
"DOI": "10.1056/NEJMoa2118542",
"doi-asserted-by": "publisher",
"key": "r8"
},
{
"DOI": "10.1136/bmj.m3379",
"doi-asserted-by": "publisher",
"key": "r9"
},
{
"DOI": "10.1126/science.abl4784",
"doi-asserted-by": "publisher",
"key": "r10"
},
{
"DOI": "10.1056/NEJMoa2116846",
"doi-asserted-by": "publisher",
"key": "r11"
},
{
"DOI": "10.1126/scitranslmed.abm3410",
"doi-asserted-by": "publisher",
"key": "r12"
},
{
"DOI": "10.1126/scitranslmed.abm7621",
"doi-asserted-by": "publisher",
"key": "r13"
},
{
"DOI": "10.1016/j.bmc.2021.116364",
"doi-asserted-by": "publisher",
"key": "r14"
},
{
"DOI": "10.1038/s41422-021-00570-1",
"doi-asserted-by": "publisher",
"key": "r15"
},
{
"DOI": "10.1038/s41401-022-00895-6",
"doi-asserted-by": "publisher",
"key": "r16"
},
{
"DOI": "10.1080/22221751.2022.2078230",
"doi-asserted-by": "publisher",
"key": "r17"
},
{
"DOI": "10.1016/S0140-6736(22)00327-0",
"doi-asserted-by": "publisher",
"key": "r19"
},
{
"DOI": "10.1016/S0140-6736(22)00838-8",
"doi-asserted-by": "publisher",
"key": "r20"
},
{
"DOI": "10.1016/j.jinf.2022.07.006",
"doi-asserted-by": "publisher",
"key": "r21"
},
{
"DOI": "10.1056/NEJMoa2108163",
"doi-asserted-by": "publisher",
"key": "r22"
},
{
"DOI": "10.1001/jama.2021.0202",
"doi-asserted-by": "publisher",
"key": "r23"
},
{
"author": "Ganatra S",
"journal-title": "Clin Infect Dis",
"key": "r24",
"year": "2022"
},
{
"author": "Najjar-Debbiny R",
"journal-title": "Clin Infect Dis",
"key": "r26",
"year": "2022"
},
{
"DOI": "10.1371/journal.pone.0063623",
"doi-asserted-by": "publisher",
"key": "r27"
}
],
"reference-count": 25,
"references-count": 25,
"relation": {},
"resource": {
"primary": {
"URL": "http://www.nejm.org/doi/10.1056/NEJMoa2208822"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "VV116 versus Nirmatrelvir–Ritonavir for Oral Treatment of Covid-19",
"type": "journal-article"
}