Efficacy of convalescent plasma therapy in the patient with COVID-19: a randomised control trial (COPLA-II trial)
et al., BMJ Open, doi:10.1136/bmjopen-2021-055189, COPLA-II, NCT04425915, Apr 2022
RCT 400 hospitalized severe COVID-19 patients in India showing no significant difference in time to clinical improvement, mortality, or other outcomes with convalescent plasma compared to standard treatment. In a subgroup analysis, results were better for patients receiving plasma within 3 days of admission. There was no difference in outcomes based on patient baseline antibody levels.
|
risk of death, 13.5% higher, RR 1.14, p = 0.62, treatment 42 of 200 (21.0%), control 37 of 200 (18.5%), day 28.
|
|
risk of death, 19.0% higher, RR 1.19, p = 0.64, treatment 25 of 200 (12.5%), control 21 of 200 (10.5%), day 7.
|
|
risk of mechanical ventilation, 12.5% higher, RR 1.13, p = 0.76, treatment 27 of 200 (13.5%), control 24 of 200 (12.0%), day 7.
|
|
ICU time, 1.7% higher, relative time 1.02, p = 0.80, treatment mean 11.1 (±7.77) n=200, control mean 10.91 (±6.96) n=200.
|
|
hospitalization time, 0.1% lower, relative time 1.00, p = 0.98, treatment mean 13.8 (±7.03) n=200, control mean 13.82 (±7.19) n=200.
|
|
risk of no recovery, 5.9% lower, RR 0.94, p = 0.75, treatment 64 of 200 (32.0%), control 68 of 200 (34.0%), NNT 50, day 28.
|
|
relative mean Ct, 1.1% worse, RR 1.01, p = 0.54, treatment mean 34.31 (±6.61) n=200, control mean 34.7 (±6.2) n=200, day 7.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Bajpai et al., 6 Apr 2022, Randomized Controlled Trial, India, peer-reviewed, mean age 55.5, 23 authors, study period June 2020 - December 2020, trial NCT04425915 (history) (COPLA-II).
Contact: shivsarin@gmail.c.
Efficacy of convalescent plasma therapy in the patient with COVID-19: a randomised control trial (COPLA-II trial)
BMJ Open, doi:10.1136/bmjopen-2021-055189
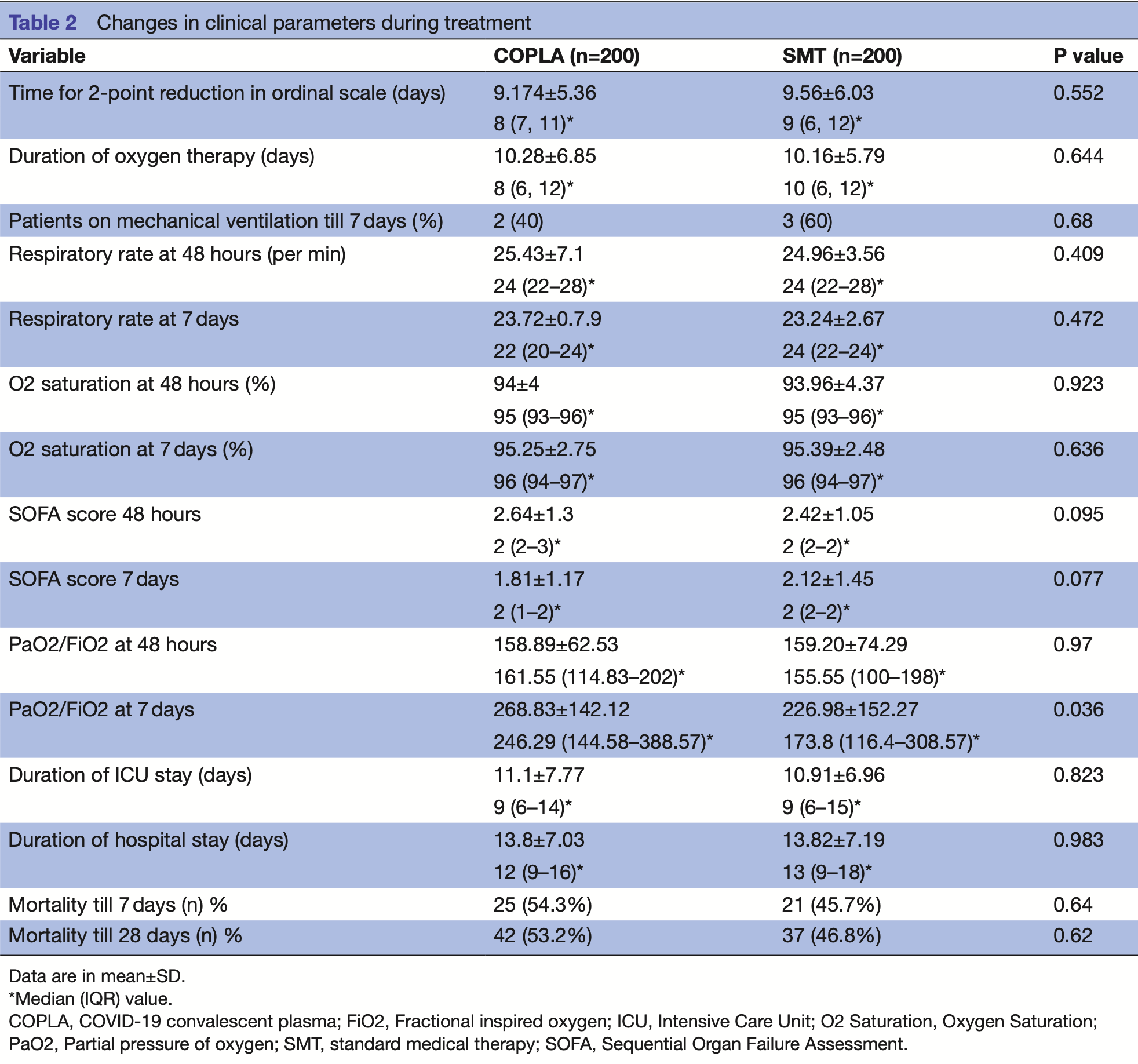
Importance No proven treatment is available for severely ill COVID-19. Therapeutic use of COVID-19 convalescent plasma (COPLA) is under investigation. Objective To compare the efficacy of COPLA with standard medical therapy (SMT) alone in severe COVID-19 patients. Design, setting and participants A multicentric, openlabelled, phase-III randomised controlled trial conducted at two treatment centres with COPLA collected at the third dedicated centre in North-India, the coordinating centre during trial from June 2020 to December 2020. The study population comprised 400 participants in the ratio of 1:1 in each treatment group. Intervention One group received COPLA with SMT (n=200), and another group received SMT only (n=200). Main outcome measures Primary outcome was time to clinical improvement measured by a two-point reduction in the ordinal scale. Secondary outcomes included duration of O 2 therapy, the proportion of patients on mechanical ventilation at day-7, mortality, SARS-CoV-2 antibody levels, cytokine levels and incidence of adverse events.
Results The median time to a two-point reduction in the ordinal scale in both groups was 9 days (IQR=7-13) (p=0.328). The median duration of O 2 therapy was 8 days (IQR=6-12) in COPLA and 10 days (IQR=6-12) in SMT group (p=0.64). The PaO 2 /FiO 2 ratio showed significant improvement at 7 days in COPLA group(p=0.036). There was no difference in mortality till 28 days in both groups (p=0.62). However, if COPLA was given within 3 days of hospital admission, a significant reduction in ordinal scale was observed (p=0.04). Neutralising antibody titres in COPLA group (80 (IQR 80-80)) were higher than SMT group (0 (IQR 0-80)) at 48 hours (p=0.001). COPLA therapy led to a significant reduction in TNF-α levels at 48 hours (p=0.048) and D-dimer at 7 days (p=0.02). Mild allergic reactions were observed in 3 (1.5%) patients in COPLA group.
Conclusion and relevance Convalescent plasma with adequate antibody titres should be transfused in COVID-19 patients along with SMT in the initial 3 days of hospitalisation for better clinical outcomes. Trial registration number NCT04425915.
Competing interests None declared. Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication Not applicable. Ethics approval This study involves human participants and was approved by Institute of Liver and Biliary Sciences, New Delhi, Reference number or ID for ethics: IEC/2020/77/MA05. Participants gave informed consent to participate in the study before taking part. Provenance and peer review Not commissioned; externally peer reviewed. Data availability statement Data are available on reasonable request. Data (unpublished data in its row form in Microsoft excel sheet) will be available with principal investigator of the study and it will be available for researcher in unidentified data form on reasonable request to principal investigator till 5 years from date of publication. Rest all data relevant to the study are included in the article or uploaded as online supplemental information Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and..
References
Agarwal, Mukherjee, Kumar, Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID trial), BMJ, doi:10.1136/bmj.m3939
Alhazzani, Møller, Arabi, Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19), Crit Care Med, doi:10.1097/CCM.0000000000004363
Cao, Wang, Wen, A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19, N Engl J Med, doi:10.1056/NEJMoa2001282
Chen, Xiong, Bao, Shi, Convalescent plasma as a potential therapy for COVID-19, Lancet Infect Dis
Chen, Zhou, Dong, Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study, Lancet
Cheng, Wong, Soo, Use of convalescent plasma therapy in SARS patients in Hong Kong, Eur J Clin Microbiol Infect Dis, doi:10.1007/s10096-004-1271-9
Duan, Liu, Li, Effectiveness of convalescent plasma therapy in severe COVID-19 patients, Proc Natl Acad Sci U S A, doi:10.1073/pnas.2004168117
Egloff, Junglen, Restivo, Convalescent plasma associates with reduced mortality and improved clinical trajectory in patients hospitalized with COVID-19, J Clin Invest, doi:10.1172/JCI151788
Gharbharan, Jordans, Geurtsvankessel, Effects of potent neutralizing antibodies from convalescent plasma in patients hospitalized for severe SARS-CoV-2 infection, Nat Commun, doi:10.1038/s41467-021-23469-2
Grein, Ohmagari, Shin, Compassionate Use of Remdesivir for Patients with Severe Covid-19, N Engl J Med, doi:10.1056/NEJMoa2007016
Group, Horby, Lim, Dexamethasone in hospitalized patients with Covid-19, doi:10.1056/NEJMoa2021436
Harms, The risks will be the same as the risks related to the transfusion of Plasma Components
Huang, Pranata, Lim, C-Reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a metaanalysis, Ther Adv Respir Dis, doi:10.1177/1753466620937175
Huang, Wang, Li, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet
Hung, To, Lee, Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection, Clin Infect Dis, doi:10.1093/cid/ciq106
Joyner, Bruno, Klassen, Safety update: COVID-19 convalescent plasma in 20,000 hospitalized patients, Mayo Clin Proc, doi:10.1016/j.mayocp.2020.06.028
Joyner, Carter, Senefeld, Convalescent plasma antibody levels and the risk of death from Covid-19, N Engl J Med, doi:10.1056/NEJMoa2031893
Joyner, Senefeld, Klassen, Effect of convalescent plasma on mortality among hospitalized patients with COVID-19: initial three-month experience, medRxiv, doi:10.1101/2020.08.12.20169359
Kermali, Khalsa, Pillai, The role of biomarkers in diagnosis of COVID-19 -A systematic review, Life Sci, doi:10.1016/j.lfs.2020.117788
Ko, Seok, Cho, Challenges of convalescent plasma infusion therapy in middle East respiratory coronavirus infection: a single centre experience, Antivir Ther, doi:10.3851/IMP3243
Kunze, Johnson, Van Helmond, Mortality in individuals treated with COVID-19 convalescent plasma varies with the geographic provenance of donors, Nat Commun, doi:10.1038/s41467-021-25113-5
Körper, Weiss, Zickler, Results of the capsid randomized trial for high-dose convalescent plasma in patients with severe COVID-19, J Clin Invest, doi:10.1172/JCI152264
Li, Zhang, Hu, Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial, JAMA, doi:10.1001/jama.2020.10044
Libster, Marc, Wappner, Early high-titer plasma therapy to prevent severe COVID-19 in older adults, N Engl J Med, doi:10.1056/NEJMoa2033700
Luke, Kilbane, Jackson, Meta-Analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment?, Ann Intern Med, doi:10.7326/0003-4819-145-8-200610170-00139
Perotti, Baldanti, Bruno, Mortality reduction in 46 severe Covid-19 patients treated with hyperimmune plasma. A proof of concept single arm multicenter trial, Haematologica, doi:10.3324/haematol.2020.261784
Salazar, Perez, Ashraf, Treatment of coronavirus disease 2019 (COVID-19) patients with convalescent plasma, Am J Pathol, doi:10.1016/j.ajpath.2020.05.014
Shen, Wang, Zhao, Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma, JAMA, doi:10.1001/jama.2020.4783
Shen, Wang, Zhao, Treatment of 5 critically ill patients with COVID-19 with convalescent plasma, JAMA, doi:10.1001/jama.2020.4783
Simonovich, Pratx, Scibona, A randomized trial of convalescent plasma in Covid-19 severe pneumonia, N Engl J Med, doi:10.1056/NEJMoa2031304
Who, Who coronavirus disease (COVID-19) dashboard
Yao, Cao, Wang, D-Dimer as a biomarker for disease severity and mortality in COVID-19 patients: a case control study, J Intensive Care, doi:10.1186/s40560-020-00466-z
Zhang, Yan, Fan, D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19, J Thromb Haemost, doi:10.1111/jth.14859
Zhou, Zhong, Guan, Treatment with convalescent plasma for influenza A (H5N1) infection, N Engl J Med, doi:10.1056/NEJMc070359
DOI record:
{
"DOI": "10.1136/bmjopen-2021-055189",
"ISSN": [
"2044-6055",
"2044-6055"
],
"URL": "http://dx.doi.org/10.1136/bmjopen-2021-055189",
"abstract": "<jats:sec><jats:title>Importance</jats:title><jats:p>No proven treatment is available for severely ill COVID-19. Therapeutic use of COVID-19 convalescent plasma (COPLA) is under investigation.</jats:p></jats:sec><jats:sec><jats:title>Objective</jats:title><jats:p>To compare the efficacy of COPLA with standard medical therapy (SMT) alone in severe COVID-19 patients.</jats:p></jats:sec><jats:sec><jats:title>Design, setting and participants</jats:title><jats:p>A multicentric, open-labelled, phase-III randomised controlled trial conducted at two treatment centres with COPLA collected at the third dedicated centre in North-India, the coordinating centre during trial from June 2020 to December 2020. The study population comprised 400 participants in the ratio of 1:1 in each treatment group.</jats:p></jats:sec><jats:sec><jats:title>Intervention</jats:title><jats:p>One group received COPLA with SMT (n=200), and another group received SMT only (n=200).</jats:p></jats:sec><jats:sec><jats:title>Main outcome measures</jats:title><jats:p>Primary outcome was time to clinical improvement measured by a two-point reduction in the ordinal scale. Secondary outcomes included duration of O<jats:sub>2</jats:sub>therapy, the proportion of patients on mechanical ventilation at day-7, mortality, SARS-CoV-2 antibody levels, cytokine levels and incidence of adverse events.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>The median time to a two-point reduction in the ordinal scale in both groups was 9 days (IQR=7–13) (p=0.328). The median duration of O<jats:sub>2</jats:sub>therapy was 8 days (IQR=6–12) in COPLA and 10 days (IQR=6–12) in SMT group (p=0.64). The PaO<jats:sub>2</jats:sub>/FiO<jats:sub>2</jats:sub>ratio showed significant improvement at 7 days in COPLA group(p=0.036). There was no difference in mortality till 28 days in both groups (p=0.62). However, if COPLA was given within 3 days of hospital admission, a significant reduction in ordinal scale was observed (p=0.04). Neutralising antibody titres in COPLA group (80 (IQR 80–80)) were higher than SMT group (0 (IQR 0–80)) at 48 hours (p=0.001). COPLA therapy led to a significant reduction in TNF-α levels at 48 hours (p=0.048) and D-dimer at 7 days (p=0.02). Mild allergic reactions were observed in 3 (1.5%) patients in COPLA group.</jats:p></jats:sec><jats:sec><jats:title>Conclusion and relevance</jats:title><jats:p>Convalescent plasma with adequate antibody titres should be transfused in COVID-19 patients along with SMT in the initial 3 days of hospitalisation for better clinical outcomes.</jats:p></jats:sec><jats:sec><jats:title>Trial registration number</jats:title><jats:p><jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"clintrialgov\" xlink:href=\"NCT04425915\">NCT04425915</jats:ext-link>.</jats:p></jats:sec>",
"alternative-id": [
"10.1136/bmjopen-2021-055189"
],
"author": [
{
"affiliation": [],
"family": "Bajpai",
"given": "Meenu",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-0716-939X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Maheshwari",
"given": "Ashish",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dogra",
"given": "Vikas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kumar",
"given": "Suresh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gupta",
"given": "Ekta",
"sequence": "additional"
},
{
"affiliation": [],
"family": "kale",
"given": "Pratibha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saluja",
"given": "Vandana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Thomas",
"given": "Sherin S",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Trehanpati",
"given": "Nirupama",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bihari",
"given": "Chhagan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Agarwal",
"given": "Reshu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bharti",
"given": "Praveen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shankar",
"given": "Prabha",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3195-0776",
"affiliation": [],
"authenticated-orcid": false,
"family": "Hussain",
"given": "Javid",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chhabra",
"given": "Karan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gupta",
"given": "Amita",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Narayanan",
"given": "Ashad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Agarwal",
"given": "Sarika",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jain",
"given": "Shruti",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bhardwaj",
"given": "Ankit",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kumar",
"given": "Guresh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yadav",
"given": "Birendra Kumar",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0544-5610",
"affiliation": [],
"authenticated-orcid": false,
"family": "Sarin",
"given": "Shiv Kumar",
"sequence": "additional"
}
],
"clinical-trial-number": [
{
"clinical-trial-number": "nct04425915",
"registry": "10.18810/clinical-trials-gov"
}
],
"container-title": "BMJ Open",
"container-title-short": "BMJ Open",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"bmj.com"
]
},
"created": {
"date-parts": [
[
2022,
5,
2
]
],
"date-time": "2022-05-02T04:37:53Z",
"timestamp": 1651466273000
},
"deposited": {
"date-parts": [
[
2023,
3,
8
]
],
"date-time": "2023-03-08T12:25:11Z",
"timestamp": 1678278311000
},
"indexed": {
"date-parts": [
[
2024,
8,
4
]
],
"date-time": "2024-08-04T14:00:53Z",
"timestamp": 1722780053748
},
"is-referenced-by-count": 22,
"issue": "4",
"issued": {
"date-parts": [
[
2022,
4
]
]
},
"journal-issue": {
"issue": "4",
"published-online": {
"date-parts": [
[
2022,
4,
12
]
]
},
"published-print": {
"date-parts": [
[
2022,
4
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "unspecified",
"delay-in-days": 5,
"start": {
"date-parts": [
[
2022,
4,
6
]
],
"date-time": "2022-04-06T00:00:00Z",
"timestamp": 1649203200000
}
}
],
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1136/bmjopen-2021-055189",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "239",
"original-title": [],
"page": "e055189",
"prefix": "10.1136",
"published": {
"date-parts": [
[
2022,
4
]
]
},
"published-online": {
"date-parts": [
[
2022,
4,
6
]
]
},
"published-print": {
"date-parts": [
[
2022,
4
]
]
},
"publisher": "BMJ",
"reference": [
{
"DOI": "10.1007/s10096-004-1271-9",
"doi-asserted-by": "publisher",
"key": "2023030803151926000_12.4.e055189.1"
},
{
"DOI": "10.1093/cid/ciq106",
"doi-asserted-by": "publisher",
"key": "2023030803151926000_12.4.e055189.2"
},
{
"DOI": "10.1056/NEJMc070359",
"doi-asserted-by": "publisher",
"key": "2023030803151926000_12.4.e055189.3"
},
{
"DOI": "10.1016/j.mayocp.2020.06.028",
"article-title": "Safety update: COVID-19 convalescent plasma in 20,000 hospitalized patients",
"author": "Joyner",
"doi-asserted-by": "crossref",
"first-page": "1888",
"journal-title": "Mayo Clin Proc",
"key": "2023030803151926000_12.4.e055189.4",
"volume": "95",
"year": "2020"
},
{
"DOI": "10.7326/0003-4819-145-8-200610170-00139",
"doi-asserted-by": "publisher",
"key": "2023030803151926000_12.4.e055189.5"
},
{
"DOI": "10.3851/IMP3243",
"doi-asserted-by": "publisher",
"key": "2023030803151926000_12.4.e055189.6"
},
{
"key": "2023030803151926000_12.4.e055189.7",
"unstructured": "Directorate General of Health Services, EMR Division, Ministry of Health and Family Welfare; Government of India . Clinical management protocol: COVID-19 V.5, 2020. Available: www.mohfw.gov.in/pdf/UpdatedClinicalManagementProtocolforCOVID19dated03072020.pdf [Accessed 17 Feb 2021]."
},
{
"DOI": "10.1097/CCM.0000000000004363",
"doi-asserted-by": "publisher",
"key": "2023030803151926000_12.4.e055189.8"
},
{
"key": "2023030803151926000_12.4.e055189.9",
"unstructured": "Department of Health and Family Welfare,, Ministry of Health and Family Welfare . Government of India. Gazette of India. Available: https://cdsco.gov.in/opencms/opencms/en/Notifications/Gazette-Notifications/ [Accessed 17 Feb 2021]."
},
{
"key": "2023030803151926000_12.4.e055189.10",
"unstructured": "WHO . Who coronavirus disease (COVID-19) dashboard. Available: https://COVID19.who.int/ [Accessed 17 Feb 2021]."
},
{
"DOI": "10.1056/NEJMoa2021436",
"doi-asserted-by": "publisher",
"key": "2023030803151926000_12.4.e055189.11"
},
{
"key": "2023030803151926000_12.4.e055189.12",
"unstructured": "COVID-19 treatment guidelines. Available: https://www.COVID19treatmentguidelines.nih.gov/anti-sars-cov-2-antibody-products/convalescent-plasma/ [Accessed 17 Feb 2021]."
},
{
"DOI": "10.1177/1753466620937175",
"article-title": "C-Reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis",
"author": "Huang",
"doi-asserted-by": "crossref",
"journal-title": "Ther Adv Respir Dis",
"key": "2023030803151926000_12.4.e055189.13",
"volume": "14",
"year": "2020"
},
{
"DOI": "10.1016/j.lfs.2020.117788",
"doi-asserted-by": "publisher",
"key": "2023030803151926000_12.4.e055189.14"
},
{
"DOI": "10.1111/jth.14859",
"doi-asserted-by": "publisher",
"key": "2023030803151926000_12.4.e055189.15"
},
{
"DOI": "10.1186/s40560-020-00466-z",
"article-title": "D-Dimer as a biomarker for disease severity and mortality in COVID-19 patients: a case control study",
"author": "Yao",
"doi-asserted-by": "crossref",
"first-page": "49",
"journal-title": "J Intensive Care",
"key": "2023030803151926000_12.4.e055189.16",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1016/j.ajpath.2020.05.014",
"article-title": "Treatment of coronavirus disease 2019 (COVID-19) patients with convalescent plasma",
"author": "Salazar",
"doi-asserted-by": "crossref",
"first-page": "1680",
"journal-title": "Am J Pathol",
"key": "2023030803151926000_12.4.e055189.17",
"volume": "190",
"year": "2020"
},
{
"DOI": "10.1136/bmj.m3939",
"doi-asserted-by": "publisher",
"key": "2023030803151926000_12.4.e055189.18"
},
{
"DOI": "10.1056/NEJMoa2031304",
"doi-asserted-by": "publisher",
"key": "2023030803151926000_12.4.e055189.19"
},
{
"DOI": "10.1001/jama.2020.1004",
"doi-asserted-by": "publisher",
"key": "2023030803151926000_12.4.e055189.20"
},
{
"DOI": "10.1038/s41467-021-23469-2",
"doi-asserted-by": "publisher",
"key": "2023030803151926000_12.4.e055189.21"
},
{
"DOI": "10.1172/JCI151788",
"article-title": "Convalescent plasma associates with reduced mortality and improved clinical trajectory in patients hospitalized with COVID-19",
"author": "Arnold Egloff",
"doi-asserted-by": "crossref",
"journal-title": "J Clin Invest",
"key": "2023030803151926000_12.4.e055189.22",
"volume": "131",
"year": "2021"
},
{
"key": "2023030803151926000_12.4.e055189.23",
"unstructured": "Evidence based Advisory to address inappropriate use of convalescent plasma in COVID-19 patients, 2020. Available: https://www.icmr.gov.in/pdf/COVID/techdoc/ICMR_ADVISORY_Convalescent_plasma_ 17112020_v1.pdf"
},
{
"DOI": "10.1056/NEJMoa2033700",
"doi-asserted-by": "publisher",
"key": "2023030803151926000_12.4.e055189.24"
},
{
"DOI": "10.1001/jama.2020.478",
"doi-asserted-by": "publisher",
"key": "2023030803151926000_12.4.e055189.25"
},
{
"article-title": "Effect of convalescent plasma on mortality among hospitalized patients with COVID-19: initial three-month experience",
"author": "Joyner",
"first-page": "2020.08.12.20169359",
"journal-title": "medRxiv",
"key": "2023030803151926000_12.4.e055189.26",
"year": "2020"
},
{
"DOI": "10.1073/pnas.2004168117",
"doi-asserted-by": "publisher",
"key": "2023030803151926000_12.4.e055189.27"
},
{
"DOI": "10.3324/haematol.2020.261784",
"article-title": "Mortality reduction in 46 severe Covid-19 patients treated with hyperimmune plasma. A proof of concept single arm multicenter trial",
"author": "Perotti",
"doi-asserted-by": "crossref",
"first-page": "2834",
"journal-title": "Haematologica",
"key": "2023030803151926000_12.4.e055189.28",
"volume": "105",
"year": "2020"
},
{
"DOI": "10.1172/JCI152264",
"article-title": "Results of the capsid randomized trial for high-dose convalescent plasma in patients with severe COVID-19",
"author": "Körper",
"doi-asserted-by": "crossref",
"journal-title": "J Clin Invest",
"key": "2023030803151926000_12.4.e055189.29",
"volume": "131",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2031893",
"doi-asserted-by": "publisher",
"key": "2023030803151926000_12.4.e055189.30"
},
{
"DOI": "10.1038/s41467-021-25113-5",
"doi-asserted-by": "publisher",
"key": "2023030803151926000_12.4.e055189.31"
}
],
"reference-count": 31,
"references-count": 31,
"relation": {},
"resource": {
"primary": {
"URL": "https://bmjopen.bmj.com/lookup/doi/10.1136/bmjopen-2021-055189"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Efficacy of convalescent plasma therapy in the patient with COVID-19: a randomised control trial (COPLA-II trial)",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1136/crossmarkpolicy",
"volume": "12"
}