The impact of asprin use on the outcome of patients admitted to the intensive care unit with COVID-19 infection
et al., Research Square, doi:10.21203/rs.3.rs-2313880/v1, Nov 2022
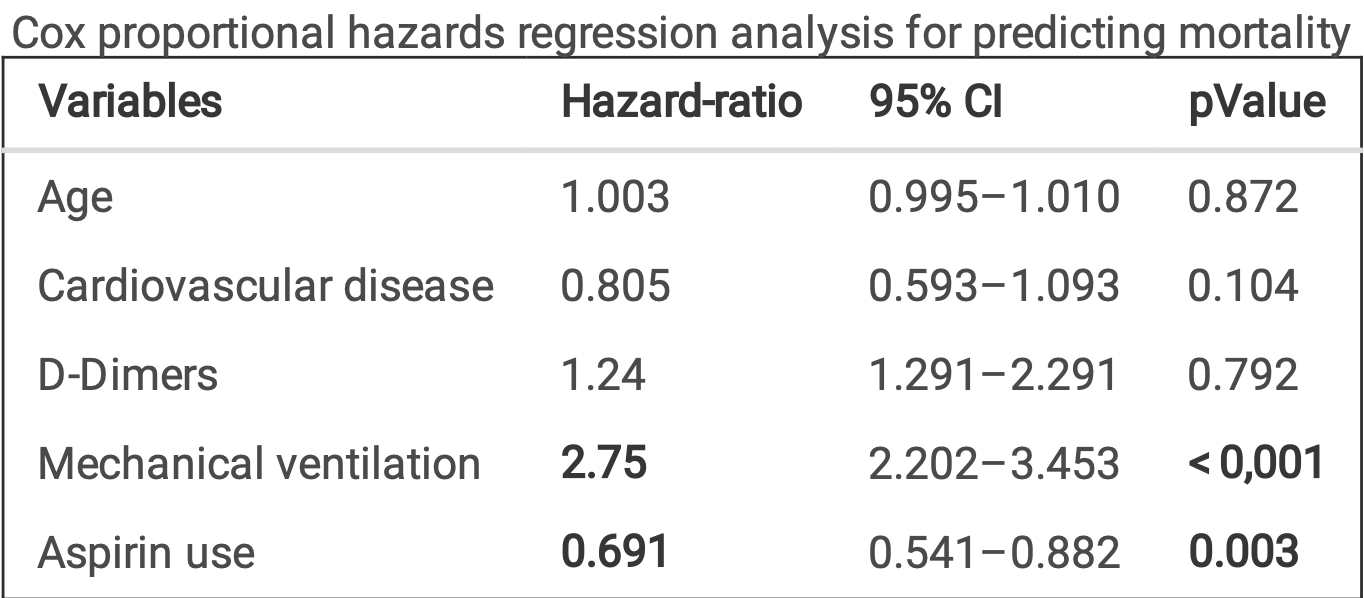
Prospective study of 1,124 COVID-19 ICU patients, showing lower mortality with aspirin treatment.
|
risk of death, 30.9% lower, HR 0.69, p = 0.003, treatment 202 of 712 (28.4%), control 165 of 412 (40.0%), NNT 8.6, adjusted per study, multivariable, Cox proportional hazards.
|
|
risk of mechanical ventilation, 9.6% lower, RR 0.90, p = 0.33, treatment 189 of 712 (26.5%), control 121 of 412 (29.4%), NNT 35.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Aidouni et al., 30 Nov 2022, prospective, Morocco, preprint, mean age 64.0, 6 authors, study period March 2020 - March 2022.
Contact: aminbouchlarhem63@gmail.com.
The impact of asprin use on the outcome of patients admitted to the intensive care unit with COVID-19 infection
doi:10.21203/rs.3.rs-2313880/v1
Background Our objective in this study is to know the impact of the use of asprin in anti-aggregation dose on the evolution during hospitalization of patients admitted in intensive care unit for a severe infection by SARS-COV-2.
Methods We conducted a prospective study of patients admitted to our department with severe COVID-19 infection during the period between March 2020 and March 2022, analyzing the difference between the placebo group and the aspirin group on the primary endpoint of all-cause hospital mortality and the composite secondary endpoint of use of mechanical ventilation and thromboembolic events.
Results Out of 1124 patients included, 32.6% died, with a protective effect of aspirin against placebo (Hazardratio = 0.691, p = 0.003), for thrombo-embolic complications, 104 events were observed, with a protective effect of aspirin (Hazard-Ratio = 0.448 and p = 0.001), nally regarding mechanical ventilation, there was no remarkable bene t on our sample.
Conclusion Given the divergence of results of studies published in the literature, the availability of results of large randomized controlled trials is a necessity.
References
Abani, Aspirin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial, Lancet, doi:10.1016/S0140-6736(21)01825-0
Ahmed, Rationales and uncertainties for aspirin use in COVID-19: A narrative review, Fam. Med. Community Heal, doi:10.1136/fmch-2020-000741
Ali, Spinler, COVID-19 and thrombosis: From bench to bedside, Trends Cardiovasc. Med, doi:10.1016/j.tcm.2020.12.004
Birnhuber, Between in ammation and thrombosis: endothelial cells in COVID-19, The European respiratory journal, doi:10.1183/13993003.00377-2021
Botton, No association of low-dose aspirin with severe COVID-19 in France: A cohort of 31.1 million people without cardiovascular disease, Res. Pract. Thromb. Haemost, doi:10.1002/rth2.12743
Carbonell, COVID-19 and thromboprophylaxis: Recommendations for our clinical practice in Primary Care, Semergen, doi:10.1016/j.semerg.2020.07.007
Dzeshka, Shantsila, Lip, Effects of Aspirin on Endothelial Function and Hypertension, Curr. Hypertens. Rep, doi:10.1007/s11906-016-0688-8
Formiga, Does admission acetylsalicylic acid uptake in hospitalized COVID-19 patients have a protective role? Data from the Spanish SEMI-COVID-19 Registry, Intern. Emerg. Med, doi:10.1007/s11739-021-02870-1
Glatthaar-Saalmüller, Mair, Saalmüller, Antiviral activity of aspirin against RNA viruses of the respiratory tract-an in vitro study, uenza Other Respi. Viruses, doi:10.1111/irv.12421
Gómez-Mesa, Galindo-Coral, Montes, Muñoz Martin, Thrombosis and Coagulopathy in COVID-19, Curr. Probl. Cardiol, doi:10.1016/j.cpcardiol.2020.100742
Ma, Does aspirin have an effect on risk of death in patients with COVID-19? A metaanalysis, Eur. J. Clin. Pharmacol, doi:10.1007/s00228-022-03356-5
Moschonas, Tselepis, SARS-CoV-2 infection and thrombotic complications: a narrative review, J. Thromb. Thrombolysis, doi:10.1007/s11239-020-02374-3
Sahai, Effect of aspirin on short-term outcomes in hospitalized patients with COVID-19, Vasc. Med. (United Kingdom), doi:10.1177/1358863X211012754
Salah, Mehta, Meta-Analysis of the Effect of Aspirin on Mortality in COVID-19, The American journal of cardiology, doi:10.1016/j.amjcard.2020.12.073
Talasaz, Recent Randomized Trials of Antithrombotic Therapy for Patients With COVID-19: JACC State-of-the-Art Review, J. Am. Coll. Cardiol, doi:10.1016/j.jacc.2021.02.035
DOI record:
{
"DOI": "10.21203/rs.3.rs-2313880/v1",
"URL": "http://dx.doi.org/10.21203/rs.3.rs-2313880/v1",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:p>Background\n Our objective in this study is to know the impact of the use of asprin in anti-aggregation dose on the evolution during hospitalization of patients admitted in intensive care unit for a severe infection by SARS-COV-2.\nMethods\n We conducted a prospective study of patients admitted to our department with severe COVID-19 infection during the period between March 2020 and March 2022, analyzing the difference between the placebo group and the aspirin group on the primary endpoint of all-cause hospital mortality and the composite secondary endpoint of use of mechanical ventilation and thromboembolic events.\nResults\n Out of 1124 patients included, 32.6% died, with a protective effect of aspirin against placebo (Hazard-ratio = 0.691, p = 0.003), for thrombo-embolic complications, 104 events were observed, with a protective effect of aspirin (Hazard-Ratio = 0.448 and p = 0.001), finally regarding mechanical ventilation, there was no remarkable benefit on our sample.\nConclusion\n Given the divergence of results of studies published in the literature, the availability of results of large randomized controlled trials is a necessity.</jats:p>",
"accepted": {
"date-parts": [
[
2022,
11,
25
]
]
},
"author": [
{
"affiliation": [
{
"name": "Mohammed I st University"
}
],
"family": "Aidouni",
"given": "Ghizlane El",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Mohammed I st University"
}
],
"family": "Bouchlarhem",
"given": "Amine",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Mohammed I st University"
}
],
"family": "Bkiyar",
"given": "Houssam",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Mohammed I st University"
}
],
"family": "Ismaili",
"given": "Nabila",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Mohammed I st University"
}
],
"family": "Ouafi",
"given": "Noha El",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Mohammed I st University"
}
],
"family": "housni",
"given": "Brahim",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
11,
30
]
],
"date-time": "2022-11-30T19:44:40Z",
"timestamp": 1669837480000
},
"deposited": {
"date-parts": [
[
2022,
11,
30
]
],
"date-time": "2022-11-30T19:44:42Z",
"timestamp": 1669837482000
},
"group-title": "In Review",
"indexed": {
"date-parts": [
[
2022,
12,
1
]
],
"date-time": "2022-12-01T06:01:35Z",
"timestamp": 1669874495833
},
"institution": [
{
"name": "Research Square"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
11,
30
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
11,
30
]
],
"date-time": "2022-11-30T00:00:00Z",
"timestamp": 1669766400000
}
}
],
"link": [
{
"URL": "https://www.researchsquare.com/article/rs-2313880/v1",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.researchsquare.com/article/rs-2313880/v1.html",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "8761",
"original-title": [],
"posted": {
"date-parts": [
[
2022,
11,
30
]
]
},
"prefix": "10.21203",
"published": {
"date-parts": [
[
2022,
11,
30
]
]
},
"publisher": "Research Square Platform LLC",
"reference": [
{
"DOI": "10.1016/j.cpcardiol.2020.100742",
"article-title": "Thrombosis and Coagulopathy in COVID-19",
"author": "Gómez-Mesa JE",
"doi-asserted-by": "publisher",
"first-page": "100742",
"issue": "3",
"journal-title": "Curr Probl Cardiol",
"key": "ref1",
"unstructured": "Gómez-Mesa JE, Galindo-Coral S, Montes MC, Muñoz Martin AJ. Thrombosis and Coagulopathy in COVID-19. Curr Probl Cardiol. Mar. 2021;46(3):100742. doi:10.1016/j.cpcardiol.2020.100742. “ ,”, , .",
"volume": "46",
"year": "2021"
},
{
"DOI": "10.1136/fmch-2020-000741",
"article-title": "Rationales and uncertainties for aspirin use in COVID-19: A narrative review",
"author": "Ahmed HAS",
"doi-asserted-by": "publisher",
"issue": "2",
"journal-title": "Fam Med Community Heal",
"key": "ref2",
"unstructured": "Ahmed HAS, et al., “Rationales and uncertainties for aspirin use in COVID-19: A narrative review,” Fam Med Community Heal, 9, 2, 2021, doi:10.1136/fmch-2020-000741.",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1016/j.tcm.2020.12.004",
"article-title": "“COVID-19 and thrombosis: From bench to bedside",
"author": "Ali MAM",
"doi-asserted-by": "publisher",
"first-page": "143",
"issue": "3",
"journal-title": "” Trends Cardiovasc Med",
"key": "ref3",
"unstructured": "Ali MAM, Spinler SA. “COVID-19 and thrombosis: From bench to bedside. ” Trends Cardiovasc Med. Apr. 2021;31(3):143–60. doi:10.1016/j.tcm.2020.12.004.",
"volume": "31",
"year": "2021"
},
{
"DOI": "10.1007/s11239-020-02374-3",
"article-title": "“SARS-CoV-2 infection and thrombotic complications: a narrative review",
"author": "Moschonas IC",
"doi-asserted-by": "publisher",
"first-page": "111",
"issue": "1",
"journal-title": "” J Thromb Thrombolysis",
"key": "ref4",
"unstructured": "Moschonas IC, Tselepis AD. “SARS-CoV-2 infection and thrombotic complications: a narrative review. ” J Thromb Thrombolysis. Jul. 2021;52(1):111–23. doi:10.1007/s11239-020-02374-3.",
"volume": "52",
"year": "2021"
},
{
"DOI": "10.1016/j.semerg.2020.07.007",
"article-title": "“[COVID-19 and thromboprophylaxis: Recommendations for our clinical practice in Primary Care]",
"author": "Piera Carbonell A",
"doi-asserted-by": "publisher",
"first-page": "479",
"issue": "7",
"journal-title": "” Semergen",
"key": "ref5",
"unstructured": "Piera Carbonell A, et al. “[COVID-19 and thromboprophylaxis: Recommendations for our clinical practice in Primary Care]. ” Semergen. Oct. 2020;46(7):479–86. doi:10.1016/j.semerg.2020.07.007.",
"volume": "46",
"year": "2020"
},
{
"DOI": "10.1183/13993003.00377-2021",
"author": "Birnhuber A",
"doi-asserted-by": "publisher",
"key": "ref6",
"unstructured": "Birnhuber A, et al., “Between inflammation and thrombosis: endothelial cells in COVID-19.,” The European respiratory journal, vol. 58, no. 3. Sep. 2021, doi: 10.1183/13993003.00377-2021.",
"year": "2021"
},
{
"DOI": "10.1007/s11906-016-0688-8",
"article-title": "Effects of Aspirin on Endothelial Function and Hypertension",
"author": "Dzeshka MS",
"doi-asserted-by": "publisher",
"first-page": "83",
"issue": "11",
"journal-title": "” Curr Hypertens Rep",
"key": "ref7",
"unstructured": "Dzeshka MS, Shantsila A, Lip GYH. Effects of Aspirin on Endothelial Function and Hypertension. ” Curr Hypertens Rep. Nov. 2016;18(11):83. doi:10.1007/s11906-016-0688-8. “, ,, .",
"volume": "18",
"year": "2016"
},
{
"DOI": "10.1111/irv.12421",
"article-title": "Antiviral activity of aspirin against RNA viruses of the respiratory tract-an in vitro study",
"author": "Glatthaar-Saalmüller B",
"doi-asserted-by": "publisher",
"first-page": "85",
"issue": "1",
"journal-title": "” Influenza Other Respi Viruses",
"key": "ref8",
"unstructured": "Glatthaar-Saalmüller B, Mair KH, Saalmüller A. Antiviral activity of aspirin against RNA viruses of the respiratory tract-an in vitro study. ” Influenza Other Respi Viruses. Jan. 2017;11(1):85–92. doi:10.1111/irv.12421. “, ,, .",
"volume": "11",
"year": "2017"
},
{
"DOI": "10.1016/S0140-6736(21)01825-0",
"article-title": "Aspirin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial",
"author": "Abani O",
"doi-asserted-by": "publisher",
"first-page": "143",
"journal-title": "Lancet",
"key": "ref9",
"unstructured": "Abani O, et al. Aspirin in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2022;399:10320, pp. 143–51. doi:10.1016/S0140-6736(21)01825-0. “,”, no.",
"volume": "399",
"year": "2022"
},
{
"DOI": "10.1007/s00228-022-03356-5",
"author": "Ma S",
"doi-asserted-by": "publisher",
"key": "ref10",
"unstructured": "Ma S, et al., “Does aspirin have an effect on risk of death in patients with COVID-19? A meta-analysis.,” Eur. J. Clin. Pharmacol., vol. 78, no. 9, pp. 1403–1420, Sep. 2022, doi: 10.1007/s00228-022-03356-5.",
"year": "2022"
},
{
"DOI": "10.1016/j.amjcard.2020.12.073",
"author": "Salah HM",
"doi-asserted-by": "publisher",
"key": "ref11",
"unstructured": "Salah HM, Mehta JL, “Meta-Analysis of the Effect of Aspirin on Mortality in COVID-19.,” The American journal of cardiology, vol. 142. pp. 158–159, Mar. 2021, doi: 10.1016/j.amjcard.2020.12.073.",
"year": "2021"
},
{
"DOI": "10.1002/rth2.12743",
"article-title": "No association of low-dose aspirin with severe COVID‐19 in France: A cohort of 31.1 million people without cardiovascular disease",
"author": "Botton J",
"doi-asserted-by": "publisher",
"first-page": "1",
"issue": "4",
"journal-title": "Res Pract Thromb Haemost",
"key": "ref12",
"unstructured": "Botton J, et al. No association of low-dose aspirin with severe COVID‐19 in France: A cohort of 31.1 million people without cardiovascular disease. Res Pract Thromb Haemost. 2022;6(4):1–6. doi:10.1002/rth2.12743. “,”, , .",
"volume": "6",
"year": "2022"
},
{
"DOI": "10.1016/j.jacc.2021.02.035",
"author": "Talasaz AH",
"doi-asserted-by": "publisher",
"key": "ref13",
"unstructured": "Talasaz AH, et al., “Recent Randomized Trials of Antithrombotic Therapy for Patients With COVID-19: JACC State-of-the-Art Review.,” J. Am. Coll. Cardiol., vol. 77, no. 15, pp. 1903–1921, Apr. 2021, doi: 10.1016/j.jacc.2021.02.035.",
"year": "2021"
},
{
"DOI": "10.1007/s11739-021-02870-1",
"article-title": "Does admission acetylsalicylic acid uptake in hospitalized COVID-19 patients have a protective role? Data from the Spanish SEMI-COVID-19 Registry",
"author": "Formiga F",
"doi-asserted-by": "publisher",
"first-page": "761",
"issue": "3",
"journal-title": "Intern Emerg Med",
"key": "ref14",
"unstructured": "Formiga F, et al. Does admission acetylsalicylic acid uptake in hospitalized COVID-19 patients have a protective role? Data from the Spanish SEMI-COVID-19 Registry. Intern Emerg Med. 2022;17(3):761–75. doi:10.1007/s11739-021-02870-1. “,”, , .",
"volume": "17",
"year": "2022"
},
{
"DOI": "10.1177/1358863X211012754",
"article-title": "Effect of aspirin on short-term outcomes in hospitalized patients with COVID-19",
"author": "Sahai A",
"doi-asserted-by": "publisher",
"first-page": "626",
"issue": "6",
"journal-title": "Vasc Med (United Kingdom)",
"key": "ref15",
"unstructured": "Sahai A, et al. Effect of aspirin on short-term outcomes in hospitalized patients with COVID-19. Vasc Med (United Kingdom). 2021;26(6):626–32. doi:10.1177/1358863X211012754. “,”, , .",
"volume": "26",
"year": "2021"
}
],
"reference-count": 15,
"references-count": 15,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.researchsquare.com/article/rs-2313880/v1"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "The impact of asprin use on the outcome of patients admitted to the intensive care unit with COVID-19 infection",
"type": "posted-content"
}