Colchicine Is Safe Though Ineffective in the Treatment of Severe COVID-19: a Randomized Clinical Trial (COLCHIVID)
et al., Journal of General Internal Medicine, doi:10.1007/s11606-021-07203-8, Nov 2021
Colchicine for COVID-19
5th treatment shown to reduce risk in
September 2020, now with p = 0.0000049 from 54 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Very late stage RCT with 56 colchicine and 60 control patients in Mexico, showing no significant differences.
Although the 29% lower mortality is not statistically significant, it is consistent with the significant 22% lower mortality [12‑31%] from meta-analysis of the 41 mortality results to date.
|
risk of death, 28.6% lower, RR 0.71, p = 0.74, treatment 4 of 56 (7.1%), control 6 of 60 (10.0%), NNT 35.
|
|
progression to critical or death, 17.0% lower, OR 0.83, p = 0.67, treatment 56, control 60, primary outcome, RR approximated with OR.
|
|
risk of no recovery, 13.0% higher, RR 1.13, p = 0.59, treatment 56, control 60, Kaplan-Meier.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Absalón-Aguilar et al., 9 Nov 2021, Double Blind Randomized Controlled Trial, placebo-controlled, Mexico, peer-reviewed, 18 authors, study period May 2020 - April 2021, dosage 1.5mg day 1, 1mg days 2-10.
Colchicine Is Safe Though Ineffective in the Treatment of Severe COVID-19: a Randomized Clinical Trial (COLCHIVID)
Journal of General Internal Medicine, doi:10.1007/s11606-021-07203-8
BACKGROUND: Colchicine is an available, safe, and effective anti-inflammatory drug and has been suggested as a COVID-19 treatment, but its usefulness in hospitalized severe COVID-19 patients has not been thoroughly demonstrated.
OBJECTIVE: To address the safety and efficacy of colchicine in hospitalized patients with severe COVID-19.
DESIGN: We conducted a triple-blind parallel nonstratified placebo-controlled clinical trial.
PARTICIPANTS: We recruited 116 hospitalized patients with severe COVID-19 in Mexico.
INTERVENTIONS: Patients were randomized to receive 1.5 mg of colchicine or placebo at the time of the recruitment in the study (baseline) and 0.5 mg BID PO to complete 10 days of treatment.
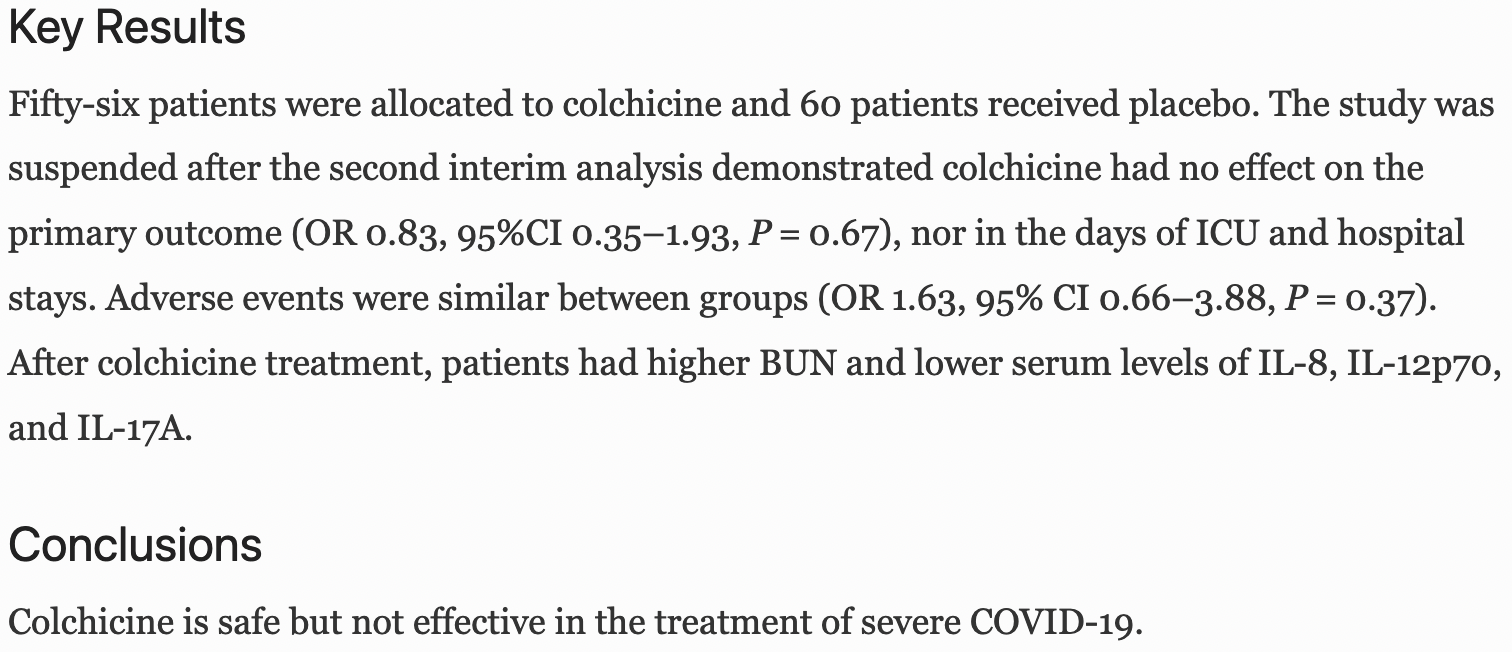
MAIN MEASURES: The primary composite outcome was the progression to critical disease or death. Besides, we evaluated immunological features at baseline and after recovery or disease progression in 20 patients. KEY RESULTS: Fifty-six patients were allocated to colchicine and 60 patients received placebo. The study was suspended after the second interim analysis demonstrated colchicine had no effect on the primary outcome (OR 0.83, 95%CI 0.35-1.93, P = 0.67), nor in the days of ICU and hospital stays. Adverse events were similar between groups (OR 1.63, 95% CI 0.66-3.88, P = 0.37). After colchicine treatment, patients had higher BUN and lower serum levels of IL-8, IL-12p70, and IL-17A.
Declarations
Conflict of Interest The authors declare that they do not have a conflict of interest. Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http:// creat iveco mmons. org/ licen ses/ by/4. 0/. Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Apostolidou, Skendros, Kambas, Mitroulis, Konstantinidis et al., Neutrophil extracellular traps regulate IL-1beta-mediated inflammation in familial Mediterranean fever, Ann Rheum Dis, doi:10.1136/annrheumdis-2014-205958.
Barnes, Adrover, Baxter-Stoltzfus, Borczuk, Cools-Lartigue et al., Targeting potential drivers of COVID-19: Neutrophil extracellular traps, J Exp Med, doi:10.1084/jem.20200652
Borges, Pithon-Curi, Curi, Hatanaka, COVID-19 and Neutrophils: The Relationship between Hyperinflammation and Neutrophil Extracellular Traps, Mediators Inflamm, doi:10.1155/2020/8829674.
Brunetti, Diawara, Tsai, Firestein, Nahass et al., Colchicine to Weather the Cytokine Storm in Hospitalized Patients with COVID-19, J Clin Med, doi:10.3390/jcm9092961.
Burrage, Koushesh, Sofat, Immunomodulatory Drugs in the Management of SARS-CoV-2, Front Immunol, doi:10.3389/fimmu.2020.01844.
Chalmers, Crichton, Goeminne, Cao, Humbert et al., Management of hospitalised adults with coronavirus disease 2019 (COVID-19): a European Respiratory Society living guideline, Eur Respir J, doi:10.1183/13993003.00048-2021
Chi, Ge, Wu, Zhang, Wu et al., Serum Cytokine and Chemokine Profile in Relation to the Severity of Coronavirus Disease 2019 in China, J Infect Dis, doi:10.1093/infdis/jiaa363.
Cicco, Cicco, Racanelli, Vacca, Neutrophil Extracellular Traps (NETs) and Damage-Associated Molecular Patterns (DAMPs): Two Potential Targets for COVID-19 Treatment, Mediators Inflamm, doi:10.1155/2020/7527953.
Deftereos, Giannopoulos, Vrachatis, Siasos, Giotaki et al., Effect of Colchicine vs Standard Care on Cardiac and Inflammatory Biomarkers and Clinical Outcomes in Patients Hospitalized With Coronavirus Disease 2019: The GRECCO-19 Randomized Clinical Trial, JAMA Netw Open, doi:10.1001/jamanetworkopen.2020.13136.
Demidowich, Levine, Apps, Cheung, Chen et al., Colchicine's effects on metabolic and inflammatory molecules in adults with obesity and metabolic syndrome: results from a pilot randomized controlled trial, Int J Obes (Lond), doi:10.1038/s41366-020-0598-3.
Elshafei, El-Bardissy, Khalil, Danjuma, Mubasher et al., Colchicine use might be associated with lower mortality in COVID-19 patients: A meta-analysis, Eur J Clin Invest, doi:10.1111/eci.13645
Group, Horby, Lim, Emberson, Mafham et al., Dexamethasone in Hospitalized Patients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2021436.
Hariyanto, Halim, Jodhinata, Yanto, Kurniawan, Colchicine treatment can improve outcomes of coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis, Clin Exp Pharmacol Physiol, doi:10.1111/1440-1681.13488.
Horby, Campbell, Spata, Colchicine in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label
Huang, Wang, Li, Ren, Zhao et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, The Lancet, doi:10.1016/s0140-6736(20)30183-5.
Kaiser, Leunig, Pekayvaz, Popp, Joppich et al., Self-sustaining interleukin-8 loops drive a prothrombotic neutrophil phenotype in severe COVID-19, doi:10.1172/jci.insight.150862.
Karatza, Ismailos, Karalis, Colchicine for the treatment of COVID-19 patients: efficacy, safety, and model informed dosage regimens, Xenobiotica, doi:10.1080/00498254.2021.1909782.
Li, Zhang, Fang, Zhao, Qian et al., The prognostic value of IL-8 for the death of severe or critical patients with COVID-19, Medicine, doi:10.1097/MD.0000000000023656.
Liu, Tao, Lei, Li, Kui et al., Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease, Chin Med J (Engl, doi:10.1097/CM9.0000000000000775
Lood, Blanco, Purmalek, Carmona-Rivera, Ravin et al., Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease, Nat Med, doi:10.1038/nm.4027
Lopes, Bonjorno, Giannini, Amaral, Menezes et al., Beneficial effects of colchicine for moderate to severe COVID-19: a randomised, double-blinded, placebo-controlled clinical trial, RMD Open, doi:10.1136/rmdopen-2020-001455
Ma, Zhang, Ye, Chen, Yu et al., High Levels of Circulating IL-8 and Soluble IL-2R Are Associated With Prolonged Illness in Patients With Severe COVID-19, Front Immunol, doi:10.3389/fimmu.2021.626235.
Manenti, Maggiore, Fiaccadori, Meschi, Antoni et al., Reduced mortality in COVID-19 patients treated with colchicine: Results from a retrospective, observational study, PLoS One, doi:10.1371/journal.pone.0248276.
Misra, Gasparyan, Zimba, Benefits and adverse effects of hydroxychloroquine, methotrexate and colchicine: searching for repurposable drug candidates, Rheumatol Int, doi:10.1007/s00296-020-04694-2.
Nawangsih, Kusmala, Rakhmat, Handayani, Juliastuti et al., Colchicine and mortality in patients with coronavirus disease 2019 (COVID-19) pneumonia: A systematic review, meta-analysis, and meta-regression, Int Immunopharmacol, doi:10.1016/j.intimp.2021.107723.
Olivas-Martinez, Jimenez, Lozano-Cruz, Ortiz-Brizuela, Tovar-Mendez, In-hospital mortality from severe COVID-19 in a tertiary care center in Mexico City; causes of death, risk factors and the impact of hospital saturation, PLoS One, doi:10.1371/journal.pone.0245772
Ong, Fong, Young, Chan, Lee et al., Persistent Symptoms and Association With Inflammatory Cytokine Signatures in Recovered Coronavirus Disease 2019 Patients, Open Forum Infect Dis, doi:10.1093/ofid/ofab156.
Panigada, Bottino, Tagliabue, Grasselli, Novembrino et al., Hypercoagulability of COVID-19 patients in Intensive Care Unit. A Report of Thromboelastography Findings and other Parameters of Hemostasis, J Thromb Haemost, doi:10.1111/jth.14850
Peter, Sandeep, Rao, Kalpana, Calming the Storm: Natural Immunosuppressants as Adjuvants to Target the Cytokine Storm in COVID-19, Front Pharmacol, doi:10.3389/fphar.2020.583777.
Pisaniello, Fisher, Farquhar, Vargas-Santos, Hill et al., Efficacy and safety of gout flare prophylaxis and therapy use in people with chronic kidney disease: a Gout, Hyperuricemia and Crystal-Associated Disease Network (G-CAN)-initiated literature review, Arthritis Res Ther, doi:10.1186/s13075-021-02416-y.
Raucci, Mansour, Casillo, Saviano, Caso et al., Interleukin-17A (IL-17A), a key molecule of innate and adaptive immunity, and its potential involvement in COVID-19-related thrombotic and vascular mechanisms, Autoimmun Rev, doi:10.1016/j.autrev.2020.102572.
Reyes, Hu, Teperman, Wampler Muskardin, Tardif et al., Anti-inflammatory therapy for COVID-19 infection: the case for colchicine, Ann Rheum Dis, doi:10.1136/annrheumdis-2020-219174
Sandhu, Tieng, Chilimuri, Franchin, A Case Control Study to Evaluate the Impact of Colchicine on Patients Admitted to the Hospital with Moderate to Severe COVID-19 Infection, Can J Infect Dis Med Microbiol, doi:10.1155/2020/8865954
Scarsi, Piantoni, Colombo, Airo, Richini et al., Association between treatment with colchicine and improved survival in a single-centre cohort of adult hospitalised patients with COVID-19 pneumonia and acute respiratory distress syndrome, Ann Rheum Dis, doi:10.1136/annrheumdis-2020-217712.
Schlesinger, Firestein, Brunetti, Colchicine in COVID-19: an Old Drug, New Use, Curr Pharmacol Rep, doi:10.1007/s40495-020-00225-6
Siemieniuk, Bartoszko, Ge, Zeraatkar, Izcovich et al., Drug treatments for covid-19: living systematic review and network meta-analysis, BMJ, doi:10.1136/bmj.m2980.
Tardif, Bouabdallaoui, Allier, Gaudet, Shah et al., Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial, Lancet Respir Med, doi:10.1016/S2213-2600(21)00222-8.
Vrachatis, Giannopoulos, Giotaki, Raisakis, Kossyvakis et al., Impact of colchicine on mortality in patients with COVID-19: A meta-analysis, Hellenic J Cardiol, doi:10.1016/j.hjc.2020.11.012.
Yuce, Filiztekin, Ozkaya, COVID-19 diagnosis -A review of current methods, Biosens Bioelectron, doi:10.1016/j.bios.2020.112752.
Yue, Liang, Gu, Zhao, Zhang et al., Comparative transcriptome analysis to elucidate the therapeutic mechanism of colchicine against atrial fibrillation, Biomed Pharmacother, doi:10.1016/j.biopha.2019.109422.
Zhao, Qin, Zhang, Li, Liang et al., Longitudinal COVID-19 profiling associates IL-1RA and IL-10 with disease severity and RANTES with mild disease, JCI Insight, doi:10.1172/jci.insight.139834
DOI record:
{
"DOI": "10.1007/s11606-021-07203-8",
"ISSN": [
"0884-8734",
"1525-1497"
],
"URL": "http://dx.doi.org/10.1007/s11606-021-07203-8",
"abstract": "<jats:title>Abstract</jats:title><jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Colchicine is an available, safe, and effective anti-inflammatory drug and has been suggested as a COVID-19 treatment, but its usefulness in hospitalized severe COVID-19 patients has not been thoroughly demonstrated.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Objective</jats:title>\n <jats:p>To address the safety and efficacy of colchicine in hospitalized patients with severe COVID-19.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Design</jats:title>\n <jats:p>We conducted a triple-blind parallel non-stratified placebo-controlled clinical trial.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Participants</jats:title>\n <jats:p>We recruited 116 hospitalized patients with severe COVID-19 in Mexico.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Interventions</jats:title>\n <jats:p>Patients were randomized to receive 1.5 mg of colchicine or placebo at the time of the recruitment in the study (baseline) and 0.5 mg BID PO to complete 10 days of treatment.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Main Measures</jats:title>\n <jats:p>The primary composite outcome was the progression to critical disease or death. Besides, we evaluated immunological features at baseline and after recovery or disease progression in 20 patients.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Key Results</jats:title>\n <jats:p>Fifty-six patients were allocated to colchicine and 60 patients received placebo. The study was suspended after the second interim analysis demonstrated colchicine had no effect on the primary outcome (OR 0.83, 95%CI 0.35–1.93, <jats:italic>P</jats:italic> = 0.67), nor in the days of ICU and hospital stays. Adverse events were similar between groups (OR 1.63, 95% CI 0.66–3.88, <jats:italic>P</jats:italic> = 0.37). After colchicine treatment, patients had higher BUN and lower serum levels of IL-8, IL-12p70, and IL-17A.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>Colchicine is safe but not effective in the treatment of severe COVID-19.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Trial Registration</jats:title>\n <jats:p>ClinicalTrials.gov Identifier: NCT04367168.</jats:p>\n </jats:sec>",
"alternative-id": [
"7203"
],
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "6 September 2021"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "4 October 2021"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "9 November 2021"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Conflict of Interest",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "The authors declare that they do not have a conflict of interest."
}
],
"author": [
{
"affiliation": [],
"family": "Absalón-Aguilar",
"given": "Abdiel",
"sequence": "first"
},
{
"affiliation": [],
"family": "Rull-Gabayet",
"given": "Marina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pérez-Fragoso",
"given": "Alfredo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mejía-Domínguez",
"given": "Nancy R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Núñez-Álvarez",
"given": "Carlos",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kershenobich-Stalnikowitz",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sifuentes-Osornio",
"given": "José",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ponce-de-León",
"given": "Alfredo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "González-Lara",
"given": "Fernanda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Martín-Nares",
"given": "Eduardo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Montesinos-Ramírez",
"given": "Sharon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ramírez-Alemón",
"given": "Martha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ramírez-Rangel",
"given": "Pamela",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Márquez",
"given": "Manlio F.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Plata-Corona",
"given": "Juan Carlos",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Juárez-Vega",
"given": "Guillermo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gómez-Martín",
"given": "Diana",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7882-1488",
"affiliation": [],
"authenticated-orcid": false,
"family": "Torres-Ruiz",
"given": "Jiram",
"sequence": "additional"
}
],
"container-title": [
"Journal of General Internal Medicine"
],
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2021,
11,
9
]
],
"date-time": "2021-11-09T18:03:40Z",
"timestamp": 1636481020000
},
"deposited": {
"date-parts": [
[
2022,
1,
6
]
],
"date-time": "2022-01-06T21:36:56Z",
"timestamp": 1641505016000
},
"indexed": {
"date-parts": [
[
2022,
1,
7
]
],
"date-time": "2022-01-07T06:43:15Z",
"timestamp": 1641537795689
},
"is-referenced-by-count": 1,
"issn-type": [
{
"type": "print",
"value": "0884-8734"
},
{
"type": "electronic",
"value": "1525-1497"
}
],
"issue": "1",
"issued": {
"date-parts": [
[
2021,
11,
9
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2022,
1
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
11,
9
]
],
"date-time": "2021-11-09T00:00:00Z",
"timestamp": 1636416000000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
11,
9
]
],
"date-time": "2021-11-09T00:00:00Z",
"timestamp": 1636416000000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1007/s11606-021-07203-8.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1007/s11606-021-07203-8/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1007/s11606-021-07203-8.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"page": "4-14",
"prefix": "10.1007",
"published": {
"date-parts": [
[
2021,
11,
9
]
]
},
"published-online": {
"date-parts": [
[
2021,
11,
9
]
]
},
"published-print": {
"date-parts": [
[
2022,
1
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1371/journal.pone.0245772",
"author": "A Olivas-Martinez",
"doi-asserted-by": "publisher",
"first-page": "e0245772",
"issue": "2",
"journal-title": "PLoS One.",
"key": "7203_CR1",
"unstructured": "Olivas-Martinez A, Cardenas-Fragoso JL, Jimenez JV, Lozano-Cruz OA, Ortiz-Brizuela E, Tovar-Mendez VH, et al. In-hospital mortality from severe COVID-19 in a tertiary care center in Mexico City; causes of death, risk factors and the impact of hospital saturation. PLoS One. 2021;16(2):e0245772. https://doi.org/10.1371/journal.pone.0245772.",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1016/s0140-6736(20)30183-5",
"author": "C Huang",
"doi-asserted-by": "publisher",
"first-page": "497",
"issue": "10223",
"journal-title": "The Lancet.",
"key": "7203_CR2",
"unstructured": "Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497–506. https://doi.org/10.1016/s0140-6736(20)30183-5.",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1155/2020/8829674",
"author": "L Borges",
"doi-asserted-by": "publisher",
"first-page": "8829674",
"journal-title": "Mediators Inflamm.",
"key": "7203_CR3",
"unstructured": "Borges L, Pithon-Curi TC, Curi R, Hatanaka E. COVID-19 and Neutrophils: The Relationship between Hyperinflammation and Neutrophil Extracellular Traps. Mediators Inflamm. 2020;2020:8829674. https://doi.org/10.1155/2020/8829674.",
"volume": "2020",
"year": "2020"
},
{
"DOI": "10.1084/jem.20200652",
"doi-asserted-by": "publisher",
"key": "7203_CR4",
"unstructured": "Barnes BJ, Adrover JM, Baxter-Stoltzfus A, Borczuk A, Cools-Lartigue J, Crawford JM, et al. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J Exp Med. 2020;217(6). https://doi.org/10.1084/jem.20200652."
},
{
"DOI": "10.1111/jth.14850",
"doi-asserted-by": "publisher",
"key": "7203_CR5",
"unstructured": "Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V, et al. Hypercoagulability of COVID-19 patients in Intensive Care Unit. A Report of Thromboelastography Findings and other Parameters of Hemostasis. J Thromb Haemost. 2020. https://doi.org/10.1111/jth.14850."
},
{
"DOI": "10.1136/bmj.m2980",
"author": "RA Siemieniuk",
"doi-asserted-by": "publisher",
"first-page": "m2980",
"journal-title": "BMJ.",
"key": "7203_CR6",
"unstructured": "Siemieniuk RA, Bartoszko JJ, Ge L, Zeraatkar D, Izcovich A, Kum E, et al. Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ. 2020;370:m2980. https://doi.org/10.1136/bmj.m2980.",
"volume": "370",
"year": "2020"
},
{
"DOI": "10.1136/annrheumdis-2020-219174",
"author": "AZ Reyes",
"doi-asserted-by": "publisher",
"journal-title": "Ann Rheum Dis.",
"key": "7203_CR7",
"unstructured": "Reyes AZ, Hu KA, Teperman J, Wampler Muskardin TL, Tardif JC, Shah B, et al. Anti-inflammatory therapy for COVID-19 infection: the case for colchicine. Ann Rheum Dis. 2020. https://doi.org/10.1136/annrheumdis-2020-219174.",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2020.01844",
"author": "DR Burrage",
"doi-asserted-by": "publisher",
"first-page": "1844",
"journal-title": "Front Immunol.",
"key": "7203_CR8",
"unstructured": "Burrage DR, Koushesh S, Sofat N. Immunomodulatory Drugs in the Management of SARS-CoV-2. Front Immunol. 2020;11:1844. https://doi.org/10.3389/fimmu.2020.01844.",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1007/s40495-020-00225-6",
"doi-asserted-by": "publisher",
"key": "7203_CR9",
"unstructured": "Schlesinger N, Firestein BL, Brunetti L. Colchicine in COVID-19: an Old Drug, New Use. Curr Pharmacol Rep. 2020:1–9. https://doi.org/10.1007/s40495-020-00225-6."
},
{
"DOI": "10.1038/s41366-020-0598-3",
"author": "AP Demidowich",
"doi-asserted-by": "publisher",
"first-page": "1793",
"issue": "8",
"journal-title": "Int J Obes (Lond).",
"key": "7203_CR10",
"unstructured": "Demidowich AP, Levine JA, Apps R, Cheung FK, Chen J, Fantoni G, et al. Colchicine's effects on metabolic and inflammatory molecules in adults with obesity and metabolic syndrome: results from a pilot randomized controlled trial. Int J Obes (Lond). 2020;44(8):1793–9. https://doi.org/10.1038/s41366-020-0598-3.",
"volume": "44",
"year": "2020"
},
{
"DOI": "10.1007/s00296-020-04694-2",
"author": "DP Misra",
"doi-asserted-by": "publisher",
"first-page": "1741",
"issue": "11",
"journal-title": "Rheumatol Int.",
"key": "7203_CR11",
"unstructured": "Misra DP, Gasparyan AY, Zimba O. Benefits and adverse effects of hydroxychloroquine, methotrexate and colchicine: searching for repurposable drug candidates. Rheumatol Int. 2020;40(11):1741–51. https://doi.org/10.1007/s00296-020-04694-2.",
"volume": "40",
"year": "2020"
},
{
"DOI": "10.1080/00498254.2021.1909782",
"author": "E Karatza",
"doi-asserted-by": "publisher",
"first-page": "643",
"issue": "6",
"journal-title": "Xenobiotica.",
"key": "7203_CR12",
"unstructured": "Karatza E, Ismailos G, Karalis V. Colchicine for the treatment of COVID-19 patients: efficacy, safety, and model informed dosage regimens. Xenobiotica. 2021;51(6):643–56. https://doi.org/10.1080/00498254.2021.1909782.",
"volume": "51",
"year": "2021"
},
{
"DOI": "10.1111/1440-1681.13488",
"author": "TI Hariyanto",
"doi-asserted-by": "publisher",
"first-page": "823",
"issue": "6",
"journal-title": "Clin Exp Pharmacol Physiol.",
"key": "7203_CR13",
"unstructured": "Hariyanto TI, Halim DA, Jodhinata C, Yanto TA, Kurniawan A. Colchicine treatment can improve outcomes of coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. Clin Exp Pharmacol Physiol. 2021;48(6):823–30. https://doi.org/10.1111/1440-1681.13488.",
"volume": "48",
"year": "2021"
},
{
"DOI": "10.1111/eci.13645",
"doi-asserted-by": "publisher",
"key": "7203_CR14",
"unstructured": "Elshafei MN, El-Bardissy A, Khalil A, Danjuma M, Mubasher M, Abubeker IY, et al. Colchicine use might be associated with lower mortality in COVID-19 patients: A meta-analysis. Eur J Clin Invest. 2021:e13645. https://doi.org/10.1111/eci.13645."
},
{
"DOI": "10.1016/j.intimp.2021.107723",
"author": "EN Nawangsih",
"doi-asserted-by": "publisher",
"first-page": "107723",
"journal-title": "Int Immunopharmacol.",
"key": "7203_CR15",
"unstructured": "Nawangsih EN, Kusmala YY, Rakhmat, II, Handayani DR, Juliastuti H, Wibowo A, et al. Colchicine and mortality in patients with coronavirus disease 2019 (COVID-19) pneumonia: A systematic review, meta-analysis, and meta-regression. Int Immunopharmacol. 2021;96:107723. https://doi.org/10.1016/j.intimp.2021.107723.",
"volume": "96",
"year": "2021"
},
{
"DOI": "10.1016/j.hjc.2020.11.012",
"author": "DA Vrachatis",
"doi-asserted-by": "publisher",
"journal-title": "Hellenic J Cardiol.",
"key": "7203_CR16",
"unstructured": "Vrachatis DA, Giannopoulos GV, Giotaki SG, Raisakis K, Kossyvakis C, Iliodromitis KE, et al. Impact of colchicine on mortality in patients with COVID-19: A meta-analysis. Hellenic J Cardiol. 2021. https://doi.org/10.1016/j.hjc.2020.11.012.",
"year": "2021"
},
{
"DOI": "10.1016/j.bios.2020.112752",
"author": "M Yuce",
"doi-asserted-by": "publisher",
"first-page": "112752",
"journal-title": "Biosens Bioelectron.",
"key": "7203_CR17",
"unstructured": "Yuce M, Filiztekin E, Ozkaya KG. COVID-19 diagnosis -A review of current methods. Biosens Bioelectron. 2021;172:112752. https://doi.org/10.1016/j.bios.2020.112752.",
"volume": "172",
"year": "2021"
},
{
"DOI": "10.1097/CM9.0000000000000775",
"doi-asserted-by": "publisher",
"key": "7203_CR18",
"unstructured": "Liu W, Tao ZW, Lei W, Ming-Li Y, Kui L, Ling Z, et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J (Engl). 2020. https://doi.org/10.1097/CM9.0000000000000775."
},
{
"DOI": "10.1056/NEJMoa2021436",
"author": "P Horby",
"doi-asserted-by": "publisher",
"first-page": "693",
"issue": "8",
"journal-title": "N Engl J Med.",
"key": "7203_CR19",
"unstructured": "Group RC, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384(8):693–704. https://doi.org/10.1056/NEJMoa2021436.",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(21)00222-8",
"author": "JC Tardif",
"doi-asserted-by": "publisher",
"journal-title": "Lancet Respir Med.",
"key": "7203_CR20",
"unstructured": "Tardif JC, Bouabdallaoui N, L'Allier PL, Gaudet D, Shah B, Pillinger MH, et al. Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial. Lancet Respir Med. 2021. https://doi.org/10.1016/S2213-2600(21)00222-8.",
"year": "2021"
},
{
"DOI": "10.1371/journal.pone.0248276",
"author": "L Manenti",
"doi-asserted-by": "publisher",
"first-page": "e0248276",
"issue": "3",
"journal-title": "PLoS One.",
"key": "7203_CR21",
"unstructured": "Manenti L, Maggiore U, Fiaccadori E, Meschi T, Antoni AD, Nouvenne A, et al. Reduced mortality in COVID-19 patients treated with colchicine: Results from a retrospective, observational study. PLoS One. 2021;16(3):e0248276. https://doi.org/10.1371/journal.pone.0248276.",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1136/rmdopen-2020-001455",
"doi-asserted-by": "publisher",
"key": "7203_CR22",
"unstructured": "Lopes MI, Bonjorno LP, Giannini MC, Amaral NB, Menezes PI, Dib SM, et al. Beneficial effects of colchicine for moderate to severe COVID-19: a randomised, double-blinded, placebo-controlled clinical trial. RMD Open. 2021;7(1). https://doi.org/10.1136/rmdopen-2020-001455."
},
{
"DOI": "10.1155/2020/8865954",
"author": "T Sandhu",
"doi-asserted-by": "publisher",
"first-page": "8865954",
"journal-title": "Can J Infect Dis Med Microbiol.",
"key": "7203_CR23",
"unstructured": "Sandhu T, Tieng A, Chilimuri S, Franchin G. A Case Control Study to Evaluate the Impact of Colchicine on Patients Admitted to the Hospital with Moderate to Severe COVID-19 Infection. Can J Infect Dis Med Microbiol. 2020;2020:8865954. https://doi.org/10.1155/2020/8865954.",
"volume": "2020",
"year": "2020"
},
{
"DOI": "10.1001/jamanetworkopen.2020.13136",
"author": "SG Deftereos",
"doi-asserted-by": "publisher",
"first-page": "e2013136",
"issue": "6",
"journal-title": "JAMA Netw Open.",
"key": "7203_CR24",
"unstructured": "Deftereos SG, Giannopoulos G, Vrachatis DA, Siasos GD, Giotaki SG, Gargalianos P, et al. Effect of Colchicine vs Standard Care on Cardiac and Inflammatory Biomarkers and Clinical Outcomes in Patients Hospitalized With Coronavirus Disease 2019: The GRECCO-19 Randomized Clinical Trial. JAMA Netw Open. 2020;3(6):e2013136. https://doi.org/10.1001/jamanetworkopen.2020.13136.",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.1136/annrheumdis-2020-217712",
"author": "M Scarsi",
"doi-asserted-by": "publisher",
"first-page": "1286",
"issue": "10",
"journal-title": "Ann Rheum Dis.",
"key": "7203_CR25",
"unstructured": "Scarsi M, Piantoni S, Colombo E, Airo P, Richini D, Miclini M, et al. Association between treatment with colchicine and improved survival in a single-centre cohort of adult hospitalised patients with COVID-19 pneumonia and acute respiratory distress syndrome. Ann Rheum Dis. 2020;79(10):1286–9. https://doi.org/10.1136/annrheumdis-2020-217712.",
"volume": "79",
"year": "2020"
},
{
"DOI": "10.3390/jcm9092961.",
"doi-asserted-by": "publisher",
"key": "7203_CR26",
"unstructured": "Brunetti L, Diawara O, Tsai A, Firestein BL, Nahass RG, Poiani G, et al. Colchicine to Weather the Cytokine Storm in Hospitalized Patients with COVID-19. J Clin Med. 2020;9(9). https://doi.org/10.3390/jcm9092961."
},
{
"key": "7203_CR27",
"unstructured": "Horby PW, Campbell M, Spata E, et al. Colchicine in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. MedRxiv. 2021."
},
{
"DOI": "10.1186/s13075-021-02416-y",
"author": "HL Pisaniello",
"doi-asserted-by": "publisher",
"first-page": "130",
"issue": "1",
"journal-title": "Arthritis Res Ther.",
"key": "7203_CR28",
"unstructured": "Pisaniello HL, Fisher MC, Farquhar H, Vargas-Santos AB, Hill CL, Stamp LK, et al. Efficacy and safety of gout flare prophylaxis and therapy use in people with chronic kidney disease: a Gout, Hyperuricemia and Crystal-Associated Disease Network (G-CAN)-initiated literature review. Arthritis Res Ther. 2021;23(1):130. https://doi.org/10.1186/s13075-021-02416-y.",
"volume": "23",
"year": "2021"
},
{
"DOI": "10.1093/ofid/ofab156",
"author": "SWX Ong",
"doi-asserted-by": "publisher",
"first-page": "ofab156",
"issue": "6",
"journal-title": "Open Forum Infect Dis.",
"key": "7203_CR29",
"unstructured": "Ong SWX, Fong SW, Young BE, Chan YH, Lee B, Amrun SN, et al. Persistent Symptoms and Association With Inflammatory Cytokine Signatures in Recovered Coronavirus Disease 2019 Patients. Open Forum Infect Dis. 2021;8(6):ofab156. https://doi.org/10.1093/ofid/ofab156.",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1172/jci.insight.150862",
"author": "R Kaiser",
"doi-asserted-by": "publisher",
"journal-title": "JCI Insight.",
"key": "7203_CR30",
"unstructured": "Kaiser R, Leunig A, Pekayvaz K, Popp O, Joppich M, Polewka V, et al. Self-sustaining interleukin-8 loops drive a prothrombotic neutrophil phenotype in severe COVID-19. JCI Insight. 2021. https://doi.org/10.1172/jci.insight.150862.",
"year": "2021"
},
{
"DOI": "10.3389/fimmu.2021.626235",
"author": "A Ma",
"doi-asserted-by": "publisher",
"first-page": "626235",
"journal-title": "Front Immunol.",
"key": "7203_CR31",
"unstructured": "Ma A, Zhang L, Ye X, Chen J, Yu J, Zhuang L, et al. High Levels of Circulating IL-8 and Soluble IL-2R Are Associated With Prolonged Illness in Patients With Severe COVID-19. Front Immunol. 2021;12:626235. https://doi.org/10.3389/fimmu.2021.626235.",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1097/MD.0000000000023656",
"author": "H Li",
"doi-asserted-by": "publisher",
"first-page": "e23656",
"issue": "11",
"journal-title": "Medicine (Baltimore).",
"key": "7203_CR32",
"unstructured": "Li H, Zhang J, Fang C, Zhao X, Qian B, Sun Y, et al. The prognostic value of IL-8 for the death of severe or critical patients with COVID-19. Medicine (Baltimore). 2021;100(11):e23656. https://doi.org/10.1097/MD.0000000000023656.",
"volume": "100",
"year": "2021"
},
{
"DOI": "10.1172/jci.insight.139834",
"doi-asserted-by": "publisher",
"key": "7203_CR33",
"unstructured": "Zhao Y, Qin L, Zhang P, Li K, Liang L, Sun J, et al. Longitudinal COVID-19 profiling associates IL-1RA and IL-10 with disease severity and RANTES with mild disease. JCI Insight. 2020;5(13). https://doi.org/10.1172/jci.insight.139834."
},
{
"DOI": "10.3389/fphar.2020.583777",
"author": "AE Peter",
"doi-asserted-by": "publisher",
"first-page": "583777",
"journal-title": "Front Pharmacol.",
"key": "7203_CR34",
"unstructured": "Peter AE, Sandeep BV, Rao BG, Kalpana VL. Calming the Storm: Natural Immunosuppressants as Adjuvants to Target the Cytokine Storm in COVID-19. Front Pharmacol. 2020;11:583777. https://doi.org/10.3389/fphar.2020.583777.",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1093/infdis/jiaa363",
"author": "Y Chi",
"doi-asserted-by": "publisher",
"first-page": "746",
"issue": "5",
"journal-title": "J Infect Dis.",
"key": "7203_CR35",
"unstructured": "Chi Y, Ge Y, Wu B, Zhang W, Wu T, Wen T, et al. Serum Cytokine and Chemokine Profile in Relation to the Severity of Coronavirus Disease 2019 in China. J Infect Dis. 2020;222(5):746–54. https://doi.org/10.1093/infdis/jiaa363.",
"volume": "222",
"year": "2020"
},
{
"DOI": "10.1016/j.autrev.2020.102572",
"author": "F Raucci",
"doi-asserted-by": "publisher",
"first-page": "102572",
"issue": "7",
"journal-title": "Autoimmun Rev.",
"key": "7203_CR36",
"unstructured": "Raucci F, Mansour AA, Casillo GM, Saviano A, Caso F, Scarpa R, et al. Interleukin-17A (IL-17A), a key molecule of innate and adaptive immunity, and its potential involvement in COVID-19-related thrombotic and vascular mechanisms. Autoimmun Rev. 2020;19(7):102572. https://doi.org/10.1016/j.autrev.2020.102572.",
"volume": "19",
"year": "2020"
},
{
"DOI": "10.1016/j.biopha.2019.109422",
"author": "H Yue",
"doi-asserted-by": "publisher",
"first-page": "109422",
"journal-title": "Biomed Pharmacother.",
"key": "7203_CR37",
"unstructured": "Yue H, Liang W, Gu J, Zhao X, Zhang T, Qin X, et al. Comparative transcriptome analysis to elucidate the therapeutic mechanism of colchicine against atrial fibrillation. Biomed Pharmacother. 2019;119:109422. https://doi.org/10.1016/j.biopha.2019.109422.",
"volume": "119",
"year": "2019"
},
{
"DOI": "10.1136/annrheumdis-2014-205958",
"author": "E Apostolidou",
"doi-asserted-by": "publisher",
"first-page": "269",
"issue": "1",
"journal-title": "Ann Rheum Dis.",
"key": "7203_CR38",
"unstructured": "Apostolidou E, Skendros P, Kambas K, Mitroulis I, Konstantinidis T, Chrysanthopoulou A, et al. Neutrophil extracellular traps regulate IL-1beta-mediated inflammation in familial Mediterranean fever. Ann Rheum Dis. 2016;75(1):269–77. https://doi.org/10.1136/annrheumdis-2014-205958.",
"volume": "75",
"year": "2016"
},
{
"DOI": "10.1155/2020/7527953",
"author": "S Cicco",
"doi-asserted-by": "publisher",
"first-page": "7527953",
"journal-title": "Mediators Inflamm.",
"key": "7203_CR39",
"unstructured": "Cicco S, Cicco G, Racanelli V, Vacca A. Neutrophil Extracellular Traps (NETs) and Damage-Associated Molecular Patterns (DAMPs): Two Potential Targets for COVID-19 Treatment. Mediators Inflamm. 2020;2020:7527953. https://doi.org/10.1155/2020/7527953.",
"volume": "2020",
"year": "2020"
},
{
"DOI": "10.1183/13993003.00048-2021",
"doi-asserted-by": "publisher",
"key": "7203_CR40",
"unstructured": "Chalmers JD, Crichton ML, Goeminne PC, Cao B, Humbert M, Shteinberg M, et al. Management of hospitalised adults with coronavirus disease 2019 (COVID-19): a European Respiratory Society living guideline. Eur Respir J. 2021;57(4). https://doi.org/10.1183/13993003.00048-2021."
},
{
"DOI": "10.1038/nm.4027",
"author": "C Lood",
"doi-asserted-by": "publisher",
"first-page": "146",
"issue": "2",
"journal-title": "Nat Med.",
"key": "7203_CR41",
"unstructured": "Lood C, Blanco LP, Purmalek MM, Carmona-Rivera C, De Ravin SS, Smith CK, et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat Med. 2016;22(2):146–53. https://doi.org/10.1038/nm.4027.",
"volume": "22",
"year": "2016"
}
],
"reference-count": 41,
"references-count": 41,
"relation": {},
"score": 1,
"short-container-title": [
"J GEN INTERN MED"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Internal Medicine"
],
"subtitle": [],
"title": [
"Colchicine Is Safe Though Ineffective in the Treatment of Severe COVID-19: a Randomized Clinical Trial (COLCHIVID)"
],
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "37"
}
